Abstract
Introduction
In a bid to mitigate growing concerns regarding the use of antibiotics in food animals
Objectives
This study determined the growth performance, haemato-biochemical status, organ development and intestinal morphology of Arbor Acre broiler chicken strain on oral administration of citrus–coconut electrolyte blend (CCEB) for 26 days.
Methods
One-hundred ninety-two chicks were brooded for 2 weeks and thereafter divided on a weight equalization basis into four groups (0, 5, 10 and 15 ml CCEB per litre of water) of six replicates each and eight birds per replicate. Phytochemical screening of CCEB was determined, while data collected for growth performance, organ proportions and intestinal morphology were subjected to a one-way analysis of variance.
Results
Phytochemical composition revealed the abundance of phenols (128.40 mg/100g) and tannins (78.10 mg/100g) in CCEB. All productive performance parameters measured were not significantly (p < 0.05) different across treatment means. However, significantly (p < 0.05) highest concentrations (134.47 and 66.48 mg/dl, respectively) for total cholesterol and high-density lipoprotein (HDL) and the lowest concentration (38.34 mg/dl) for low-density lipoprotein (LDL) were recorded in birds on 15 ml of CCEB/litre of water. Furthermore, a progressive reduction (p < 0.05) in the bursa of Fabricius was observed with increasing CCEB/litre of water. The supplementation of CCEB did not influence (p > 0.05) duodenal morphological parameters.
Conclusion
The study concluded that 15 ml of CCEB/litre of water enhanced the production of HDL, reduced LDL, and improved immunity via the reduction of the bursa of Fabricius in broiler chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the affordability, nutrient density and compatibility of poultry meat with many religious dietary laws, the consumption of poultry as a source of animal proteins has recently increased globally (Shah et al. 2021). In a bid to cater to this demand, many farmers are incorporating antibiotic growth promoters (AGPs) via feed and water of poultry birds to improve meat production through improved feed conversion ratio, growth rate promotion and disease prevention (Suresh et al. 2018). Antibiotics used at sub-therapeutic dosages can promote growth and modify the immune status of broiler chickens (Lee et al. 2012; Mehdi et al. 2018). This is primarily due to the control of gastrointestinal infections and the modification of microbiota within the intestines (Torok et al. 2011; Singh et al. 2013). However, AGPs are not effective against fungal and viral pathogens and the consequence of their long-term usage in poultry has been found to alter intestinal microbiota, thereby resulting in the production of antibiotic-resistant bacteria (Mehdi et al. 2018). Bacterial resistance to AGPs is a public health issue as poultry meat plays a major role in human infections (Manges et al. 2007; Diarra et al. 2010; Hur et al. 2011). In addition, waste from poultry may contain antibiotic-resistant bacteria and active antibiotics that may contaminate the environment which may in turn foster the emergence of antibiotic resistance in bacteria other than those found in live animals and their products (Wongsuvan et al. 2018). Antibiotic abuse has also resulted in drug residues in animal products (Gonzalez Ronquillo and Angeles Hernandez 2017). These residues can adversely impact the health and well-being of consumers, for example, tetracycline, which interferes with teeth development in children (Kummerer 2009). Also, the use of clenbuterol by some breeders to produce meat with less fat and more protein is sometimes responsible for food poisoning and muscle tremors (Chan 1999). Gassner and Wuethrich (1994) also demonstrated a possible link between the presence of chloramphenicol metabolites in meat products and the occurrence of aplastic anaemia in humans.
Furthermore, as a result of the rapid consumer concerns regarding the use of antibiotics in food animals, restrictions were placed on the use of AGPs in several countries (Ajuwon 2016). This prompted research on suitable alternatives to AGPs that can support poultry development and maintain minimal mortality while preserving the environment and consumers’ health (Diarra and Malouin 2014; Mehdi et al. 2018). Consequently, natural phytochemicals derived from medicinal plants have gained significant recognition in the potential management of livestock and human diseases. Herbs, spices and other plant extracts are being evaluated as alternatives to antibiotics and some have shown growth-promoting effects, antimicrobial properties and other health-related benefits (Diaz-Sanchez et al. 2015). However, a dearth of information exists on the use of natural electrolytes—a blend of different nutraceuticals rich in minerals and vitamins as a natural growth promoter to replace AGPs in poultry production. Citrus-coconut electrolytes blend (CCEB) is a mixture of ingredients with antioxidant properties such as citrus, coconut water and honey. Citrus fruits are well-known for their health-promoting values such as antioxidant, anti-inflammation, anti-mutagenicity, anti-carcinogenicity, and anti-ageing properties (Zou et al. 2016). Coconut water contains biologically active compounds that are required for cellular functions and have the ability to scavenge free radicals in the body as well as help ease a number of severe health conditions (Agbafor et al. 2015). Honey is a natural product that contains compounds such as vitamin C, phenol compounds, catalase, peroxides, flavonoids, carotenoids, and glucose oxidase (Khalil et al. 2010).
