Abstract
Urban expansion to rural and natural areas is a global process. Although several studies have analyzed bird community attributes along urbanization gradients, little is known on the impact of urbanization on temporal variability of bird communities. Rural areas show higher seasonal and interannual variability in environmental conditions and resources than do urban areas. Our objectives are to determine how seasonal and interannual variability in bird assemblages change along an urban–rural gradient, and how interannual variability in bird assemblages changes with season. Low seasonal and interannual variability of bird communities is expected in urbanized areas that show a process of temporal homogenization. Seasonal variability of bird richness and abundance were positively related to the percent cover of crops. Seasonal and interannual variability in community composition were positively related to coverage of herbaceous vegetation and crops, and negatively related to coverage of impervious areas. Interannual variability of bird richness and abundance were highest during the non-breeding season. We conclude that highly urbanized areas allow bird communities to have a more stable composition over time, promoting temporal homogenization. Our results emphasize that urbanization alters the temporal dynamics of resources and, therefore, the temporal variability of bird communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 21st century, over 80 % of the human population is expected to live in urban areas in Latin America and the Caribbean (United Nations Fund 2012). The expansion of urban areas can exceed population growth (Szlavecz et al. 2011); thus, the impact of urbanization on biodiversity has major conservation and management implications. Many studies that have focused on the influence of urbanization on bird communities have shown that high levels of urbanization reduces bird species richness at the local scale (Chace and Walsh 2006; Faeth et al. 2011). However, little is known about the impact of urbanization on the seasonal and interannual variability of bird communities (Jokimäki and Suhonen 1998; Barrett et al. 2008; Jokimäki and Kaisanlahti-Jokimäki 2012; Leveau and Leveau 2012).
Seasonality -- or the predictable change in environmental conditions of a site throughout the year -- is relevant to bird community dynamics because it determines the proportions of resident and migratory species in a community (Herrera 1978; Hurlbert and Haskell 2003). Sites with the greatest difference in resources availability between the winter and the most productive period of the year, such as those located in cold and temperate climates, are expected to have the greatest proportion of migrant species (MacArthur 1959; Hurlbert and Haskell 2003). Previous studies suggested that seasonal variation in bird communities may be affected by urbanization. The degree of seasonal change in community composition has been found to be negatively associated with level of urbanization (Catterall et al. 1998; Clergeau et al. 1998; Caula et al. 2008; La Sorte et al. 2014). Migratory birds were negatively affected by high levels of urbanization (Blair and Johnson 2008; MacGregor-Fors et al. 2010; Leveau 2013). Migrants may be more excluded from urban areas than the resident species due to low tree cover in forested areas (MacGregor-Fors et al. 2010). Alternatively, the lowest seasonal variation of bird communities may be associated to lower environmental seasonality within urban areas than in rural areas, which is related to favorable microclimate and food availability during the winter (Hwang and Turner 2005; Caula et al. 2008). In temperate climates, seasonal environmental variability is reduced in urban centers compared to the landscape matrix in which urbanization is developed (White et al. 2002; Shochat et al. 2006; Faeth et al. 2011; Buyantuyev and Wu 2012). Thus, seasonal variability in bird community richness, abundance and composition are also expected to be reduced.
Interannual variability in environmental conditions also influences bird communities. Previous studies have shown that bird communities responded to year-to-year variability in habitat structure and resources, climate variation and predictability, primary productivity and species diversity (Järvinen 1979). Also, abundance of the dominant species was positively related to community stability (Sasaki and Lauenroth 2011). Moreover, the pattern of interannual variability in bird community attributes may differ among seasons (Wiens 1989); several previous studies found higher interannual variability of communities during the non-breeding than during the breeding season (Rice et al. 1983; Leveau and Leveau 2012). This pattern was related to a more restricted habitat use during the reproductive season (Alatalo 1981).
Urban areas have a constant supply of food resources for omnivorous species. Moreover, urban areas in temperate and cold climates may offer relatively mild microclimatic conditions during winter (Suhonen et al. 2009). Residential areas have also a constant supply of resources, which can stabilize the interannual variability of primary productivity compared to rural areas (Leveau 2014), promoting stability in community composition. Given the reduction in environmental variability in urban environments compared to rural areas (Shochat et al. 2006; Buyantuyev and Wu 2009), there can also be expected reductions in the variability of bird community attributes among years as well as a seasonal effect on observed interannual patterns.
Studies that relate the interannual variability of bird communities to urbanization levels are few and show contrasting results (Sodhi 1992; Suhonen et al. 2009; Leveau and Leveau 2012). To our knowledge, no previous study describes a year-to-year variation in bird community composition along urban–rural gradients. By including rural areas (i.e., horticulture, agriculture, pastures), we searched on the contrast of interannual variability in resources and habitat structure between urban and rural areas (Buyantuyev and Wu 2012). This greater contrast can shed new insight concerning the interannual variability of bird communities along urban–rural gradients.
