Abstract
Soil nitrogen (N) mineralization is an important process determining terrestrial N availability, and evidence suggests elevated temperatures will enhance N mineralization rates. Along a 40 km urban-rural gradient of chestnut oak forest stands in Louisville, KY, we expected N mineralization rates would be higher in urban than in rural forests in part due to increased temperatures caused by the urban heat island. However, a 12-month field study along this Louisville gradient showed that annual N mineralization rates were lower in urban than in rural stands. Since variation in precipitation inputs and other factors across this land-use gradient may be influencing soil N mineralization rates, we conducted a three-month soil incubation experiment in the lab to determine the extent to which a + 2 °C temperature difference could affect soil N mineralization in urban and rural soils. Across the range of temperatures tested, rural soils mineralized N at twice the rate of urban soils under base (7.86 vs. 3.65 mg N kg−1 AFDW soil d−1) and elevated (9.08 vs. 4.76 mg N kg−1 AFDW soil d−1) temperatures (p < 0.01). A 2 °C temperature difference, did not significantly alter total inorganic N production in urban (p = 0.272) or rural soils (p = 0.293). The proportion of nitrate produced was lower in the urban (15.1 %) than in the rural soils (72.3 %; p < 0.01). These results suggest that differences in soil organic matter quality and potentially decomposer community composition are the primary explanatory factors for forests along this Louisville gradient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban environments experience multiple anthropogenic effects that act singly and synergistically to modify ecosystem processes. Temperature, carbon dioxide (CO2), nitrogen (N), and ozone (O3) are four important abiotic factors causing environmental change at local, regional, and global levels (Grimm et al. 2008). Land-use change and human activities associated with cities are important drivers of these global changes but also alter these environmental factors at local scales. For example, cities are well known to be warmer than surrounding areas and create ‘urban heat islands’ largely due to conversion of soil and vegetation to impervious surfaces (Oke 1995; Bonan 2002). Since temperature is a strong controlling variable for many ecosystem processes, including ecosystem respiration, evapotranspiration and soil processes such as nitrogen (N) mineralization, the urban heat island may affect the net ecosystem productivity and species composition of natural areas within the urban landscape.
Natural areas, such as forests, are embedded within many land uses (i.e., city centers, suburbs, and rural agriculture areas), thus impacts from surrounding land on these natural systems can be studied using an urban-to-rural gradient approach (McDonnell and Pickett 1990; Medley et al. 1995; McDonnell et al. 1997). While there are now additional methods for studying urban ecosystems as whole systems (i.e., ecology of cities; Grimm et al. 2000) and as complex social-ecological systems (Pickett et al. 2011), the urban-rural gradient approach is a useful comparative method for investigating the combined effects of multiple environmental factors on ecosystem function (Groffman et al. 2006) and for generating hypotheses about more proximate controls on ecosystem behavior that can be tested using manipulated experiments (e.g., Gregg et al. 2003). Several studies have shown that forest remnants surrounded by urban land use display altered ecosystem function relative to nearby rural forest remnants (e.g., increased litter decay rates, atmospheric deposition fluxes, and soil nitrogen cycling; McDonnell et al., 1997; Carreiro et al. 2009; Pouyat et al. 2009). Understanding how urban land-use context affects forest soils and their processes may also be useful for predicting impacts on rural areas as they become converted to urban land, or potentially for predicting how global environmental change might affect rural habitats in their region in the future (Carreiro and Tripler 2005).
Soil temperature and moisture are considered the two most important abiotic factors influencing soil microbial activity and therefore microbially-mediated processes such as nitrogen mineralization. In situ soil warming experiments (Peterjohn et al. 1994; Rustad et al. 2001; Butler et al. 2012), as well as laboratory incubation studies (Gonçlaves and Carlyle 1994; Dalias et al. 2002; Franzluebbers et al. 2001), have shown increased soil mineralization rates under elevated temperatures. Since urban environments experience higher temperatures than nearby rural areas, such a temperature differential may have an important direct effect on soil processes and stimulate faster N production rates, when all other factors are equal.