Based on the foregoing, this study determined the effect of citrus-coconut electrolytes blend on growth performance, haemato-biochemical parameters, organs development, and intestinal morphology of broiler chickens.
Materials and methods
Experimental locations
Experimental birds were reared at the Poultry Unit of the Teaching and Research Farm of the Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria. The site is located in the tropical rainforest zone of Nigeria on latitude 5° 29′ N and longitude 7° 33ʹ E with an elevation of 122 m above sea level, annual rainfall of about 2177 mm a monthly ambient temperature range of 22–36 °C and relative humidity of 50–95% depending on the season and location. Laboratory studies were carried out in the Animal Nutrition and Veterinary Pathology laboratories of the College of Animal Science and Animal Production, Michael Okpara University of Agriculture, Umudike.
Preparation of citrus-coconut electrolyte blend (CCEB)
The ingredients for the citrus-coconut electrolyte blend which consisted of 52.27% of orange juice, 4.05% of lime juice, 40.45% of coconut water, 3.01% of honey and 0.22% of Himalayan salt were sourced from nearby markets in Umudike, Abia State. The juices from oranges and lemons were extracted using a cold-press juice extractor, while a metal skewer was used to poke a hole at the top of the coconuts so the water can be poured into a container. Afterward, all ingredients were blended together and stored in airtight single-serving mason jars (8 oz). The jars were stored in a refrigerator at 4 °C prior to use.
Experimental birds and management
Day-old broiler chicks (Arbor acre, n = 192) were purchased from a reputable hatchery in Ibadan, Oyo State, and brooded for 2 weeks before dividing them equally into 4 groups (six replicates per group and eight birds per replicate) after balancing for body weight. The chicks were placed in separate pens on deep litter, and all chicks became acclimatised to the experimental conditions after 2 days. To assess the efficacy of CCEB, it was added via drinking water provided daily for each group of birds at 0, 5, 10 and 15 ml per litre of water, respectively for 26 days. All experimental birds were fed the same single basal diet (Table 1) ad libitum throughout the experiment and vaccinated against Newcastle disease (on the 5th and 24th day) and infectious bursal disease (on the 10th and 20th day).
Data collection
Phytochemical screening of CCEB
A concentration of 10 mg CCEB/ml distilled water was prepared for the screening of various phytochemical constituents using standard methods described by Trease and Evans (1989), Sofowora (1993), and Harborne (1998). The analyses involved the detection of saponins, glycosides, alkaloids, phenol, tannins, flavonoids, oxalate, steroids and terpenoids.
Growth performance
Initial weights of birds were measured per replicate at the beginning of the study, while body weights were measured every 9th day. Feed intake per day was determined by subtracting the amount of feed remaining in each replicate from the specified quantity they received the day prior. Feed intake per bird was determined by dividing the total amount of feed consumed by the total number of birds in each replicate. The feed conversion ratio was calculated as the ratio of feed intake to weight gain. The mortality rate was calculated as the ratio of the number of dead to the total number of birds stocked, with the resultant figure expressed in percentage.
Haemato-biochemical parameters
Approximately, 4 ml of blood samples were drawn from the brachial vein of two randomly selected birds of each replicate on day 36 of the study into anticoagulant tubes (EDTA) for haematological analysis and plain tubes for cholesterol profile analysis. Determination of haematological parameters (packed cell volume, haemoglobin concentration, red blood cell, white blood cell, and its differentials) using a standard procedure (Jain 1986). Blood samples in the plain tubes were centrifuged at 3000 rpm for 10 min to obtain serum, and levels of total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) were estimated using kits by Randox laboratories, United Kingdom (Model BT294QY).
