Abstract
The negative impact of heat stress on cattle growth, development, reproduction and production has been quite alarming across the world. Climate change elevates earth surface temperature which exacerbates the wrath of heat stress on cattle. Moreover, cattle in tropical and sub-tropical countries are most commonly affected by the menace of heat stress which severely wane their production and productivity. In general, cattle exhibit various thermoregulatory responses such as behavioural, physiological, neuro-endocrine and molecular responses to counteract the terrible effects of heat stress. Amongst the aforementioned thermoregulatory responses, behavioural, physiological and neuro-endocrine responses are regarded as most conventional and expeditious responses shown by cattle against heat stress. Furthermore, molecular responses serve as the major adaptive response to attenuate the harmful effects of heat stress. Therefore, present review highlights the significance of behavioural, physiological, neuro-endocrine and molecular responses which act synergistically to combat the deleterious effects of heat stress thereby confer thermo-tolerance in cattle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
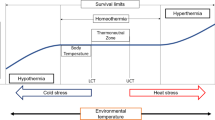
Thermo-neutral zone (TNZ) is considered the most comfort zone for all livestock including cattle, where they use minimum energy to maintain their core body temperatures. On the other hand, upper critical temperature (UCT) is the temperature above which livestock must use energy to dissipate body heat to maintain their core body temperature (Mishra 2021). Livestock experience heat stress when environmental temperature emulates TNZ and UCT (Mishra and Palai 2014; Collier et al. 2019). Consistent spike of greenhouse gases in the atmosphere has been the prime factor for climate change (Afsal et al. 2018). IPCC envisages an increase in earth surface temperature from 1.4 to 4.8°C by the end of the twenty-first century. This noticeable rise in earth surface temperature might lead to heat stress which disturbs the equilibrium between livestock and their ecosystem resulting in awful decline in livestock production throughout the world including India (Das et al. 2011; Stocker et al. 2013; Mishra 2020). Moreover, climate change amplifies the detrimental impact of heat stress on livestock production and productivity (Gaughan et al. 2013; Bharati et al. 2017; Sahu et al. 2019; Lees et al. 2019). High ambient temperature in combination with high relative humidity represent the most severe form of heat stress which could be more deleterious and life threatening to livestock species (Mishra 2020). Furthermore, severity of heat stress in livestock species is commonly estimated by temperature humidity index (THI) (Yadav et al. 2021; Mishra 2021). It has been well established that THI less than 72, within 73–77, between 78 and 89 and more than 90 is considered no heat stress, mild heat stress, moderate heat stress and severe heat stress respectively (Somparn et al. 2004; Gantner et al. 2011; Kohli et al. 2014). High THI precludes heat loss via evaporative cooling thereby exposes livestock to the adverse effect of heat stress (Nardone et al. 2010; Thornton 2010). However, THI has some flaws as it does not include environmental variables such as solar radiations and wind velocity (Moretti et al. 2017). To overcome the flaws encountered in THI, some more indices have been emerged to quantify the intensity of heat stress such as black globe temperature humidity index (BGTHI), equivalent temperature index (ETI) and heat load index (HLI) (Lenis Sanin et al. 2015; Silva and Passini 2017). BGTHI includes ambient temperature, relative humidity and solar radiation while ETI includes ambient temperature, relative humidity and wind velocity (Lenis Sanin et al. 2015). In addition, HLI includes black globe temperature, relative humidity and wind velocity (Silva and Passini 2017).
Various studies reported that livestock in tropics and sub-tropics including India are hugely affected by the deleterious effects of heat stress (Collier et al. 2017; Polsky and Von Keyserlingk 2017). Nonetheless, livestock have their own intrinsic thermoregulatory responses to withstand the rigours of heat stress. Livestock basically adapt to heat insults by displaying different thermoregulatory responses like behavioural, physiological, neuro-endocrine and molecular responses (Collier and Gebremedhin 2015; Mishra 2020). Earlier reports indicated that behavioural responses are most immediate responses exhibited by livestock on exposure to heat stress (Baumgard and Rhoads 2013; De Andrade Ferrazza et al. 2017). After behavioural responses, physiological responses are swiftly manifested by livestock against heat stress and categorised as rectal temperature, respiratory rate, heart rate, skin temperature and sweating rate (Indu and Pareek 2015; Bharati et al. 2017; Ahmad et al. 2018). After behavioural and physiological responses, neuro-endocrine responses play a pivotal role to adapt livestock against heat stress (Kumar et al. 2015; Chen et al. 2018). If behavioural, physiological and neuro-endocrine responses are inadequate to revive homeostasis, then livestock manifest molecular responses to cope up with the devastating effects of heat stress (Sahu et al. 2019; Mishra 2020). Despite the aforementioned thermo-regulatory responses, livestock production and productivity tend to reduce during summer heat stress (Gaughan et al. 2013). Amongst tropical and subtropical countries, India is exalted with 192 millions of cattle, contributing around 12.5% of world’s total cattle population (Das et al. 2012; Das et al. 2016). For decades, India has been remained at zenith vis a vis cattle milk production and known to be world’s largest milk-producing country, contributing around 22% of global milk production. However, vast population of cattle have been protractedly exposed to summer heat stress resulting in decline in milk production which markedly affects farmer’s economy as well the socio-economic status of India. It is high time to critically look into this issue and develop some productive mitigation strategies along with some farmer’s friendly technologies which could minimise the impact of heat stress in cattle. Before that, it is imperative to have a deep insight into the inherent adaptive mechanisms expressed by cattle on exposure to heat stress. Therefore, this review provides an update on different adaptive responses manifested by heat-stressed cattle, which could be helpful to design suitable mitigation strategies and technologies to ameliorate the adverse effects of heat stress in cattle.