During the growing season, urban forest remnants within Louisville, KY were measured to have mean monthly air and soil temperatures (10 cm depth) 1–2 °C greater than rural stands of similar tree composition, stand age, aspect and soil characteristics that were 40 km away from the city center (Carreiro et al., unpublished results). The primary goal of this study was to isolate and estimate the effect of elevated temperature on soil N mineralization rates of urban and rural forest soils collected from an urban-to-rural land-use gradient in Louisville, KY. We conducted this experiment in the lab to remove potential influences of precipitation and pollutant deposition differences that can occur during soil incubations in the field. Potential soil legacy differences between urban and rural soils, such as organic matter quality and quantity or microbial community differences, would remain to influence the temperature effects. We asked the following questions: 1) Once removed from the field, will urban soils mineralize N at rates similar to rural soils?, and 2) Does a temperature elevation of 2 °C from different baseline temperatures increase N mineralization and nitrate production in urban and rural forest soils and in a similar proportion? We hypothesized that lab-incubated urban soils would mineralize N at similar rates to rural soils once removed from site conditions, suggesting that other site factors like soil moisture are important in controlling N mineralization rates in these forest soils. Once isolated from site factors, we also expected a 2 °C increase in temperature would both increase N mineralization rates and the relative proportion of nitrate production in urban and rural forest soils. Since soil N production may be altered by other abiotic (i.e., N deposition, precipitation) and biotic (i.e., litter quantity and quality) factors, it is important to experimentally isolate temperature differential, such as that caused by the urban heat island effect, as a potential factor altering soil N mineralization in similar habitats at local-to-regional scales.
Methods
Study sites
The study area is located in the Interior Low Plateau, Bluegrass Section and the Eastern Broadleaf Forest biome (National Atlas of the U.S. 2013). Louisville (equivalent to Jefferson County since the city-county governmental merger in 2003) has a population of 741,000 and a mean density of 750 people km−1 (U.S. Census Bureau 2008). The mean annual precipitation for this region is 113 cm, and the mean annual maximum and minimum temperatures are 30.6 °C and −3.9 °C, respectively (National Climate Data Center 2009).
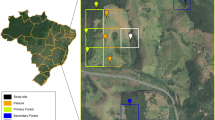
Study sites were located along a 40 km urban-rural gradient established in Louisville, KY (Jefferson County) extending southward into Bullitt County. All forest sites were located on erosional remnant hills that comprise “the Knobs” physiographic region of the Outer Bluegrass of Kentucky. The urban forest sites in Iroquois Park (300 ha) were located approximately 8 km south of the city center, and the rural forest sites in Bernheim Arboretum and Research Forest (5660 ha) were approximately 40 km south of the city center. We established three 400-m2 forest sites dominated by Quercus montana (Chestnut oak), which were at least 300 m – 1 km apart in the urban park and more than 1 km apart in the rural park. Sites were chosen to minimize variation in site factors except those related to surrounding land use. Tree composition, stand age, basal area, and soil characteristics were similar between urban and rural forest sites (Table 1). Plots were located on the eastern side of the Knob Hills on silt-dominated soils in the Tilsit Soil Series. To provide more information on the degree of urban-rural contrast for these forests, population density, built land, and road length were quantified within either 1.5 km of the forest boundary (population) or within a 2 km radius of each forest site (built land and road length) using Geographic Information System and data obtained from the U.S. Census Bureau (2000), the KY Natural Resources and Environmental Protection Cabinet, and KY Geological Survey (2001; Table 2).
Soil collection and analyses
Soil samples were collected from urban and rural forest sites (n = 3 sites per land use) underneath two chestnut oak trees, 1 m from the trunk, in September, October and November (n = 6 soil cores per land use per month). At each sampling date, loose litter was removed from the soil surface, and a soil core (5 cm diameter; referred to as “initial”) from the top 10 cm of mineral soil was collected to provide data about initial soil ammonium (NH4 +) and nitrate (NO3 −) content. Two additional soil cores were collected close to the first using a soil impact corer with 5.8 cm diameter × 10 cm long polycarbonate sleeves and removed for intact core lab incubation. Cores were transported in a cooler, and refrigerated in the lab at 4 °C until they could be processed.