Carcass yield and organs development
Two birds per replicate were randomly selected, weighed and slaughtered on day 36 of the study by severing the jugular vein and carotid arteries. Afterward, birds were de-feathered and had their heads, necks, shanks, feet and viscera carefully removed. The resulting carcasses were dressed and weighed with the dressing percentages estimated as enunciated by Safiyu et al. (2019). Organs (lungs, heart, liver, gizzard, pancreas, thymus, spleen, proventriculus, and bursa of Fabricius) were each weighed and expressed as a percentage of the live weight.
Intestinal morphology
A long portion 2 cm of the duodenum from slaughtered carcasses was cut and placed into a sample bottle containing 10% formalin saline (100 ml formalin, 900 ml distilled water, 0.4 g sodium dihydrogen phosphate, 0.65 g disodium hydrogen phosphate) after washing the contents with normal saline. The duodenal cut samples were prepared as slides for light microscopy, as described by Shamoto and Yamauchi (2000) with the following histological parameters (villus height, villus width, crypt depth, and the villus height: crypt depth ratio) measured and histological slides presented.
Statistical analysis
Data collected in this study were analysed by one-way analysis of variance using general linear model as contained in the Minitab® software version 17.1.0. Significant (p < 0.05) differences among treatment means were separated using the Tukey test of the same software. The statistical model is as follows:
where Yij is the individual observation ij, µ is the overall mean effect, Ti is the treatment effect i, and Eij is the experimental error effect.
Results
Phytochemical constituents of CCEB
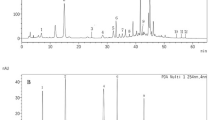
The phytochemical constituents of the citrus-coconut electrolyte blend are depicted in Fig. 1. The result revealed presence of the following bioactive compounds and their quantities in CCEB: saponins (15.30 mg/100 g), glycosides (32.53 mg/100 g), alkaloid (3.00%), phenol (128.40 mg/100 g), tannin (78.10 mg/10 mg/100 g), flavonoid (28.99 mg/100 g), oxalate (8.39), steroid (8.54 mg/100 g) and terpenoid (19.00 mg/100 g).
Growth performance
The effect of CCEB on the growth performance of broiler chickens is shown in Table 2. All productive parameters measured were not significantly (p < 0.05) different across treatment means.
Haemato-biochemical status
In Table 3, the effect of CCEB on haemato-biochemical parameters revealed all parameters measured except total cholesterol, HDL and LDL were not significantly (p > 0.05) influenced. Total cholesterol was significantly (p = 0.017) highest (134.47 mg/dl) in birds supplemented with 15 ml CCEB/litre of water and lowest (96.97 mg/dl) in birds in the control. Comparable means (56.56 and 66.48 mg/dl) for HDL recorded in birds supplemented with 10 and 15 ml CCEB/litre of water, respectively, were significantly (p = 0.021) higher than 28.15 mg/dl recorded in birds in the control. Significantly (p = 0.019) highest values (48.61 and 46.15 mg/dl) for LDL were obtained in groups supplemented with 0 and 5 ml CCEB/litre of water, respectively, and the lowest (38.34 mg/dl) in birds supplemented with 15 ml CCEB/litre of water.
Carcass yield and organs development
The effect of CCEB on carcass yield and organs development of broiler chickens is presented in Table 4. The results indicated that the citrus-coconut electrolyte blend had no effect (p > 0.05) on all parameters measured except the bursa of Fabricius. Significantly (p = 0.019) highest value (0.22%) for the bursa of Fabricius was recorded in the control group, while the lowest value (0.11%) was recorded at 15 ml CCEB/litre of water.
Intestinal morphology
The supplementation of citrus-coconut electrolyte blend did not influence (p > 0.05) duodenal morphological parameters measured (Table 5). Furthermore, photomicrographs depicted in Fig. 2 showed intestinal tissues from birds administered 0, 5, 10 and 15 ml CCEB/litre of water with evenly distributed, well and orderly differentiated glands occupying approximately 18, 22, 33 and 32%, respectively, of the mucosal thickness.
