Abstract
Coccidiosis is an important global chickens’ disease which can cause serious economic losses in the poultry industry worldwide. Little is known about the extent of infection or diversity, of the causative agent Eimeria spp., in Algeria. A priority, therefore, is to determine the prevalence and species composition to inform strategies on treatments and control measures. Samples were collected from 187 broiler farms, located in 7 Northeastern Algerian provinces (Jijel, Constantine, Skikda, Mila, Setif, Batna, Bordj bou-Arreridj), and Internal Transcribed Spacer 1 PCR (ITS1-PCR) was used to determine the prevalence and composition of Eimeria species in chickens. The survey revealed the presence of all seven species of Eimeria at different prevalences (E. maxima (69%), E. acervulina (68.4%), E. necatrix (11.2%), E. tenella (8%), E. praecox (4.3%), E. mitis (2.1%), E. brunetti (2.1%). Multiple infections, with up to 4 different Eimeria species present on a single farm, were the most frequent situation in our samples (51.9% mixed infections versus 47.6% single infections). All farms revealed infected samples, and we conclude that this parasite is a significant problem in these provinces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis is the most important chickens’ parasitic disease (Gallus gallus domesticus), with a global cost estimated at around £10.36 billion in 2016, including losses during production and costs for prophylaxis and treatment (Blake et al. 2020). Caused by intestinal protozoan parasites, belonging to the Phylum Apicomplexa, the genus Eimeria manifests its disease by causing enteritis. It is found worldwide in all types of poultry production and the disease can take many clinical forms (McDougald and Reid 1997).
It is generally accepted that seven species of Eimeria (E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella) parasitize chickens and different species have different degrees of pathogenicity (Williams et al. 1996). E. tenella, E. maxima, and E. acervulina are regarded as the most economically significant species (Dodd et al. 2014). Co-infection of Eimeria species (Haug et al. 2008; Jenkins et al. 2008) is common in coccidiosis which contributes not only to pathogenicity but can also result in misleading diagnoses (Fatoba and Adeleke 2018). Many factors, including the age and diet of the birds, the effectiveness of prophylactic anticoccidial drugs, and intercurrent infections and stress, determine the degree of the clinical manifestation of coccidial infection (Williams et al. 1996). To our knowledge, only a few other studies have been conducted to ascertain the prevalence of infection in Algeria (Debbou-louknane et al. 2018). These previous studies have focused on using traditional parasitological identification of parasites to determine species identity. This is the first study to use a molecular approach (ITS1-PCR) (Jenkins et al. 2006a, b) to determine the prevalence and species identity for each of the seven species of Eimeria spp. Conducted on 187 broiler farms across 7 provinces in northeastern Algeria, we show that this parasite could be a significant problem in this area of Algeria.
Materials and methods
Study area and sample collection
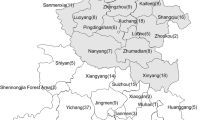
The survey was carried out on 187 broiler farms located in seven north-eastern provinces of Algeria: Jijel (44 farms), Constantine (31 farms), Skikda (10 farms), Mila (17 farms), Setif (35 farms), Batna (42 farms), and Bordj bou-Arreridj (8 farms) (Fig. 1). Sampling was conducted over a period from June 2009 to March 2014. In all the selected flocks, samples were taken from the litter at the frequency of one fresh individual chicken dropping per 100 birds (wet areas of the litter was avoided) and this, one day per week in the following weeks of birds’ age: 3 or 4, 5 or 6, 8 or 9 as recommended previously (Williams 2006).
The total size of the chicken population presents in all the selected farms was 1,000,580 animals distributed across 187 broiler flocks with each flock having a range of capacities from 4000 to 10,000 chickens (the number of broilers varies from one farm to another). The birds were selected from different broiler-breeder flocks and commercial hatcheries located in these different provinces of the country. The broiler feeds distributed in all selected farms are composed of corn, soybean meal, wheat bran, and premix (three ionophores are used in the surveyed farms: monensin, salinomycin, lasalocid). All flocks were kept on litter composed of straw and wood shavings. In terms of husbandry, the age at slaughter for chickens on these farms was between the 50th and 55th days for an average weight of about 3.1 kg and the breeds of broilers used were Cobb 500, Arbor Acre, and Hubbard F15.
Oocyst preparation
For each broiler farm, the sampled feces were mixed into a single pool (one pool/broiler farm; knowing that the weight of each pool of samples per farm ranges from 300 to 500 g, depending on the size of the sampled broiler facilities). Unsporulated oocysts of Eimeria were suspended in 2.5% potassium dichromate (with a dose of 500 to 1200 oocysts/ml of potassium dichromate solution) and were stored at 4 °C until use.