Initial soils were sieved (4 mm) within 48 h of return to the lab. The lab-incubated soils were sieved at the end of each incubation period. To determine the NO3 − and NH4 + content of the soil, a 10 g subsample was extracted with 1 M KCl and analyzed for NO3 − and NH4 + by colorimetric spectrophotometry (Bundy & Meisinger 1994), using a Skalar San Plus segmented flow water analysis system (Breda, the Netherlands) and cadmium reduction and phenate digestion methods (APHA 1999). Net N mineralization was calculated as the change in NO3 −-N plus NH4 +-N (total inorganic nitrogen, TIN) and net nitrification as the change in NO3 −-N between initial and incubated extracts, respectively. A second 10 g subsample was oven-dried at 105 °C for at least six hours to calculate the gravimetric soil moisture content. For the lab-incubated soils, the remaining soil was dried at 70 °C for 48 h to calculate bulk density (g cm−3) for each soil core. Ash-free dry mass (soil organic matter; SOM) was determined gravimetrically and by loss on ignition (LOI) after placing 7 g dry mass soil in a muffle oven at 500 °C for 4 h.
Lab incubation experiment and temperature regime
Soil cores used for the lab incubations were weighed upon arrival at the lab to determine the total combined weight of plastic sleeve and soil. After weighing, the intact cores were incubated in the dark in a Percival incubator (Percival Intellus I-36VL, Percival Scientific Inc., Perry, IA). Periodically during the incubation, the cores were reweighed and any mass lost was assumed to be moisture loss. In this case, the soil was brought up to the initial weight with distilled water applied to the top of the core using a pipet. The incubation period lasted approximately 28 days. Soils were collected monthly over a three-month period, because we sought to understand if the N-mineralization response to a +2 °C difference in temperature might vary with changes in the base temperature measured in the forests over that period. Therefore, the temperature regime for the incubations varied between months and was determined using the previous months’ urban soil temperature measured at 10 cm depth using iButton data loggers (Dallas Semiconductor, Dallas, TX). Temperatures were not held constant over any single monthly incubation period, but instead were set to mimic diurnal fluctuations (changed in three-hour time steps) that had been measured the previous month in the urban stands over a 24-h cycle (Fig. 1). One incubator was set to urban field soil temperatures (base temperature) and the other set to +2 °C above the base temperature. The average base temperatures for September, October, and November incubations were 25.8 °C, 24.5 °C, and 17.4 °C, respectively. These autumn months were chosen to represent a seasonal transition when warm summer temperatures (i.e., September) change to cool fall temperatures (i.e., November) in order to capture potential seasonal differences. Elevation above the urban baseline would also allow us to observe how N mineralization rates in these urban and rural soils may respond to warmer temperatures that may occur as the heat island intensifies or expands into present rural areas, or if global warming increases seasonal baseline temperatures over time.
Statistical analyses
Soil organic matter (SOM) and soil moisture were analyzed separately for each core using a three-factor model (ANOVA) to partition variation according to time (incubation month), site (urban vs. rural), and temperature (base vs. +2 °C). Initial soil NH4 +-N and NO3 −-N content were quantified prior to incubation using a separate core and the same initial value applied to both sets of incubated soil cores; thus, a two-factor model was used to assess the effects of time (incubation month) and site (urban vs. rural) on N mineralization rate. Total N mineralization and relative proportion of nitrate in TIN produced were analyzed separately for urban and rural soils using a two-factor model to assess variation due to time (incubation month) and temperature treatment (base vs. +2 °C). Comparisons between urban and rural soil N mineralization were analyzed separately for each temperature treatment using a two-factor model to partition variation according to time (incubation month) and site (urban vs. rural). Where tests for normality and homoscedasticity failed, data were log transformed prior to ANOVA analysis. All statistical tests were performed using Systat 10.2.
Results
Soil water, SOM, and initial TIN content
Soil moisture in the cores was held as constant as possible during the laboratory incubation and as expected no significant differences in soil water content (g H2O g−1 soil) were detected between urban and rural lab-incubated soils at the end of the incubation period (p > 0.05; Table 3). Mean urban soil organic matter (SOM) content was 20 to 26 % greater than rural SOM for base and +2 °C temperature incubations (base =0.069 vs. 0.057 g OM g−1 soil, +2 °C = 0.072 vs. 0.057 g OM g−1 soil, respectively; p < 0.01; Table 3). However, mean initial TIN content in urban soils was approximately 50 % less than in rural soils on a dry weight (p = 0.018) and ash free dry weight basis (p = 0.014; Table 3).