Discussion
In this study, the preliminary phytochemical composition of the citrus-coconut electrolyte blend revealed the abundance of phenols (128.40 mg/100 g) and tannins (78.10 mg/100 g) in CCEB. This implies that CCEB possesses effective antioxidant activity suitable for therapeutic and prophylactic purposes since phenolic compounds are the largest class of secondary metabolites with bioactive potential that accounts for most of the antioxidant activity of many plant extracts (Thabrew et al. 1998). Tannins have also been acknowledged to be efficient antioxidants, anti-carcinogenic and antimicrobial agents (Lai and Roy 2004). The presence of glycosides and flavonoids in CCEB is proof of its aptness as a biological response modifier and antimicrobial agent against a wide array of microorganisms. Glycosides are valuable in the pharmaceutical industry for the treatment of a number of illnesses such as cardiac glycoside which is an important ingredient in drugs and antitoxins (Trease and Evans 1989). Previous in vitro studies (Belay and Sisay 2014; Adebayo-Tayo et al. 2016) also revealed flavonoids possess antimicrobial, antiallergic and anti-inflammatory properties due to the ability to form complexes with bacterial cell walls, extracellular and soluble proteins. Moreover, the presence of other bioactive components such as saponins, alkaloids, oxalate, steroids and terpenoids in CCEB accentuates the importance of citrus-coconut electrolyte blend for ethnomedicine. The results obtained were in concurrence with the findings of Oikeh et al. (2016) who observed the presence of bioactive compounds such as alkaloids, saponins, glycosides, phenols, flavonoids and terpenoids in citrus juices. However, plant metabolites should not only be clinically effective but non-toxic. Therefore, it is worth noting that CCEB is safe for both poultry and human consumption as the ingredients (orange, lime, coconut water, honey and Himalayan salt) are natural healthy foods. The quantities of investigated bioactive compounds especially phenols and tannins which are in abundance in CCEB are below the lethal doses of 140 mg/kg to 500 mg/kg for oral phenolic compounds in animals (Wiley–VCH 2003), as well as 5 and 6 g/kg for tannins in rats and mice, respectively (Robles 2005).
The statistical similarities in all growth parameters measured among experimental birds in the control and their counterparts supplemented varying levels of CCEB is an indication that CCEB posed no negative impact on poultry performance as experimental birds showed no signs of loss of appetite, depressed weight, weakness and/or, illness. Although the use of many herbal products has been explored in the livestock industry as growth promoters and digestion stimulants (Frankic et al. 2009), a dearth of information exists on the application of citrus-coconut electrolyte mix in broiler production. However, the results of this study were partially in line with the findings of Khaligh et al. (2011) who observed no differences in feed intake and FCR of birds fed different medicinal plant blends as compared to control birds from 22 to 42 days of age, but body weight gain of the birds differed significantly across treatments. Furthermore, the efficacy of different blends of herbal plants to improve growth indices in broiler chickens still remains controversial; from one standpoint, some researchers (Botsoglou et al. 2002; Zhang et al. 2005; Jang et al. 2007) documented no significant effect of phytobiotics supplementation on performance of chickens while other reports indicated improvement in growth performance in poultry of different ages (Jamroz et al. 2003; Hernandez et al. 2004; Cross et al. 2007; Gheisar et al. 2015).
The effect of CCEB on haemato-biological parameters revealed packed cell volume, haemoglobin concentration, red blood cell count, white blood cell count, triglycerides and very low-density lipoprotein of experimental birds were not significantly (p > 0.05) influenced. This implies that the immune system of the experimental birds was functioning adequately. The PCV and Hb were within the normal range similar to the findings of Qamar et al. (2015) who reported herbal medicines supplementation via drinking water did not influence the blood haemoglobin level, packed cell volume and white blood cell count of broiler chickens, whereas concentrations of total cholesterol, high-density lipoprotein, and low-density lipoprotein blood differed significantly (p < 0.05) across treatments in the present study. Citrus-coconut electrolyte blend supplementation of up to 15 ml per litre of water increased the total cholesterol and high-density lipoprotein concentrations. On the other hand, low-density lipoprotein concentration was significantly lowest in birds supplemented with 15 ml of CCEB/litre of water. This trend in serum HDL and LDL may point towards the possible hypocholesterolemic and hypolipidemic mechanisms of bioactive constituents in CCEB. The most abundant phytochemical component in CCEB—phenol—is known to inhibit the expression and the activity of 3-hydroxy-3-methylglutaryl-coenzyme A, a critical enzyme in cholesterol synthesis (Ricketts and Ferguson 2018). Our results further confirm reports by previous researchers (Kermanshahi and Riasi 2006; Al-Kassie and Jameel 2009) on the cholesterol-lowering effects of a number of natural feed additives.