Genomic DNA extraction from oocysts
Genomic DNA from purified sporulated oocysts was extracted using a phenol chloroform extraction process as described by Duncanson et al. (2001) and Bajnok et al. (2015) with modifications for small tissue samples (Dodd et al. 2014) and carried out at the laboratory GBBV (Laboratoire de Génétique Biochimie et Biotechnologies Végétales, University of Constantine 1, Algeria).
Identification of Eimeria species by PCR
Genomic DNA was transferred to the University of Salford, Manchester UK, and was used as a template to PCR amplify the ITS-1 region of specific Eimeria species (Jenkins et al. 2006a, b) in order to identify the different species of Eimeria present in the different pools of oocyst isolates. The reaction mixtures (25 μL of master mix) for each sample that contained 25 pmol forward (Eurofins; Forward primer, UK) and ITS1 reverse primer (Eurofins; Reverse primer, UK), 200 nM dNTP (Amersham, Piscataway, NJ), 20 mM Tris pH 8.4, 50 mM KCl, 3.0 mM MgCl2, and 1 U Taq polymerase ((Bioline-BIOTAQTMDNA Polymerase, UK). Add at the end 1 μL of the DNA of the sample (an amount of total Eimeria DNA equivalent to more than 4000 oocysts) to be identified in a reaction mixture (in the negative control, add 1 μL of water). DNA amplification was done in a thermal cycler (Stratagene Gradient RobocyclerTM96, UK). Reaction conditions were as follows: 1 cycle—95 C, 7 min; 35 cycles—95 C, 20 s, 44–60 C, 30 s, 72 C, 1 min; 1 cycle—72 °C, 5 min (Jenkins et al. 2006a, b).
Statistical analysis
The data were collected and calculated in Microsoft Excel 2019 (version 16.27). Chi square tests between the percentages found for the different types of infection were performed with RStudio environment version 1.2.5033 (RStudio Team, 2019).
Results
The prevalence of chicken Eimeria spp in all of our samples is in the order of 99.5%. The proportions of each Eimeria species present in the pooled samples differed between farms and between the 7 provinces (Table 1); however, E. maxima and E. acervulina were the most commonly found species, as follows (Table 2): E. maxima (69%), E. acervulina (68.4%), E. necatrix (11.2%), E. tenella (8%), E. praecox (4.3%), E. mitis (2.1%), and E. brunetti (2.1%). In an overview, the differences in the prevalences between the identified species were statistically significant (p < 0.001) (Table 2). Table 3 shows the status of mixed infections on single farms—these ranged from no mixtures to up to 4 species observed in the same sample. E. maxima was the most prevalent species (in 42 cases; 22.5%) (p < 0.001) when only one Eimeria species was found in the sample pools, followed respectively (p < 0.001) by E. acervulina (38 cases; 20.3%), E. tenella (5 cases; 2.7%), E. necatrix (3 cases; 1.6%), and E. praecox (one case; 0.53%) (Table 3).
The prevalence of single infections was 47.6% compared with 51.9% for mixed infections (41.2%, 7.5%, and 3.2%, respectively, for double, triple, and quadruple infections). There was a significant difference between the prevalences of single, double, triple, and quadruple infections (p < 0.001) (Table 3).
Mixed infections (double, triple, or quadruple infections) were found in 97 (51.9%) flocks. Numerically, the most prevalent combinations were E. acervulina + E. maxima (60/187; 32.1%), followed respectively by E. acervulina + E. maxima + E. necatrix (6/187; 3.2%), E. maxima + E. necatrix (5/187; 2.7%), E. acervulina + E. necatrix (4/187; 2.1%), and E. acervulina + E. maxima + E. tenella (4/187; 2.1%), E acervulina + E. maxima + praecox (3 /187; 1.6%) (Table 3).
Double infections E. acervulina + E. tenella, E. acervulina + E. mitis, and E. maxima + E. tenella, each have a prevalence of 1.1%, while all quadruple infections each has a frequency of the order of 0.5% (Table 3).