Urbanization is considered one of the most homogenizing forces on bird communities (McKinney 2006). As urbanization gets more intense, similarity in species composition between sites increases regardless of the geographic location (Clergeau et al. 2001; Blair and Johnson 2008; La Sorte and McKinney 2007). If bird communities in the most urbanized areas have lower seasonal and interannual variability of composition, the biotic homogenization might have a temporal component in the seasonal (La Sorte et al. 2014) and interannual time scales, which we call “temporal homogenization”.
Our objective was to analyze seasonal and interannual patterns in the variability of bird assemblages along an urban–rural gradient in central Argentina. The degree of temporal variability in species richness, abundance and composition of bird assemblages were compared along a gradient from the core urban area of Mar del Plata city (Pampean region of Argentina) to the agricultural area during three consecutive years. It is expected that the least variability in richness, abundance and composition of bird assemblages to be in the most urbanized sites and during the breeding season.
Materials and methods
Study area
Bird surveys were conducted at the coastal city of Mar del Plata (>600,000 inhabitants, Instituto Nacional de Estadísticas y Censos 2012) and nearby rural areas in Buenos Aires province, Argentina (38° 00′S, 57° 33′W). Mean monthly temperature is the lowest in July (6.7 °C) and the highest in January (21.1 °C). Maximum mean rainfall occurs in January (124.2 mm) and minimum in June (21.5 mm) (Servicio Meteorológico Nacional). The city is surrounded by agricultural areas (the landscape matrix) comprising cropfields, pastures, tree plantations and small fragments of seminatural grasslands and forests.
We defined five habitat types based on coverage of primary land uses along the urban–rural gradient: 1) core urban areas with concentration of commercial and administrative activities and having mean of 61 % of ground cover being buildings (see Electronic Supplementary information) along transects; 2) suburban areas composing mainly detached houses with managed vegetation such as gardens with lawn, trees and shrubs, and having mean of 27 % of ground cover being buildings; 3) periurban areas of houses and extensive parks located at the edges of town with mean of 25 % of ground cover being buildings; 4) horticulture sector located 2 km from the city edge with mostly lettuce, carrot and tomato crops, with mean of 6 % of ground cover being buildings and 5) agricultural areas located 1 km from city edge composed mainly of soybean and wheat crops on fields larger than those in the horticulture areas, with mean of 0.10 % of ground cover being buildings.
Bird surveys
Birds were surveyed along 100 × 50-m strip transects (sampling units) within 4 h of sunrise. Surveys were conducted from May 2010 to February 2013. Following a stratified sampling design, each of the five habitat types along this urban–rural gradient were surveyed along 15 strip transects separated at least by 100 m, and visited three times during the non-breeding (April-September) and three times during the breeding (October-March) seasons. All transects were located along streets within the city and along secondary roads in horticulture and agriculture habitats. All birds seen or heard while walking transects were recorded, excluding flying high birds. Surveys were conducted by the same observer (LML) and lasted 3 to 5 min in each transect. For more information about the location of transects see Leveau (2013).
Habitat variables
In each habitat type, two circles of 25 m radius were located, one in the center of the first 50 m along transects and the other in center of the remaining 50 m. In each circle, the following variables were estimated visually: 1) percentage coverage of trees, shrubs, lawn (managed herbaceous vegetation), buildings, non-managed herbaceous vegetation, cultivated land and paved roads; 2) the number of trees > 5 m in height and < 5 m in height; and 3) the number of pedestrian and vehicles (cars, bicycles and motorcycles) every three min during the bird surveys (Fernández-Juricic 2000). Coverage of vegetation types and buildings sometimes exceeded 100 % because vegetation types were overlapped. Habitat diversity in each transect was estimated using the Shannon index, which incorporated the percent cover of trees, shrubs, lawn, herbaceous vegetation, cultivated land and buildings. When the percentage cover of habitat components exceeded 100 %, values were corrected for up to 100 %. Occasionally, habitat coverage changed between years; in these cases, average coverage among years were estimated. Pedestrian and vehicle traffic for each season and year were also averaged. Artificial nest boxes and feeding sites were very rare in the study area.
Data analysis
Environmental variables were analyzed by Principal Component Analysis (PCA). The number of axes was determined choosing eigenvalues greater than one (Kaiser 1960). We characterized each axis based on those variables with correlations greater than or equal to 0.40. For each variable, greatest correlation to the axes was considered to interpret each factor (Pérez and Medrano 2010). Scores for each axis were used as independent variables in statistical tests. Species richness for each transect was determined using the COMDYN program (Hines et al. 1999). This program estimates richness and associated variance taking into account possible differences in detectability among species (Hines et al. 1999). The outcomes of COMDYN are the estimated species richness, its standard error and an estimated detection probability of species in the transect. COMDYN considers the information of species detected and undetected in a series of visits to a given transect.