The progressive reduction (p < 0.05) in the bursa of Fabricius of the birds with increasing supplementation of CCEB was in accordance with reports by Wang et al. (2021) who observed reduced bursa of Fabricius in chicks administered herbal blends. The bursa of Fabricius is a primary lymphoid organ for B cell lymphopoiesis, lymphocyte maturation, and differentiation and development of the antibody repertoire (Ifrah et al. 2017). Although birds used in the present study were in a normal state of health, however, the findings may indicate improved immunity in birds administered CCEB as less production of antibodies against pathogenic organisms will be necessary. This is in consonance with the study of Sadler and Glick (1962) where bursa size influenced antibody production with chicks with larger bursas producing more antibodies than their counterparts with smaller bursa size. The non-significance finding on carcass parts was in accord with earlier reports of Khattak et al. (2014) who observed no differences (p > 0.05) in live and carcass weights across treatments of broiler chickens supplemented with a natural blend of essential oils. Sinurat et al. (2002) and Mehala and Moorthy (2008) also reported no significant effect of herbal mixtures on carcass yield and internal organs. On the contrary, the study of David et al. (2015) revealed herbal dietary supplements increased (p < 0.05) live weights and dressing percentages in broiler chickens. This variation may be a result of differences in the herbal blends used as well as their mode of administration.
The supplementation of CCEB did not influence duodenal morphological parameters. This result may imply supplementation of CCEB improved duodenal health since villus height and crypt depth play crucial roles in nutrient digestion and absorption. Though literature is still limiting on the effect of CCEB on intestinal growth and function in broiler chickens, the result of the present study was contrary to the findings of Viveros et al. (2011) who observed a significant (p < 0.05) reduction in villus height and crypt depth in birds at 21 days of age fed dietary polyphenol-rich grape products compared with birds fed control diet. On the other hand, the supplementation of herbal extract and mixed prebiotics significantly (p < 0.05) increased the villus height of the duodenum in broilers at 35 days of age according to reports by Karukarach et al. (2016). Thus, conflicting results may be a result of differences in the herbal blends, mode of administration and age of poultry used. Furthermore, photomicrographs showed intestinal tissues from birds administered 0, 5, 10 and 15 ml CCEB/litre of water with evenly distributed, well and orderly differentiated glands occupying approximately 18, 22, 33 and 32%, respectively, of the mucosal thickness. This is quite difficult to explain because the muscularis mucosa of intestinal tissues of each treatment is of normal thickness and the intervening stroma were thinly fibrocollagenous and infiltrated by florid population of mononuclear inflammatory cells, predominantly lymphocytes. According to Miles et al. (2006), the inclusion of feed antibiotics in the diets of chicken reportedly leads to a decreased thickness of intestinal walls.
From this study, it could be concluded that CCEB can replace antibiotic use in broiler production due to the presence of bioactive compounds with phenols and tannins in abundance. It was found that 15 ml of CCEB per litre of water enhanced the production of HDL, reduced LDL and improved immunity via the reduction of the bursa of Fabricius, and it is hereby recommended for broiler chicken production.
Data availability
All data generated or analysed during this study are included in this manuscript.
References
Adebayo-Tayo, B.C., Akinsete, T.O. and Odeniyi, O.A., 2016. Phytochemical composition and comparative evaluation of antimicrobial activities of the juice extract of Citrus aurantifolia and its silver nanoparticles, Nigerian Journal of Pharmaceutical Research, 12(1), 59-64.
Agbafor, K.N., Elom, S.O., Ogbanshi, M.E., Oko, A.O., Uraku, A.J., Nwankwo, V.U.O., Ale, B.A. and Obiudu, K.I., 2015. Antioxidant Property and Cardiovascular Effects of Coconut (Cocos nucifera) Water, International Journal of Biochemistry Research and Review, 5(4), 259-263
Ajuwon, K., 2016. Toward a better understanding of mechanisms of probiotics and prebiotics action in poultry species, Journal of Applied Poultry Reasearch, 25(2), 277–283.