Discussion
In this study, the prevalence of Eimeria spp was measured using molecular approaches (ITS1-PCR) and found to be very high (99.5%) across all farms. which is probably due to the poor hygienic conditions and/or poor control of rearing techniques, in particular, the high stocking density. Generally, in Algeria, the stocking density of broilers is greater than 10/m2 around the seventh week which may be a factor accounting for the high prevalence. Few other studies exist on Eimeria prevalence in Algerian broilers to our knowledge. Debbou-louknane et al. (2018) found a much lower prevalence of 93/147 (63.3%) of litter samples or 78/109 (71.6%) chicken intestinal contents with an overall prevalence rate of 54.3% farms infected. This study was conducted in the Bejaia province (adjoining Jijel, Setif, and Bordj bou-Arreridj), and it involved parasitological examination and identification of Eimeria species using traditional characteristics such as size, shape, and pathology. They found E. acervulina and E. tenella to be the most prevalent species but did not detect E. necatrix or E. praecox. Our study is the first to use molecular tools and, while it is tempting to speculate that the 100% infection rate observed in this study indicates that molecular tools may be more sensitive, robust comparative studies would be needed to establish this. However, other molecular studies have also reported high prevalences, for example, Györke et al. (2013), who obtained two prevalences: 100% and 91%, obtained respectively by flotation of oocysts and PCR. However, this latter study was conducted in Romania—a significant geographical distance from Algeria—and may not be directly comparable.
Our study confirms the presence of all seven species of chicken Eimeria in the Algerian field; with E. maxima (69%) and E. acervulina (68.4%) being the most frequent species. This has also been found by numerous other studies in other locations. Moraes et al. (2015) obtained the same results (E. maxima was 63.7%; E. acervulina was 63.3%) in a study conducted in Brazil on 251 broiler farms. Jeffers (1974) confirms that these two species of Eimeria are ubiquitous and their occurrence is largely unaffected by the anticoccidial medication employed. However, E. acervulina is the most prevalent species in France (Williams et al. 1996), in the UK (Williams 2006) and in Norway (Haug et al. 2008). This is probably due to the very high reproductive potential of this species (Williams 2001).
In this study, E. acervulina and E. maxima were recovered as single species from 44 (23.5%) and 36 (19.3%) of the farms sampled, respectively; this observation corroborates other studies (Jeffers 1974; Haug et al. 2008; Györke et al. 2013; Moraes et al. (2015); who have also shown that E. acervulina and/or E. maxima also dominate single infections in broilers.
Interestingly, the prevalence of E necatrix (11.2%: third place in the species ranking in this study) was higher than that observed in previous European studies (Warren et al. 1966; Hodgson et al. 1969; Williams et al. 1996, 1999) which suggest that this species of Eimeria seems to be generally uncommon (or of low prevalence) in Europe. Similarly, McDonald and Shirley (2009) attest that E. necatrix is rare in North America. However, studies carried out in Africa, the Middle East, and Asia show that E. necatrix is one of the most frequent species (4–30%) reported in broilers (Lobago et al. 2005; Aarthi et al. 2010; Shirzad et al. 2011; Awais et al. 2012). Our studies are consistent with this.
Contrary to what is reported in this study (it ranks fourth with a relatively low prevalence 8%), E. tenella is one of the predominant species in broilers (Lee et al. 2010; Al-Natour et al. 2002; Awais et al. 2012; Györke et al. 2013; Moraes et al. 2015) and also has a very high reproductive potential (Williams 2001).
The species less frequently detected in our survey were E. praecox (4.3%), E. mitis (2.1%), and E. brunetti (2.1%). Jeffers (1974) observed a prevalence of around 2.3% of E. brunetti in litter from the major broiler-producing regions of the USA. However, according to several authors (Lobago et al. 2005; Aarthi et al. 2010; Lee et al. 2010), E. brunetti is one of the most frequent species in broiler chickens. On 18 samples from broiler farms, Aarthi et al. (2010) obtained the following results: E. necatrix (100%), E. brunetti (83.33%), E. tenella (83.33%), E. maxima (77.77%), E. acervulina (55.55%), E. praecox (16.66%), and E. mitis (11.11%).
E. mitis and E. praecox are generally underestimated and underdiagnosed species, due to the less identifiable lesion manifestations (McDougald and Reid 1997). However, these two species have been frequently detected in certain surveys, for example, those carried out by Kučera (1990) in Czechoslovakia (50% and 31.25% respectively for E. mitis and E. praecox), Williams et al. (1996) in France (82% and 45%, respectively, for E. mitis and E. praecox), Williams (2006) in the UK, and McDougald et al. (1997) in Argentina (67% and 51%, respectively, for E. mitis and E. praecox).
Multiple infections with different Eimeria species affecting chickens were the most frequent situation in our samples (51.9% mixed infections versus 47.6% single infections). This conclusion is also generally reached in previous studies (Williams et al. 1996; Haug et al. 2008; Györke et al. 2013). Double infection E. acervulina + E. maxima predominates mixed infections with a prevalence of around 60%, while E. acervulina dominates single infections (50% of single infections).