Seasonal and interannual variability in species richness were determined by the coefficient of variation (CV). The standard deviation of the temporal variability of bird richness, which takes into account the sampling variance associated with species richness according to the COMDYN results, was estimated (Link and Nichols 1994). Thus, the “real” temporal variance is equal to the estimated variance in time using estimates of bird richness from COMDYN minus the average sampling variance associated with seasonal and annual estimates of bird richness (Link and Nichols 1994; Newmark 2006). Negative values were set to 0. The variability in bird abundance was also quantified by the CV.
The variation in bird community composition was estimated by ‘persistence’ (Fernández-Juricic 2000), which is the number of species recorded in each transect during every season (or year) divided by the total number of species recorded in all seasons (or years). Persistence values range from 0 (no species was recorded during either seasons or the three consecutive years) to 1 (all species were recorded in each season or year of sampling). For example, considering four species (A, B, C and D) of which A and B were recorded during the three breeding periods, whereas species C was only observed in the first period and D during the second breeding period; then persistence is 2/4 equal to 0.50. Furthermore, the temporal variation in species composition was quantified by the new adjusted abundance-based Sorensen index of similarity between seasons (Chao et al. 2005, 2006), which takes into account unobserved species due to differences in detectability between seasons. For the interannual variability among the three years, the new abundance-based Morisita index was applied to evaluate the overall similarity among years (Chao et al. 2008). Similarity indices were applied from SPADE program (Chao and Shen 2010).
Difference in bird richness and abundance between seasons was tested with a paired student t-test (Zar 1999). Differences in the seasonal CV of bird richness, abundance, persistence and the Sorensen index among habitat types were analyzed with one-factor ANOVA, whereas differences in the interannual CV of bird richness, abundance, persistence and the Morisita index between seasons and habitat types were analyzed applying a two-way factor ANOVA. Indices of seasonal variability of assemblages were averaged for the three years. The assumption of normality of the data was analyzed with the Kolmogorov-Smirnov test, whereas the homoscedasticity was tested by the Levene test. In cases of heteroscedasticity, an alpha value of 0.025 (Tabachnick and Fidell 2001) was used.
Generalized Additive Models (GAM) were used to relate temporal variability of bird communities with habitat variables. Additive models allow establishing non-linear relationships (Zuur et al. 2009). The relationships between habitat variables, CV of bird richness and abundance, and similarity indices were determined assuming a Gaussian distribution. When necessary, the dependent variables were arcsine square root transformed to approximate assumptions of normality and homoscedasticity (Zar 1999). Because persistence may be influenced by the level of detectability in the different transects of the urban–rural gradient, the estimated probability of detection calculated by COMDYN was selected as a linear predictor in the GAM. Statistical analyzes were performed with R using the mgcv package (Wood 2011; The R Development Core Team 2013). Models were obtained using a backward procedure to eliminate non-significant variables (P < 0.05) from the full model using the ANOVA function. Plots of the regression models were constructed with visreg package (Breheny and Burchett 2013).
Results
A total of 72 bird species were recorded (Table 1), of which 9 were recorded only during the non-breeding season and 20 only during the breeding season. Five species were exotics: Rock Dove, European Starling, European Greenfinch, European Goldfinch and House Sparrow (see Table 1 for scientific names). Eleven bird species were migrants, 8 of them are part of the South American Temperate-Tropical group, two were South American Cool-Temperate migrants and one was a Pan New World migrant (see Joseph 1997).
Environmental axes
The PCA generated four environmental axes representing 83 % of the variance. Component 1 (PC1) explained 37 % of the variance and was negatively related to variables that indicate highly urbanized areas with high proportion of constructed areas and pedestrian and vehicular traffic (Table 2). PC2 explained 30 % of the variance and was positively related to the proportion of residential vegetation (trees, shrubs, grass, trees > 5 m) and habitat diversity, and negatively correlated to the proportion of crops. PC3 represented 8 % of the variance and was positively related to density of trees < 5 m. Finally, PC4 represented 7 % of the variance and was negatively related to the percentage of herbaceous vegetation and positively related to the proportion of paved roads.
Seasonal variations in bird communities
Bird richness and abundance were higher during the breeding season (\( \overline{\mathrm{X}} \) = 12.15, SD = 5.47; \( \overline{\mathrm{X}} \) = 13.41, SD = 5.46, respectively) than during the non-breeding season (\( \overline{\mathrm{X}} \) = 9.07, SD = 3.98; \( \overline{\mathrm{X}} \) = 12.16, SD = 6.48, respectively) (t = 7.95, P < 0.001; t = 2.69, P = 0.009, respectively). Seasonal variability of bird assemblages was the highest in the agricultural habitat (Table 3).