Al-Kassie, G.A.M. and Jameel, Y.J., 2009. The effect of adding Thyme vulgaris and Cinnamomum zeylanicum on production performance and some blood traits in broiler chicken, The Iraqi Journal of Veterinary Medicine, 33(2), 84–90.
Belay, K. and Sisay, M.J.C.M.R., 2014. Phytochemical constituents and physicochemical properties of medicinal plant (Moringa Oleifera) around bule hora, Chemistry and Materials Research, 6(7), 61-72.
Botsoglou, N.A., Florou-Paner, P. Chiristaki, E. Fletouris, D.J. and Spais, A.B., 2002. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues, British Poultry Science, 43: 223-230.
Chan, T.Y., 1999. Health hazards due to clenbuterol residues in food, Journal of Toxicology: Clinical Toxicology, 37: 517-519
Cross, D.E., McDevitt, R.M., Hillman, K. and Acamovic, T., 2007. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age, British Poultry Science, 48, 496–506.
David, L.S., Vidanarachchi, J.K., Samarasinghe, K., Cyril, H.W. and Dematawewa, C.M.B., 2015. Effects of moringa based feed additives on the growth performance and carcass quality of broiler chicken, Tropical Agricultural Research, 24(1), 12–20
Diarra, M.S. and Malouin, F., 2014. Antibiotics in canadian poultry productions and anticipated alternatives, Frontiers in Microbiology, 5, 282
Diarra, M.S., Rempel, H., Champagne, J., Masson, L., Pritchard, J. and Topp, E., 2010. Distribution of antimicrobial resistance and virulence genes in enterococcus spp. and characterisation of isolates from broiler chickens, Applied and Environmental Microbiology, 76, 8033-8043
Diaz-Sanchez, S., D'Souza, D., Biswas, D. and Hanning, I., 2015. Botanical alternatives to antibiotics for use in organic poultry production, Poultry Science, 94(6), 1419-1430
Frankic, T., Voljg, M., Salobir, J., Rezar, V., 2009. Use of Herbs and spices and their extracts in animal nutrition, Acta Agriculturae Slovenica, 92(2), 95-102
Gassner, B. and Wuethrich, A., 1994. Pharmacokinetic and toxicological aspects of the medication of beef-type calves with an Oral formulation of chloramphenicol palmitate, Journal of Veterinary Pharmacology and Therapeutics, 17: 279-283
Gheisar, M.M., Hosseindoust, A. and Kim, I.H., 2015. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chickens diet, Journal of Applied Poultry Research, 24(4), 511-519.
Gonzalez Ronquillo, M. and Angeles Hernandez, J.C., 2017. Antibiotic and synthetic growth promoters in animal diets: review of impact and analytical methods, Food Control, 72, 255-267
Harborne, J.B., 1998. Phytochemical methods guide to modern Technique of Plant analysis. 3rd Edition, (Chapman and Hall, London)
Hernandez, F., Madrid, J., Garcia, V., Orengo, J. and Megias, M.D., 2004. Influence of two plant extracts on broiler performance, digestibility and digestive organ size, Poultry Science, 83, 169–174.
Hur, J., Kim, J.H., Park, J.H., Lee, Y.J. and Lee, J.H., 2011. Molecular and virulence characteristics of multi-drug resistant salmonella enteritidis strains isolated from poultry, The Veterinary Journal, 189, 306-311
Ifrah, M.E., Perelman, B., Finger, A. and Uni, Z., 2017. The role of the bursa of Fabricius in the immune response to vaccinal antigens and the development of immune tolerance in chicks (Gallus domesticus) vaccinated at a very young age. Poultry Science, 96(1), 51-57.
Jain, N. C. 1986. Schalm's Veterinary Hematology. 4th edition, (Lea and Febiger, Philadelphia)
Jamroz, D., Orda, J., Kamel, C., Wiliczkiewicz, A., Wertelecki, T. and Skorupinska. J., 2003. The influence of phytogenic extracts on performance, nutrient digestibility, carcass characteristics, and gut microbial status in broiler chickens, Journal of Animal and Feed Sciences, 12, 583–596.