Despite the presence of highly pathogenic species, including E. tenella, E. brunetti, and E. necatrix, we have not found severe episodes of clinical coccidiosis across the 187 farms we have studied. This observation of subclinical infections is probably the result of the addition of ionophore coccidiostats in the food (three ionophores are used in the surveyed farms: monensin, salinomycin, lasalocid). However, there are concerns that resistance to these coccidiostats may be developing (Djemai et al. 2016). The high background levels of parasite prevalence provide a worryingly large potential baseline on which resistance can evolve. There is little current data concerning subclinical coccidiosis in this region, and the baseline data reported here offer the opportunity to be followed up with future studies to investigate the possible progression of resistance.
Data availability
Data will be made available upon reasonable request.
Code availability
Not applicable.
References
Aarthi, S., Dhinakar, R.G., Raman, M., Gomathinayagam, S., and Kumanan, K., 2010. Molecular prevalence and preponderance of Eimeria spp among chickens in Tamil Nadu India. Parasitology Research, 107(4), 1013–1017.
Al-Natour, M.Q., Suleiman, M.M., and Abo-Shehada, M.N., 2002. Flock level prevalence of Eimeria species among broiler chicks in northern Jordan. Preventive Veterinary Medicine, 53 (4), 305–310.
Awais, M.M., Akhtar, M., Iqbal, Z., Muhammad, F., and Anwar, M.I., 2012. Seasonal prevalence of coccidiosis in industrial broiler chickens in Faisalabad Punjab Pakistan. Tropical Animal Health and Production, 44, 323–328.
Bajnok, J., Boyce, K., Rogan, M.T., Craig, P.S., Lun, Z.R., and Hide, G., 2015. Prevalence of Toxoplasma gondii in localized populations of Apodemus sylvaticus is linked to population genotype not to population location. Parasitology, 142 (5), 680–690.
Blake, D. P., Knox, J., Dehaeck, B., Huntington, B., Rathinam, T., Ravipati, V., Ayoade, S., Gilbert, W., Adebambo, A. O., Jatau, I. D., Raman, M., Parker, D., Rushton, J., and Tomley, F.M., 2020. Re‑calculating the cost of coccidiosis in chickens. Veterinary Research, 51: 115, 2–14.
Debbou-Iouknane, N., Benbarek, H.,and Ayad, A., 2018. Prevalence and aetiology of coccidiosis in broiler chickens in Bejaia province, Algeria. Onderstepoort Journal of Veterinary Research, 85(1), 1–6.
Djemai, S., Mekroud, A., and Jenkins., M.C., 2016. Evaluation of ionophore sensitivity of Eimeria acervulina and Eimeria maxima isolated from the Algerian to Jijel province poultry farms. Veterinary Parasitology, 224, 77–81.
Dodd, N.S., Lord, J.S., Jehl, R., Parker, S., Parker, F., Brooks, D.R., and Hide, G., 2014. Toxoplasma gondii: Prevalence in species and genotypes of British bats (Pipistrellus pipistrellus and P. pygmaeus). Experimental Parasitology, 139, 6–11.
Duncanson, P., Terry, R.S., Smith, J.E., and Hide, G., 2001. High levels of congenital transmission of Toxoplasma gondii in a commercial sheep flock. International Journal for Parasitology, 31 (14), 1699–1703.
Fatoba, A.J., and Adeleke, M.A., 2018. Diagnosis and control of chicken coccidiosis: a recent update. Journal of Parasitic Diseases, 42(4), 483–493.
Györke, A., Pop, L., and Cozma, V., 2013. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite, 20 (50), 1–8.
Haug, A., Gjevre, A.G., Thebo, P., Mattsson, J.G., and Kaldhusdal, M., 2008. Coccidial infections in commercial broilers: epidemiological aspects and comparison of Eimeria species identification by morphometric and polymerase chain reaction techniques. Avian Pathology, 37(2), 161–170.
Hodgson, J.N., Ball, S.J., Ryan, K.C., and Warren, E.W.,1969. The incidence of drug resistant strains of Eimeria in chickens in Great Britain, 1966. British Veterinary Journal, 125(1), 31–35.
Jeffers, T.K., 1974. E. acervulina and E. maxima: Incidence and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Diseases, 18 (3), 331–342.
Jenkins, M.C., Miska, K., and Klopp, S., 2006a. Improved polymerase chain reaction technique for determining the species composition of Eimeria in poultry litter. Avian Diseases, 50 (4), 632–635.