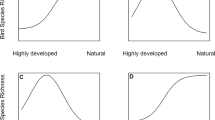
Seasonal variability of bird richness was greatest in transects dominated by cultivated land (F = 5.38, P < 0.001, estimated degrees of freedom [edf] = 5.83) and negatively related to the amount of low trees (F = 4.68, P = 0.034, edf = 1.00; model r 2 = 0.42) (Fig. 1a). On the other hand, the seasonal variability of abundance was greatest in transects with a high percentage of cultivated land (F = 8.10, P < 0.001, edf = 6.07; model r 2 = 0.43) (Fig. 1b). Persistence increased with the proportion of constructed areas, vehicular and pedestrian traffic (F = 34.10, P < 0.001, edf = 2.02), and also increased with greater proportion of residential vegetation and habitat diversity (F = 2.93, P < 0.001, edf = 4.82) and with the proportion of paved roads (F = 29.73, P < 0.001, edf = 1.00; model r 2 = 0.72) (Fig. 1c). Finally, seasonal similarity of community composition according to the abundance-based Sorensen index increased with the proportion of constructed areas, vehicular and pedestrian traffic (F = 10.84, P < 0.001, edf = 1.69), with greater proportion of residential vegetation and habitat diversity (F = 4.83, P < 0.001, edf = 4.14), and with the proportion of paved roads (F = 14.14, P < 0.001, edf = 1.00; model r 2 = 0.56) (Fig. 1d).
Partial effects of environmental axes and seasonal variability in bird assemblages for: a) the coefficient of variation (CV) of bird richness, b) CV of abundance, c) persistence, and d) Sorensen index. Gray areas represent the confidence intervals at 95%. In axis x are indicated the habitat components related to each environmental axis
Interannual variability in bird communities
Interannual variability of bird richness did not vary among habitat types (F4, 70 = 0.90, P = 0.47), but changed between seasons (F1, 70 = 5.91, P = 0.018) being highest during the non-reproductive season (Fig. 2a). Interannual variability of bird abundance differed among habitat types (F4, 70 = 3.14, P = 0.020) and between seasons (F 1, 70 = 14.06, P < 0.001), being highest in agriculture habitats and during the non-breeding season (Fig. 2b). Persistence varied between habitat types (F4, 70 = 13.37, P < 0.001) increasing toward the most urbanized sites (Fig. 2c), and it did not vary between seasons (F1, 70 = 2.35, P = 0.130). The Morisita index was the highest in the more urbanized habitat types (F 4, 70 = 5.89, P < 0.001) and it was similar between seasons (F 1, 70 = 0.22, P = 0.64).
Interannual variability of bird assemblages along the urban-rural gradient of Mar del Plata in different seasons of the year. Bars indicate means and vertical lines indicate standard deviations. Showing: a) the CV of bird richness; b) the CV of abundance; c) persistence; and d) the Morisita index. NOREP: non-reproductive season, REP: reproductive season. Different letters indicate significant differences among habitats (LSD test, P < 0.05)
Interannual variability of both bird richness and abundance were not significantly related to any environmental variable. Interannual persistence increased in those transects with a high coverage of constructed areas, and pedestrian and vehicle traffic (F = 92.12, P < 0.001, edf = 1.00), being highest in places with abundant low trees (F = 6.30, P = 0.014, edf = 1.00) and increasing in transects with paved roads (F = 23.47, P < 0.002, edf = 1.00; model r 2 = 0.62) (Fig. 3a). Interannual similarity of composition according to the abundance-based Morisita index increased in transects with higher proportion of constructed areas and pedestrian and vehicle traffic (F = 9.89, P = 0.001, edf = 1.00), with the lowest numbers of low trees (F = 2.37, P = 0.064, edf = 3.05) and increasing in areas with residential vegetation and habitat diversity (F = 3.83, P = 0.052, edf = 1.04; model r 2 = 0.28) (Fig. 3b).
Discussion
Seasonal variability in bird communities
Seasonal changes in bird communities are partially determined by the fluctuation in number of migratory species and residents that make local movements following temporal variation of resource availability (Avery and Van Ripper 1989; Cueto and Lopez de Casenave 2000a). In general, bird communities in central Argentina show an increase in richness and abundance in the spring and summer partially due to the arrival of migratory species (Cueto and Lopez de Casenave 2000b; Isacch and Martinez 2001; Isacch et al. 2003; Codesido et al. 2008; Leveau and Leveau 2011). However, urban environments may negatively impact migratory species because of low availability of nesting sites and food may be limited (Croci et al. 2008; Marzluff and Rodewald 2008; MacGregor-Fors et al. 2010; Leveau 2013). Our results show that community attributes of urban bird assemblages have lower seasonal variability than farmland assemblages.