Potential net N mineralization
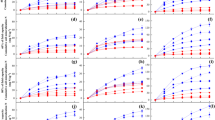
Potential N mineralization rates on a dry weight basis were approximately one order of magnitude lower than those expressed on an ash free dry weight basis. No differences in mineralization directional trends were observed in lab-incubated soils between data expressed on dry weight (mg N kg−1 DW soil d−1), ash free dry weight (mg N kg−1 AFDW soil d−1), or area (g N m−2 y−1) basis (Tables 4 and 5). Thus, only data expressed on an ash-free dry weight basis were statistically analyzed. Across all base temperatures, net N mineralization rates were about twice as high in rural (7.86 mg N kg−1 AFDW soil d−1) than in urban soils (3.65 mg N kg−1 AFDW soil d−1, p = 0.001; Fig. 2a). A 2 °C temperature elevation across all months did not alter this N mineralization disparity between rural (9.08 mg N kg−1 AFDW soil d−1) and urban soils (4.76 mg N kg−1 AFDW soil d−1, p < 0.001; Fig. 2b). Although across all months mean N mineralization rates at +2 °C were higher, they were not statistically different for either urban (base vs. elevated, respectively: 3.65 vs. 4.76 N mg kg−1 AFDW soil d−1, p = 0.272; Fig. 3a) or rural soils (7.86 vs. 9.08 N mg kg−1 AFDW soil d−1 respectively, p = 0.293; Fig. 3b).
Potential net NO3 −-N production
The percentage of TIN production as NO3 −-N was lower in urban than in rural soils (15.1 % vs. 72.3 % NO3 −, respectively; p < 0.001). Elevating temperature by 2 °C did not change the percentage of TIN comprised of NO3 −-N in urban soils (14.3 % vs. 15.9 % NO3 —N, for base and elevated temperature, respectively; p = 0.380; Fig. 4a), or in rural soils (77.5 % vs. 67.0 % NO3 −-N for base and elevated temperature, respectively; p = 0.398; Fig. 4b).
Discussion
The urban forests in Louisville, KY exhibited air and soil temperatures that were 1 to 2 °C warmer during the growing season than those measured in their rural forest counterparts, likely due to the urban heat island effect. Despite warmer temperatures, net N mineralization rates measured in situ in these forests were lowest in urban stands (Carreiro et al. 2009). This soil core incubation experiment, which was conducted concurrently with the field experiment, enabled us to isolate urban and rural soils from potential variation in other local environmental factors (e.g., pollutant deposition, differences in soil moisture), and evaluate the potential of a realistic increase in temperature to affect N mineralization in these forest soils.
Effect of temperature
The expectation that removing urban soils from the field and incubating them at the same temperatures as rural soils would increase urban N mineralization rates to the levels observed in rural soils was not supported. Rural soils continued to mineralize faster than urban soils across a range of temperatures experienced in those forest soils from September to November (Fig. 2). Additionally, the hypothesis that a 2 °C increase in temperature would increase N mineralization rates to a statistically detectable extent was not supported. While mean soil N mineralization rates were greater with a 2 °C increase temperature in all months in the urban soils and in September and November in the rural soils, we found no significant differences in urban or rural soil N mineralization rates between the base and elevated temperature regimes due to high variability (Fig. 3). Furthermore, the effect of a 2 °C rise in temperature on either nitrate production or nitrate production as a proportion of total N mineralized was not statistically detectable in either urban or rural soils at any month (Fig. 4, Tables 4 and 5). Within the spatial sampling limitations of a soil core experiment, all of these responses to temperature suggest that variation in both SOM quantity and quality, and perhaps differences in microbial and invertebrate communities, are more important determinants of N mineralization rates across this urban-rural gradient. While monthly temperature difference during the three-month period affected N mineralization rates (Tables 4 and 5), a 2 °C rise in temperature does not appear a substantial enough elevation to overcome soil organic matter legacy effects in these particular systems.