Jenkins, M.C., Miska, K., and Klopp, S., 2006b. Application of polymerase chain reaction based on ITS1 rDNA to speciate Eimeria. Avian Diseases, 50(1), 110–114.
Jenkins, M.C., Allen, P., Wilkins, G., Klopp, S., and Miska, K., 2008. Eimeria praecox infection ameliorates effects of Eimeria maxima infection in chickens. Veterinary Parasitology, 155 (1-2), 10–14.
Kučera, J., 1990. Identification of Eimeria species in Czechoslovakia. Avian Pathology, 19 (1), 59–66.
Lee, B.H., Kim, W.H., Jeong, J., Yoo, J., Kwon, Y.K., Jung, B.Y., Kwon, J.H., Lillehoj, H.S., and Min, W., 2010. Prevalence and cross-immunity of Eimeria species on Korean chicken farms. The Journal of Veterinary Medical Science, 72(8), 985–989.
Lobago, F, Worku, N., and Wossene, A., 2005. Study on coccidiosis in Kombolcha poultry farm Ethiopia. Tropical Animal Health and Production, 37, 245–251.
McDonald, V., and Shirley, M.W., 2009. Past and future: vaccination against Eimeria. Parasitology, 136, 1477–1489.
McDougald, L.R., Fuller, L., and Mattiello R., 1997. A survey of Coccidia on 43 poultry farms in Argentina. Avian Diseases, 41(4), 923– 929.
McDougald, L.R. and Reid, W.M., 1997. Coccidiosis. In: B.W. Calnek (Eds). Diseases of Poultry. 10th Ed. (Iowa State University Press, Ames), pp 865–883.
Moraes, J. C., França, M., Sartor, A. A., Bellato, V., Barbosa de Moura, A., Borba Magalhães, M. L., Pereira de Souza, A., and Miletti, L. C., 2015. Prevalence of Eimeria spp. in Broilers by Multiplex PCR in the Southern Region of Brazil on Two Hundred and Fifty Farms. Avian Diseases, 59(2):277–281.
Ryley, J.F, Meade, R, Hazelhurst, J., and Robinson, T.E., 1976. Methods in coccidiosis research: separation of oocysts from faeces. Parasitology, 73 (3), 311–326.
Shirzad, M.R, Seifi, S, Gheisari, H.R., Hachesoo, BA, Habibi, H., and Bujmehrani, H., 2011. Prevalence and risk factors for subclinical coccidiosis in broiler chicken farms in Mazandaran province Iran. Tropical Animal Health and Production, 43, 1601–1604.
Warren, E.W., Ball, S.J., and Mackenzie, D.R., 1966. The incidence of drug-resistant strains of Eimeria species in chickens in Great Britain, 1964/65. British Veterinary Journal, 122, (12), 534–543.
Williams, R.B., 2001. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. International Journal for Parasitology, 31(10), 1056–1069.
Williams, R.B., 2006. Tracing the emergence of drug-resistance in coccidia (Eimeria spp) of commercial broiler flocks medicated with decoquinate for the first time in United Kingdom. Veterinary Parasitology, 135(1), 1–14.
Williams, R.B., Bushell, A.C., Répérant, J.M., Doy, T.G., Morgan, J.H., Shirley, M.W., Yvoré, P., Carr, M.M., and Frémont, Y., 1996. A survey of Eimeria species in commercially-reared chickens in France during 1994. Avian Diseases, 25, 113–130.
Williams, R.B., Carlyle, W.W., Bond, D.R., and Brown, I.A., 1999. The efficacy and economic benefits of Paracox, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom. International Journal for Parasitology, 29 (2), 341–355.
Author information
Authors and Affiliations
Contributions
Samir Djemai: conceived the idea for this study, methodology, analyzed the data, wrote the manuscript. Ouarda Ayadi: data collection, analyzed the data, wrote the manuscript. Daoudi Khelifi and Ines Bellil: extraction and purification of parasitic DNA. Geoff Hide: analyzed the data, wrote the manuscript, supervision.
Corresponding author
Ethics declarations
Ethics approval
The manuscript does not contain clinical studies, which means that no animal was harmed during the course of this research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Djemai, S., Ayadi, O., Khelifi, D. et al. Prevalence of Eimeria species, detected by ITS1-PCR, in broiler poultry farms located in seven provinces of northeastern Algeria. Trop Anim Health Prod 54, 250 (2022). https://doi.org/10.1007/s11250-022-03252-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03252-1