We found that seasonality of community attributes declined to more highly urbanized areas and to areas with managed vegetation, whereas seasonal variability of communities increased to areas with greater coverage of herbaceous vegetation and cultivated areas. During the autumn and winter, crop fields are usually stubble or had been plowed, which may influence seasonal variation of bird communities (Delgado and Moreira 2000). Herbaceous vegetation can be highly related to seasonal changes in temperature and precipitation. Conversely, habitat structure in residential areas was relatively stable between seasons because vegetation is managed (Faeth et al. 2011; Buyantuyev and Wu 2012). Areas with high building concentration and pedestrian traffic should insure a constant provision of food for omnivorous birds such as Rock Doves, Eared Doves and House Sparrows.
Bird species living in rural areas use habitats differently between seasons according to both food and nesting availability (Leveau and Leveau 2011). Certain species such as the Rufous-collared Sparrow and Grassland Yellow-finch congregate in flocks during winter and concentrate at certain sites (Fjeldså and Krabbe 1990; Narosky and Yzurieta 2003); thus, the occupation of sampling units may vary considerably between breeding and non-breeding season.
Our results are consistent with other studies showing a decrease in the seasonality of bird communities in highly urbanized areas (Catterall et al. 1998; Clergeau et al. 1998; Caula et al. 2008). However, the relationship between urbanization and seasonal variability in bird communities may vary with the geographic location of particular cities and their effects on migratory species. For example, Juri and Chani (2009) showed that the greatest seasonal variability in species richness in core urban areas of San Miguel de Tucumán coincided with the arrival of the migratory Southern Martin (Progne elegans).
Interannual variability in bird communities
Our results showed that the interannual variability of bird abundance and community composition varied significantly along the urban–rural gradient. However, the degree of urbanization did not affect the interannual variability in species richness. These results are consistent with recent studies on the impact of urban gradients on bird communities, which have found the highest interannual similarity in composition to be in the most urbanized areas (Suhonen et al. 2009; Leveau and Leveau 2012). In contrast to our study, Leveau and Leveau (2012) found non- significant differences in the interannual variability of bird abundance among habitat types; however those authors analyzed interannual variability of bird communities within Mar del Plata city, whereas we extended the spatial scale to include rural areas achieving a greater contrast in environmental variability (Buyantuyev and Wu 2012). In contrast to our results, Suhonen et al. (2009) found that the variation in abundance was greater in the most urbanized areas of 31 Finnish villages and cities.
The stability of bird communities seems to be mainly affected by environmental variability, species richness and the abundance of dominant species (Järvinen 1979; Grime 1998; Collins 2000; Therriault and Kolasa 2000; Tilman 1999; Sasaki and Lauenroth 2011). Present results indicate that environmental variability and the presence of a few dominant bird species may be the main factors influencing a community’s stability. Species richness is not related to stability in community composition, because interannual similarity in community composition increased to more urbanized areas in which bird richness declines.
In this report, the Rock Pigeon, Eared Dove and House Sparrow dominated the most urbanized areas, comprising 98 % of individuals recorded. Various factors such as food availability, nesting site availability and microclimate conditions may favor the permanence of only a few species in highly urbanized areas during the winter (Roth et al. 1989; Suhonen and Jokimäki 1988; Devictor et al. 2007). An omnivorous diet, gregarious behavior and the capability of nesting in buildings allow for high density of urban-tolerant species (Kark et al. 2007; Leveau 2013), which therefore increases their annual persistence (Tellería and Santos 1997). Thus, the most urbanized areas have greatest stability in bird community composition (Suhonen et al. 2009; Leveau and Leveau 2012). Because urban exploiters tend to share specific traits, it is likely that the temporal variability of functional diversity will be affected by urbanization.
The proportion of herbaceous vegetation and crop fields were correlated to the variability in bird community composition. Specifically, unmanaged herbaceous vegetation was naturally affected by year-to-year variations in rainfall, as opposed to managed vegetation in residential areas (Blair 1996; Faeth et al. 2011). Moreover, lands devoted to agricultural and horticultural practices vary between years due to crop rotation (e.g., soybean-wheat) that influence resources for birds. For example, some transects in the agricultural area changed from soybean in one year to sunflower in the next year; sunflower fields may offer more resources for birds than offered by soybean fields (Leveau and Leveau 2004).
Our study found that community stability varied during the year. There was less interannual variability in species richness and abundance of birds during the breeding than during the non-breeding season. That pattern may be related to a more specialized habitat use and spatial constraints related to the reproductive behavior of birds (Delgado and Moreira 2000; Caula et al. 2008). During the breeding season birds need to select habitats with suitable nesting sites, singing spots and shelter (Hildén 1965; Alatalo 1981). On the other hand, during the non-breeding season habitat use is more relaxed because individuals increase movements to alternative habitats in the process of dispersal (Tellería and Santos 1997; Murgui 2007).