Surprisingly, soil organic matter quantity differed significantly between urban and rural soils, with SOM being 20–26 % greater in the urban. Expectations are that N mineralization rates on a dry weight basis would be positively correlated with greater organic matter content. However, this was not observed. Possible explanations for these differences in soil organic matter are: 1) greater organic matter inputs to the urban soils, 2) decreased microbial mineralization of organic matter in the urban soils, or 3) both. While we found that aboveground organic matter inputs to the soil from the two dominant tree species in these forest stands (Quercus montana and Acer saccharum) were not significantly different between the urban and rural forests (Carreiro et al., unpublished results), rhizodeposition (i.e., plant root cells sloughed off into the rhizosphere) and dead root inputs as sources were not measured and could occur particularly during summer droughts. Rates of rhizodeposition and dead root input could be greater in urban stands if the heat island results in greater evapotranspiration in summer even if precipitation differences between urban and rural stands are negligible. However, when soil temperature was held constant and soil moisture differences were minimal, as occurred in this lab incubation experiment, the slower N mineralization rates on a per gram AFDW basis indicate that the quality of SOM in urban soils was poorer than in rural soils. This suggests that decreased microbial activity may be a more plausible explanation for increased SOM quantity in the urban soils as well.
The quality of the litter inputs to the soil may help explain the differences in N mineralization between these urban and rural soils. Organic matter inputs to the soil may explain some of this expected variation in SOM quality between urban and rural soils. While not found to be statistically significant, the total biomass inputs from the recalcitrant Quercus montana litter was greater in the urban forests compared to the rural forests (60.6 g versus 47.8 g, respectively), whereas the total biomass inputs from the higher quality litter of Acer saccharum was significantly greater in the rural than urban stands (41.6 g versus 10.74 g, respectively; p < 0.01; Carreiro et al., unpublished results). Furthermore, the litter N in Q. montana was significantly greater in the rural stands (68.2 μg N cm−2 leaf) than in the urban stands (51.1 μg N cm−2 leaf; p < 0.05), and, while not statistically significant, litter N in A. saccharum was also greater in rural stands (37.7 μg N cm−2 leaf) than in urban stands (29.7 μg N cm−2 leaf; p > 0.05). These differences in the contribution of the two species to the total litter biomass inputs to the soil and in the litter N concentration between urban and rural stands could likely explain the SOM quality differences between the urban and rural soils. This in turn may help explain the differences in soil N mineralization observed between these urban and rural forests.
The carbon-to-nitrogen ratios (C:N) of soil are indicative of soil organic matter quality and of N availability to microbes and plants and can predict nitrification rates in soils. As expected from the N mineralization data, the urban soils had higher C:N (C:N = 26.2 vs. 21.2) and significantly less initial soil N content than rural soils, providing additional support that the urban soils may have lower SOM quality compared to the rural soils. C:N ratios above 25 may reduce microbial activity and limit their ability to produce nitrogen (Gundersen et al., 1998; Lovett et al. 2002; Ollinger et al. 2002). Thus, the urban soil C:N may be above this threshold where microbes are not able to net mineralize N even when temperatures are increased by +2 °C or moisture is sufficient. Less nitrogen production in the urban soils suggests less N is available for plant growth and foliar production, which may feed back to produce SOM of lower quality.
While removing soils from current environmental conditions (e.g., air and soil pollution) did not alter urban soil N production, the legacy of pollution on with-in soil factors, such as soil organic matter quality and the soil microbial community, may affect urban soil N mineralization rates as well. Ozone damage to cottonwood leaves altered leaf litter quality (Findlay et al. 1996), which reduced subsequent litter decomposition rates. The New York City urban-to-rural gradient study of forest remnants demonstrated that the quality of red oak litter was more recalcitrant in urban than in rural stands (Carreiro et al. 1999; Pouyat and Carreiro 2003). Heavy metal deposition to urban soils may also decrease decomposer activity, resulting in lower N mineralization rates (Bääth 1989). In this study, the urban soils had higher heavy metal concentrations (Pb + Cu + Ni) than the rural soils (289 vs. 107 μmol kg−1 ODW soil), and these heavy metal concentrations in the urban soil approach concentrations that may negatively affect soil microbial activity in forests (Pouyat et al. 1997). Lower soil organic matter quality, different microbial community composition, and higher heavy metal concentrations may be several factors that can account for lower N mineralization rates in urban soils; however, further research is needed to determine the extent to which each of these biotic and abiotic factors are potentially controlling soil N production in these urban and rural forest soils.