Jang, I.S., Ko, Y.H., Kang, S.Y. and Lee, C.Y., 2007. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens, Animal Feed Science and Technology, 134, 304–315
Karukarach, T., Rakangthong, C., Bunchasak, C. and Poeikhampha, T., 2016. Comparative Effects of Blended Herbal Extracts and Mixed Prebiotics on Growth Performance, Carcass Yields and Intestinal Morphology of Broiler Chickens, International Journal of Poultry Science, 15, 264-270.
Kermanshahi, H. and Riasi, A., 2006. Effect of turmeric rhizome powder (Curcuma longa) and soluble NSP degrading enzyme on some blood parameters of laying hens, International Journal of Poultry Science, 5, 494-498.
Khaligh, F., Sadeghi, G., Karimi A. and Vaziry, A., 2011. Evaluation of different medicinal plants blends in diets for broiler chickens, Journal of Medicinal Plants Research, 5(10), 1971-1977
Khalil, M. I., Sulaiman, S. A. and Boukraa, L., 2010. Antioxidant properties of honey and its role in preventing health disorder, The Open Nutraceuticals Journal, 3(1), 6-16
Khattak, F., Ronchi, A., Castelli, P. and Sparks, N., 2014. Effects of natural blend of essential oil on growth performance, blood biochemistry, caecal morphology, and carcass quality of broiler chickens. Poultry science, 93(1), 132-137
Kummerer, K., 2009. Antibiotics in the aquatic environment–a review–part I, Chemosphere, 75, 417-434
Lai, P. K. and Roy, J., 2004. Antimicrobial and chemopreventive properties of herbs and spices, Current medicinal chemistry, 11(11), 1451-1460.
Lee, K.W., Ho Hong, Y., Lee, S.H., Jang, S.I., Park, M.S. and Bautista, D.A., 2012.Effects of anticoccidial and antibiotic growth promoter programs on broiler performance and immune status, Research in Veterinary Science, 93, 721-728
Manges, A.R., Smith, S.P., Lau, B.J., Nuval, C.J., Eisenberg, J.N. and Dietrich, P.S., 2007. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia Coli causing urinary tract infections: a case-control study, Foodborne Pathogens and Disease, 4: 419-431
Mehala, C. and Moorthy, M., 2008. Effect of Aloe vera and Curcuma longa (Turmeric) on carcass characteristics and biochemical parameters of broilers, International Journal of Poultry Science, 7(9), 857-861
Mehdi, Y., Létourneau-Montminy, M., Gaucher, M., Chorfi, Y., Suresh, G., Rouissi, T., Brar, S.K., Côté, C., Ramirez, A.A. and Godbout, S., 2018. Use of antibiotics in broiler production: Global impacts and alternatives. Animal Nutrition, 4(2), 170-178
Miles, R.D., Butcher, G.D., Henry, P.R. and Littell, R.C., 2006. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters and qualitative morphology. Poultry Science, 85, 476-485
Oikeh, E. I., Omoregie, E. S., Oviasogie, F. E. and Oriakhi, K., 2016. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates, Food Science and Nutrition, 4(1), 103-109.
Qamar, S.H., Haq, A.U., Asghar, N., Rehman, S.U., Akhtar, P. and Abbas, G., 2015. Effect of herbal medicine supplementations (Arsilvon Super, Bedgen40 and Hepa-cure Herbal Medicines) on growth performance, immunity and haematological profile in broilers, Advances in zoology and botany, 3(2), 17-23.
Ricketts, M.L. and Ferguson, B.S., 2018. Polyphenols: novel signaling pathways, Current Pharmaceutical Design, 24(2), 158-170
Robles, H., 2005. Tannic Acid. In P. Wexler (Ed.), Encyclopedia of Toxicology (Second Edition). New york: Elsevier. 134-136
Sadler, C.R. and Glick, B. 1962. The relationship of the size of the bursa of Fabricius to antibody production, Poultry Science, 41(2), 508-510.
Safiyu, K.K., Sogunle, O.M., Egbeyale, L.T., Shittu, T.A., 2019. An exploratory study on the effects of rearing system and plumage colour on performance, carcass characteristics and meat quality of local turkeys, International Journal of Health, Animal Science and Food Safety, 6(1), 1-15
Shah, S.M.T., Islam, M.T., Zabin, R., Roy, P.C., Meghla, N.S. and Jahid, I.K., 2021. Assessment of novel probiotic strains on growth, hematobiochemical parameters, and production costs of commercial broilers in Bangladesh, Veterinary World. 14(1), 97-103
Shamoto, K. and Yamauchi, K., 2000. Recovery responses of chick intestinal villus morphology to different refeeding procedures, Poultry Science, 79(5): 718-723.