Unfortunately, neither the interannual variability of food resources, such as fruits, seeds or arthropods, nor of the interannual variability of predators such as domestic cats, were not measured. However, the interannual variability of the normalized difference vegetation index (NDVI), a proxy of primary productivity and resource availability for birds (Hurlbert and Haskell 2003), during the study period was significantly lower in suburban, periurban and horticulture habitats than its value for agriculture and urban habitats (Leveau 2014). This pattern suggests that vegetation management in moderate and lowest levels of urbanization decreases the interannual variability of resources for birds. In urban habitats, despite the high interannual variability of NDVI, birds may take advantage of food provided by humans.
Our results on interannual variability are limited to three consecutive years, which may not be sufficient to capture the climatic variability that regularly affects central Argentina. In Buenos Aires province, El Niño Southern Oscilation (ENSO) events influences rainfall patterns (Podestá et al. 1999; Sierra and Pérez 2001; Isla et al. 2003). El Niño phase is related to an increase in rainfall during the breeding season whereas La Niña phase is related to a decrease in rainfall. During the three years of our study, El Niño phase was recorded until May 2010, whereas La Niña phase and the neutral phase occurred during the rest of the study period (NOAA 2014). Therefore, our study covered a relatively wide spectrum of rainfall variability. However, it has been considered that the level of interannual variability in bird communities is positively correlated with the number of monitoring years (Bengtsson et al. 1997). In that sense, long-term studies are needed to test the urbanization effects on temporal variability of bird communities.
Concluding remarks
In agreement with previous studies, we found that highly urbanized areas have a relatively low seasonal and interannual variability in bird community composition along an urban–rural gradient in a temperate coastal city of South America. Temporal stability of bird community composition in highly urbanized areas, compared to less urbanized zones, occurred at both the seasonal and interannual scales showing that urbanization promotes a temporal homogenization. Finally, the level of interannual variability in bird richness and abundance depended on the season; given that variability was greater during the non-breeding than during the breeding season, those results are likely related to a relaxation of habitat requirements by birds.
References
Alatalo RV (1981) Habitat selection of forest birds in the seasonal environment of Finland. Ann Zool Fenn 18:103–114
Avery ML, Van Ripper C III (1989) Seasonal changes in bird communities of the chaparral and blue-oak woodlands in central California. Condor 91:288–295
Barrett K, Romagosa CM, Williams MI (2008) Long-term bird assemblage trends in areas of high and low human population density. Res Lett Ecol, Article ID 202606, 4 p, doi:10.1155/2008/202606
Bengtsson J, Baillie SR, Lawton J (1997) Community variability increases with time. Oikos 78:249–256
Blair RB (1996) Land use and avian species diversity along an urban gradient. Ecol Appl 6:506–519
Blair RB, Johnson EM (2008) Suburban habitats and their role for birds in the urban–rural habitat network: points of local invasion and extinction? Landsc Ecol 23:1157–1169
Breheny P, Burchett W (2013) Visualizing regression models using visreg. http://mywebuiowa.edu/pbreheny/publications/visreg.pdf
Buyantuyev A, Wu J (2009) Urbanization alters spatiotemporal patterns of ecosystem primary production: a case study of the phoenix metropolitan region, USA. J Arid Environ 73:512–520
Buyantuyev A, Wu J (2012) Urbanization diversifies land surface phenology in arid environments: interactions among vegetation, climatic variation, and land use pattern in the phoenix metropolitan region, USA. Landsc Urban Plan 105:149–159
Catterall CP, Kingston MB, Park K, Sewell S (1998) Deforestation, urbanisation and seasonality: interacting effects on a regional bird assemblage. Biol Conserv 84:65–81
Caula S, Marty P, Martin JL (2008) Seasonal variation in species composition of an urban bird community in Mediterranean France. Landsc Urban Plan 87:1–9
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69
Chao A, Shen, T –J (2010) Program SPADE (species prediction and diversity estimation). Program and user’s guide published at http://chao.stat.nthu.edu.tw
Chao A, Chazdon RL, Colwell RK, Shen T–J (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159
Chao A, Chazdon RL, Colwell RK, Shen T–J (2006) Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 62:361–371
Chao A, Jost L, Chiang SC, Jiang Y–H, Chazdon R (2008) A Two- stage probabilistic approach to multiple-community similarity indices. Biometrics 64:1178–1186