Multiple city comparisons
In several other field studies, soil N mineralization rates in remnant forests along an urban-rural gradient were found to be greater in the urban compared to the rural soils (Pouyat et al. 1997; Pouyat and Turechek 2001; Pavao-Zuckerman and Coleman 2005; Szlavecz et al. 2006; Chen et al. 2010; Enloe et al. 2015). The elevated in-situ N mineralization in these other urban forests have been partially attributed to higher temperatures in the urban forests (Chen et al. 2010; urban 1 °C warmer Pavao-Zuckerman and Coleman 2005; urban 0.63 °C warmer Enloe et al. 2015). However, in a lab incubation study of soils along the New York City urban-rural gradient, urban soils demonstrated increased net N mineralization relative to rural soils under the same temperature regime (Zhu and Carreiro 1999). The importance of nonnative earthworm activity was shown to be the important driver of soil N cycling in these urban soils (Zhu and Carreiro 2004). Furthermore, the presence of exotic earthworms in urban forest soils along this gradient was shown to be an important factor explaining differences observed between urban and rural litter decay rates as well. It appears then these earthworms were able to override the potential negative effects of lower litter and SOM quality on microbial activity on urban soil N cycling processes (Pouyat and Turechek 2001; Pouyat and Carreiro 2003; Steinberg et al. 1997). To our knowledge, this is the first study to document slower soil N mineralization rates in urban forests relative to rural forest counterparts. The uniqueness of our results demonstrates the importance of conducting urban ecological research across multiple cities, since the primary controls on ecosystem functions can differ across cities, regions and continents, changing both the magnitude and direction of these ecosystem processes.
Implications for global change
One of the advantages of studying urban ecosystems is the potential of urban environments to provide insight about ecosystem responses to global climate change since the conditions in urban environments are comparable to predicted climate alterations (e.g., increased temperature; altered precipitation regime; Grimm et al. 2008). While forests remnants embedded along urbanization gradients offer an opportunity to study the multiple factors attributed to global change (e.g., elevated temperature from urban heat islands, CO2, and N deposition), the inherent limitation of this approach is the inability to separate the direct impacts of individual factors on forest function (Carreiro and Tripler 2005). Controlled lab experiments conducted simultaneously with urban-to-rural gradient studies, as the one conducted in this study, provide valuable information about how one aspect of urbanization and global climate change (i.e., elevated temperature) may affect ecosystem function. Soil warming experiments have demonstrated increased in situ net N mineralization and nitrification when temperatures were elevated by 3–5 °C and 0.6–1.2 °C, respectively (Verburg and van Breemen 2000; Xu et al. 2010), and net N mineralization was shown to remain elevated even after 7 years of continuous experimental warming (Butler et al. 2012). These experimental manipulations suggest urban soil ecosystem processes should be accelerated with elevated temperatures. However, results from our incubation experiment demonstrated that a +2 °C temperature differential, as was measured along this gradient, was not the primary factor controlling soil N cycling differences between forest remnants at either end of an urban-rural gradient in Louisville, KY. We conclude that other factors associated with our urbanization gradient (e.g., differences in SOM quality) were more important than temperature differences in controlling the magnitude and direction of soil N mineralization and nitrification in these forests. This implies that ecosystem responses to urbanization and global change are complex and difficult to predict.
Conclusion
A +2 °C difference in soil temperature, as can be observed along urban-rural gradients, did not influence soil N production rates in our urban or rural forest soils since increased temperature regimes had no statistically detectable effect on soil N mineralization rates or proportion of nitrate production. Furthermore, urban soils removed from current climatic conditions and pollutant deposition did not mineralize at similar rates to the rural forest soils, indicating soil legacy factors are more important in controlling N production in these forests. SOM quantity and quality inputs to forest soils along this urban-rural gradient were found to be more important factors controlling soil N production rates than temperature differences. If slower N mineralization rates persist in urban forests, then this reduction in N availability may negatively feed back to above- and below-ground organisms, in turn resulting in tight N cycling in these urban forests (e.g., increased foliar N resorption) or an increased dependence on N inputs to the system (e.g., N deposition). Lower N mineralization rates in urban soils also suggests these forests are not contributing as much to N export from the system as are their rural counterparts 40 km away and are likely more retentive with respect to N inputs.
References
APHA (American Public Health Association) (1999) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, D.C.