Singh, P., Karimi, A., Devendra, K., Waldroup, P.W., Cho, K.K. and Kwon, Y.M., 2013. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens, Poultry Science, 92, 272-276
Sinurat, A.P., Purwadaria, T., Togatorop, M.H., Pasaribu, T., Bintang, I.A.K., Sitompul, S. and Rosida, J., 2002. Responses of broilers to Aloe vera bioactives as feed additive: the effect of different forms and levels of bioactives on performances of broilers, Jurnal Ilmu Ternak dan Veteriner, 7, 69-75
Sofowora, A., 1993. Medicinal plants and Traditional medicine in Africa, (John Wiley and Sons, New York)
Suresh, G., Das, R.K., Brar, S.K., Rouissi, T., Ramirez, A.A., Chorfi, Y. and Godbout, S., 2018. Alternatives to antibiotics in poultry feed: Molecular perspectives, Critical Reviews in Microbiology, 44(3), 318–335.
Thabrew, M.I., Hughes, R.D. and McFarlane, I.G., 1998. Antioxidant activity of Osbeckia aspera. Phytotherapy Research, 12, 288-290
Torok, V.A., Allison, G.E., Percy, N.J., Ophel-Keller, K. and Hughes R.J., 2011. Influence of antimicrobial feed additives on broiler commensal post-hatch gut microbiota development and performance, Applied and Environmental Microbiology, 77, 3380-3390
Trease, G.E. and Evans, W.C., 1989. Pharamacognosy, (W.B. Scandars Company Ltd. London)
Viveros, A., Chamorro, S., Pizarro, M., Arija, I., Centeno, C. and Brenes, A., 2011. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks, Poultry Science, 90(3), 566-578
Wang, Y., Li, J., Xie, Y., Zhang, H., Jin, J., Xiong, L. and Liu, H. 2021. Effects of a probiotic-fermented herbal blend on the growth performance, intestinal flora and immune function of chicks infected with Salmonella pullorum, Poultry Science, 100(7), 1-9
Wiley-VCH. 2003. Phenol, Ullmann’s Encyclopedia of industrial chemistry, 25: 589-604
Wongsuvan, G., Wuthiekanun, V., Hinjoy, S., Day, N.P. and Limmathurotsakul, D., 2018. Antibiotic use in poultry: a survey of eight farms in Thailand, Bulletin of World Health Organiztion, 96(2), 94-100
Zhang, K.Y., Yan, F., Keen, C.A. and Waldroup, P.W., 2005. Evaluation of microencapsulated essential oils and organic acids in diets for broiler chickens, International Journal of Poultry Science, 4, 612–619.
Zou, Z., Xi, W., Hu, Y., Nie, C. and Zhou, Z., 2016. Antioxidant activity of Citrus fruits, Food Chemistry, 196, 885-896
Acknowledgements
The authors would like to extend their appreciation to the Teaching and Research Farm, Animal Nutrition, and Veterinary Pathology laboratories of the Michael Okpara University of Agriculture, Umudike, Nigeria, for providing all facilities used for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the data collection, statistical analyses and final revision and approved the submission of this manuscript. KKS and KLA contributed to the study conception and design as well as the statistical analyses of the data collected. AJI, CEA and RSO carried out the preparation of CCEB, management of experimental birds, laboratory studies, and data collection. OMS carried out the final revision. KKS wrote the manuscript.
Corresponding author
Ethics declarations
Ethical statement
This study was carried out according to the Animal Use and Care Committee guidelines of the Federal Republic of Nigeria (A17 LFN, 2004). Efforts were made to lessen the pain and harm to experimental birds.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safiyu, K.K., Akinsola, K.L., Ibedu, A.J. et al. Effect of citrus-coconut electrolyte blend on growth performance, haemato-biochemical status, organs development and intestinal morphology of broiler chickens. Trop Anim Health Prod 55, 56 (2023). https://doi.org/10.1007/s11250-023-03463-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03463-0