Clergeau P, Savard J–P, Mennechez G, Falardeau G (1998) Bird abundance and diversity along an urban–rural gradient: a comparative study between two cities on different continents. Condor 100:413–425
Clergeau P, Jokimäki J, Savard JPL (2001) Are urban bird communities influenced by the bird diversity of adjacent landscapes? J Appl Ecol 38:1122–1134
Codesido M, González-Fischer CM, Bilenca DN (2008) Asociaciones entre diferentes patrones de uso de la tierra y ensambles de aves en agroecosistemas de la Región Pampeana, Argentina. Ornitol Neotropical 19:575–585
Collins ST (2000) Disturbance frequency and community stability in native tallgrass prairie. Am Nat 155:311–325
Croci S, Butet A, Clergeau P (2008) Does urbanization filter birds on the basis of their biological traits? Condor 110:223–240
Cueto VR, Lopez de Casenave J (2000a) Seasonal changes in bird assemblages of coastal woodlands in east-central Argentina. Stud Neotropical Fauna Environ 35:173–177
Cueto VR, Lopez de Casenave J (2000b) Bird assemblages of protected and exploited coastal woodlands in east-central Argentina. Wilson Bull 112:395–402
Delgado A, Moreira F (2000) Bird assemblages of an Iberian cereal steppe. Agric Ecosyst Environ 78:65–76
Devictor V, Julliard R, Couvet D, Lee A, Jiguet F (2007) Functional homogenization effect of urbanization on bird communities. Conserv Biol 21:741–751
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann N Y Acad Sci 1223:69–81
Fernández-Juricic E (2000) Avifaunal use of linear strips in an urban landscape. Conserv Biol 14:513–521
Fjeldså J, Krabbe N (1990) Birds of the high Andes. University of Copenhagen and Apollo Books, Svendborg, Denmark
Grime JP (1998) Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol 86:902–910
Herrera CM (1978) On the breeding distribution pattern of European migrant birds: MacArthur’s theme reexamined. Auk 95:496–509
Hildén O (1965) Habitat selection in birds. Ann Zool Fenn 2:53–75
Hines JE, Boulinier T, Nichols JD, Sauer JR, Pollock KH (1999) COMDYN: software to study the dynamics of animal communities using a capture-recapture approach. Bird Study 46:209–217
Hurlbert AH, Haskell JP (2003) The effect of energy and seasonality on avian species richness and community composition. Am Nat 161:83–97
Hwang C, Turner BD (2005) Spatial and temporal variability of necrophagous diptera from urban to rural areas. Med Vet Entomol 19:379–391
Instituto Nacional de Estadísticas y Censo (2012) Censo Nacional de Población, Hogares y Viviendas 2010. Instituto Nacional de Estadísticas y Censo, Buenos Aires
Isacch JP, Martinez MM (2001) Estacionalidad y relaciones con la estructura del hábitat de la comunidad de aves de pastizales de paja colorada (Paspalum quadrifarium) manejados con fuego en la provincia de Buenos Aires, Argentina. Ornitol Neotropical 12:345–354
Isacch JP, Bo MS, Maceira NO, Demaría MR, Peluc S (2003) Composition and seasonal changes of the bird community in the west pampa grasslands of Argentina. J Field Ornithol 74:59–65
Isla FI, Ruiz Barlett E, Marquez J, Urrutia A (2003) Efectos ENSO en la transición entre el espinal y la pradera cultivada en la diagonal sudamericana, Argentina Central. Cuaternario y Geomorfología: Rev Soc Esp Geomorfología y Asoc Esp Estud del Cuaternario 17:63–74
Järvinen O (1979) Geographic gradients of stability in European land bird communities. Oecologia 31:51–69
Jokimäki J, Kaisanlahti-Jokimäki ML (2012) The role of residential habitat type on the temporal variation of wintering bird assemblages in northern Finland. Ornis Fenn 89:20–33
Jokimäki J, Suhonen J (1998) Distribution and habitat selection of wintering birds in urban environments. Landsc Urban Plan 39:253–263
Joseph L (1997) Towards a broader view of neotropical migrants: consequences of a re-examination of austral migration. Ornitol Neotropical 8:31–36
Juri MD, Chani JM (2009) Variación estacional en la composición de las comunidades de aves en un gradiente urbano. Ecología Aust 19:175–184
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Meas 20:141–151
Kark S, Iwaniuk A, Schalimtzek A, Banker E (2007) Living in the city: can anyone become an ‘urban exploiter’? J Biogeogr 34:638–651
La Sorte FA, McKinney ML (2007) Compositional changes over space and time along an occurrence–abundance continuum: anthropogenic homogenization of the North American avifauna. J Biogeogr 34:2159–2167
La Sorte FA, Tingley MW, Hurlbert AH (2014) The role of urban and agricultural areas during avian migration: an assessment of within‐year temporal turnover. Glob Ecol Biogeogr 23:1225–1234
Leveau LM (2013) Bird traits in urban–rural gradients: how many functional groups are there? J Ornithol 154:655–662
Leveau LM (2014) Los efectos de la vegetación y temperatura sobre los ensambles de aves en gradientes urbano-rurales. PhD thesis, Universidad Nacional de Mar del Plata, Mar del Plata.