Bääth E (1989) Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut 47:335–379
Bonan GB (2002) Ecological climatology. Concepts and applications. Cambridge University Press, Cambridge
Bundy LG, Meisinger JJ (1994) Nitrogen availability indices. In: Weaver RW, Angle JS, Bottomly PS (eds) Methods of soil analysis. Part 2. Soil Science Society of America, Madison, pp. 951–984
Butler SM, Melillo JM, Johnson JE, Mohan J, Steudler PA, Lux H, Burrows E, Smith RM, Vario CL, Scott L, Hill TD, Aponte N, Bowles F (2012) Soil warming alters nitrogen cycling in a New England forest: implications for ecosystem function and structure. Oecologia 168:819–828
Carreiro MM, Howe K, Parkhurst DF, Pouyat RV (1999) Variation in quality and decomposability of red oak leaf litter along an urban-rural gradient. Biol Fertil Soils 30:258–268
Carreiro MM, Tripler CE (2005) Forest remnants along urban-rural gradients: examining their potential for global change research. Ecosystems 8:568–582
Carreiro MM, Pouyat RV, Tripler CE, Zhu W (2009) Carbon and nitrogen cycling in soils of remnant forests along urban-rural gradients: case studies in the New York metropolitan area and Louisville, Kentucky. In: McDonnell MJ, Hahs AK, Breuste JH (eds) Ecology of cities and towns: a comparative approach. Cambridge University Press, New York, pp. 308–328
Chen F-S, Fahey TJ, M-Y Y, Gan L (2010) Key nitrogen cycling processes in pine plantations along a short urban-rural gradient in Nanchang, China. For Ecol Manag 259:477–486
Dalias P, Anderson JM, Bottner P, Coûteaux M-M (2002) Temperature responses of net nitrogen mineralization and nitrification in conifer forest soils incubated under standard laboratory conditions. Soil Biol Biochem 34:691–701
Enloe HA, Lockaby BG, Zipperer WC, Somers GL (2015) Urbanization effects on soil nitrogen transformations and microbial biomass in the subtropics. Urban Ecosyst 18:963–976
Findlay S, Carreiro MM, Krischik V, Jones CG (1996) Effects of damage to living plants on leaf litter quality. Ecol Appl 6:269–275
Franzluebbers AJ, Haney RL, Honeycutt CW, Arshad MA, Schomberg HH, Hons FM (2001) Climatic influences on active fractions of soil organic matter. Soil Biol Biochem 33:1103–1111
Gonçlaves JLM, Carlyle JC (1994) Modelling the influence of moisture and temperature on net nitrogen mineralization in a forested sandy soil. Soil Biol Biochem 26(11):1557–1564
Gregg JW, Jones CG, Dawson TE (2003) Urbanization effects on tree growth in the vicinity of New York City. Nature 424:183–187
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760
Grimm NB, Grove JM, Pickett STA, Redman CL (2000) Integrated approaches to long-term studies of urban ecological systems. Bioscience 50(7):571–584
Groffman PM, Pouyat RV, Cadenasso ML, Zipperer WC, Szlavecz K, Yesilonis ID, Band LE, Brush GS (2006) Land use context and natural soil controls on plant community composition and soil nitrogen and carbon dynamics in urban and rural forests. For Ecol Manag 236:177–192
Gundersen P, Callesen I, de Vries W (1998) Nitrate leaching in forest ecosystems is related to forest floor C/N ratios. Environ Pollut 102:403–407
KY Geological Survey (2001) Maps and GIS. University of Kentucky. Available at: http://www.uky.edu/KGS/gis
Lovett GM, Weathers KC, Arthur MA (2002) Control of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosystems 5:712–718
McDonnell MJ, Pickett STA (1990) Ecosystem structure and function along urban-rural gradients: an unexploited opportunity for ecology. Ecology 71:1232–1237
McDonnell MJ, Pickett STA, Groffman P, Bohlen P, Pouyat RV, Zipperer WC, Parmelee RW, Carreiro MM, Medley K (1997) Ecosystem processes along an urban-to-rural gradient. Urban Ecosyst 1:21–36
Medley KE, McDonnell MJ, Pickett STA (1995) Forest-landscape structure along an urban-to-rural gradient. Prof Geogr 47:159–168
The National Atlas of the United States (2013) Available at: http://www.nationalatlas.gov/mld/ecoomrp.html