Leveau LM, Leveau CM (2004) Riqueza y abundancia de aves en agroecosistemas pampeanos durante el período post-reproductivo. Ornitol Neotropical 15:371–380
Leveau LM, Leveau CM (2011) Uso de bordes de cultivo por aves durante invierno y primavera en la Pampa Austral. Hornero 26:149–157
Leveau LM, Leveau CM (2012) The role of urbanization and seasonality on the temporal variability of bird communities. Landsc Urban Plan 106:271–276
Link WA, Nichols JD (1994) On the importance of sampling variance to investigations of temporal variation in animal population size. Oikos 69:539–543
MacArthur RH (1959) On the breeding distribution pattern of North American migrant birds. Auk 76:318–325
MacGregor-Fors I, Morales-Pérez L, Schondube JE (2010) Migrating to the city: responses of neotropical migrant bird communities to urbanization. Condor 112:711–717
Marzluff JM, Rodewald AD (2008) Conserving biodiversity in urbanizing areas: nontraditional views from a bird’s perspective. Cities and the environment 1, article 6. http://escholarship.bc.edu/cate/vol1/iss2/6/
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
Murgui E (2007) Effects of seasonality on the species–area relationship: a case study with birds in urban parks. Glob Ecol Biogeogr 16:319–329
Narosky T, Yzurieta D (2003) Guía para la identificación de las aves de Argentina y Uruguay. Asociación Ornitología del Plata y Vazquez Manzini Editores, Buenos Aires
Newmark WD (2006) A 16-year study of forest disturbance and understory bird community structure and composition in Tanzania. Conserv Biol 20:122–134
NOAA (2014) ENSO cycle: recent evolution, current status and predictions. Climate prediction center / NCEP, http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/lanina/enso_evolution-status-fcsts-web.pdf, accessed 30 March 2014
Pérez ER, Medrano L (2010) Análisis factorial exploratorio: bases conceptuales y metodológicas. Rev Argent Cienc Comportamiento 2:58–66
Podestá GP, Messina CD, Grondona MO, Magrin GO (1999) Associations between grain crop yields in central-eastern Argentina and El Niño-Southern Oscillation. J Appl Meteorol 38:1488–1498
Rice J, Ohmart RD, Anderson BW (1983) Turnovers in species composition of avian communities in contiguous riparian habitats. Ecology 64:1444–1455
Roth M, Oke TR, Emery WJ (1989) Satellite-derived urban heat islands from three coastal cities and the utilization of such data in urban climatology. Int J Remote Sens 10:1699–1720
Sasaki T, Lauenroth WK (2011) Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 166:761–768
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191
Sierra EM, Pérez SP (2001) Efecto del ENOS sobre el régimen de lluvias en Junín [norte de la provincia de Buenos Aires, Argentina]. Rev Argent Agrometeorología 1:51–57
Sodhi NS (1992) Comparison between urban and rural bird communities: urbanization and short-term population trends in prairie Saskatchewan. Can Field Nat 106:210–215
Suhonen J, Jokimäki J (1988) A biogeographical comparison of the breeding bird species assemblages in twenty Finnish urban parks. Ornis Fenn 65:76–83
Suhonen J, Jokimäki J, Kaisanlahti-Jokimäki ML, Hakkarainen H, Huhta E, Inki K, Jokinen S, Suorsa P (2009) Urbanization and stability of a bird community in winter. Ecoscience 16:502–507
Szlavecz K, Warren P, Pickett S (2011) Biodiversity on the urban landscape. In: Concotta RP, Gorenflo LJ (eds) Human populations, its influences on biological diversity. Ecological studies 214. Springer-Berlag, Berlin
Tabachnick BG, Fidell LS (2001) Using multivariate statistics. Harper & Row, New York
Tellería JL, Santos T (1997) Seasonal and interannual occupation of a forest archipelago by insectivorous passerines. Oikos 78:239–248
The R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation Project, GNU project, Boston, USA
Therriault TW, Kolasa J (2000) Patterns of community variability depend on habitat variability and habitat generalists in natural aquatic microcosms. Community Ecol 1:196–203
Tilman D (1999) The ecological consequences of biodiversity: a search for general principles. Ecology 80:1455–1474
United Nations Population Division (2012) World urbanization prospects: the 2011 revision. United Nations, New York
White MA, Nemani RR, Thornton PE, Running SW (2002) Satellite evidence of phenological differences between urbanized and rural areas of the eastern United States deciduous broadleaf forest. Ecosystems 5:260–273
Wiens JA (1989) The ecology of bird communities. Volume 2: processes and variations. Cambridge University Press, Cambridge
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B 73:3–36
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We appreciate the improvements in English writing made by Sarah Knutie and Peter Lowther through the Association of Field Ornithologists’ program of editorial assistance. We thank to two anonymous reviewers for helpful comments. Lucia Gonzalez Salinas helped during fieldwork. LM Leveau is a posdoc fellow of CONICET.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 45.0 KB)
Rights and permissions
About this article
Cite this article
Leveau, L.M., Isla, F.I. & Bellocq, M.I. Urbanization and the temporal homogenization of bird communities: a case study in central Argentina. Urban Ecosyst 18, 1461–1476 (2015). https://doi.org/10.1007/s11252-015-0469-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-015-0469-1