National Climatic Data Center (NCDC) (2009) NOAA Satellite and Information Service. Available at: http://www.ncdc.noaa.gov/oa/ncdc.html
Oke TR (1995) The heat island of the urban boundary layer: characteristics, causes and effects. In: Cermak JE, Davenport AG, Plate EJ, Viegas DX (eds) Wind climate in cities. Kluwer Academic Publishers, Dordrecht, pp. 81–107
Ollinger SV, Smith ML, Martin ME, Hallett RA, Goodale CL, Aber JD (2002) Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 83:339–355
Pavao-Zuckerman MA, Coleman DC (2005) Decomposition of chestnut oak (Quercus prinus) leaves and nitrogen mineralization in an urban environment. Biol Fertil Soils 41:343–349
Peterjohn WT, Melillo JM, Steudler PA, Newkirk KM, Bowles FP, Aber JD (1994) Responses of trace gas fluxes and N availability to experimentally elevated soil temperatures. Ecol Appl 4:617–625
Pickett STA, Cadenasso ML, Grove JM, Boone CG, Groffman PM, Irwin E, Kaushal SS, Marshall V, McGrath BP, Nilon CH, Pouyat RV, Szlavecz K, Troy A, Warren P (2011) Urban ecological systems: scientific foundations and a decade of progress. J Environ Manag 92:331–362
Pouyat RV, Carreiro MM (2003) Controls on mass loss and nitrogen dynamics of oak leaf litter along an urban-rural land-use gradient. Oecologia 135:288–298
Pouyat RV, Turechek WW (2001) Short- and long-term effects of site factors on net N-mineralization and nitrification rates along an urban-rural gradient. Urban Ecosyst 5:159–178
Pouyat RV, McDonnell MJ, Pickett STA (1997) Litter decomposition and nitrogen mineralization in oak stands along an urban-rural land use gradient. Urban Ecosyst 1:117–131
Pouyat RV, Yesilonis ID, Golubiewski NE (2009) A comparison of soil organic carbon stocks between residential turf grass and native soil. Urban Ecosyst 12:45–62
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Steinberg DA, Pouyat RV, Parmelee RW, Groffman PM (1997) Earthworm abundance and nitrogen mineralization rates along an urban-rural land use gradient. Soil Biol Biochem 29:427–430
Szlavecz K, Placella SA, Pouyat RV, Groffman PM, Csuzdi C, Yesilonis I (2006) Invasive earthworm species and nitrogen cycling in remnant forest patches. Appl Soil Ecol 32:54–62
U.S. Census Bureau (2000) Geography. Maps and Data. Available at: https://www.census.gov/geo/maps-data/
U.S. Census Bureau (2008) The 2009 Statistical Abstract. Available at: http://www.census.gov/prod/2008pubs/09statab/stlocgov.pdf/
Verburg PSJ, van Breemen N (2000) Nitrogen transformations in a forested catchment in southern Norway subjected to elevated temperature and CO2. For Ecol Manag 129:31–39
Xu Z-F, Hu R, Xiong P, Wan C, Cao G, Liu Q (2010) Initial soil responses to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China: nutrient availabilities, microbial properties and enzyme activities. Appl Soil Ecol 46:291–299
Zhu W-X, Carreiro MM (1999) Chemoautotrophic nitrification in acidic forest soils along an urban-to-rural transect. Soil Biol Biochem 31:1091–1100
Zhu W-X, Carreiro MM (2004) Temporal and spatial variations in nitrogen transformations in deciduous forest ecosystems along an urban-rural gradient. Soil Biol Biochem 36:267–278
Acknowledgments
The University of Louisville Research Foundation supported this study. We thank Chad Ladusaw and Juliane Amlacher for field and laboratory assistance and Rich Schultz for soil nitrogen analysis. We also thank Louisville Metro Parks and the Bernheim Arboretum and Research Forest for permission to conduct research in urban and rural forests, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trammell, T.L.E., Tripler, C.E., Carper, S.C. et al. Potential nitrogen mineralization responses of urban and rural forest soils to elevated temperature in Louisville, KY. Urban Ecosyst 20, 77–86 (2017). https://doi.org/10.1007/s11252-016-0580-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-016-0580-y