Behavioural responses of cattle against heat stress
Behavioural responses seem to be the immediate responses manifested by cattle to counteract the negative effects of heat stress (Fig. 1). In this section, different behavioural responses of cattle against heat stress are comprehensively described. In addition, different behavioural responses exhibited by different breeds of cattle against heat stress are presented in Table 1.
Dry matter intake
Generally, heat stress reduces dry matter intake (DMI) thereby negatively affects livestock’s health and production (El-Koja et al. 1980; West 2003; Baumgard and Rhoads 2013). Rise in ambient temperature from 25 to 27°C reduced DMI in dairy cattle (Beede and Collier 1986). NRC (1989) documented decline in DMI by 40% when cattle were exposed to environmental temperature at 40°C. McGuire et al. (1991) found a significant decline in DMI in heat-stressed Holstein Friesian (HF) cows (11.1 kg/day) than those within TNZ (15.1 kg/day). Likewise, feed intake was significantly reduced in Egyptian HF calves during summer season than winter season (Marai et al. 1995). Reduction in DMI reported by various authors could be attributed to the inhibition of arcuate nuclei (ARC), para-ventricular nuclei (PVN) and lateral hypothalamic area (LHA) in heat-stressed cattle. Reduction in DMI might reduce the body metabolism resulting in lower heat production in heat-stressed cattle. These reports also suggest the fact that DMI might be inversely related to ambient temperature. An interestingly finding was given by Ahmed and El-Amin (1997) that every 1°C hike in environmental temperature tends to decline DMI by 0.24 and 0.06/kg/h in HF and Boran cows respectively. Consistently, consumption of hay and concentrates were reduced by 56% and 88% respectively in lactating HF cows exposed to ambient temperature at 28°C (Itoh et al. 1998). More reduction in consumption of concentrates could be attributed to its less water content than hay. Similarly, DMI was reduced by 5% per day in lactating cows exposed to short-term and moderate heat stress (Ominski et al. 2002). Kadzere et al. (2002) also found a reduction in DMI in lactating cows during prolonged hyperthermia. Consistently, Spiers et al. (2004) found a decline in the DMI by 14.6 kg in HF cows following 3 days after heat exposure at high THI (between 76.4 and 78) compared to low THI (between 62.5 and 65). In another study, heat stress reduced feed intake and feed conversion rate in beef cattle (Brown-Brandl et al. 2005). Nonaka et al. (2008) found a reduction in DMI by 9% in HF heifers exposed to heat load at 33°C than at 28 or 20°C. Marked decrease in DMI could be due to inhibition of appetite centre located in hypothalamic ARC and PVN which might relay negative signal to LHA. Based on these reports on reduction in DMI, it is logical to speculate that high THI might be deleterious to dairy and beef cattle production and welfare. Similarly, DMI was significantly reduced in Angus cattle on exposure to prolonged heat stress at 32°C for 15 days, while no significant change in DMI was noticed in Brahman cattle under similar environmental conditions (Beatty et al. 2006), indicating better thermo-tolerance ability of Brahman cattle than Angus cattle. In another study, Pereira et al. (2008) did not notice any change in DMI in Mertolenga and HF but found decrease in DMI by 10% and 9.6% in Alentejana and Limousine breed respectively during afternoon than morning session under THI at 85. No change in DMI in Mertolenga breed might be due their ability to withstand the heat stress conditions. Even the lowest increase in rectal temperature (RT) in Mertolenga breed (described later) could be the reason behind their high thermotolerant ability compared to rest of the cattle breeds. Unchanged DMI in HF could be attributed to their high water intake (WI) (described later) which might have maintained their core body temperature thereby allowing them to continue feeding. Identically, Kim et al. (2010) reported reduction in DMI by 14% in lactating HF cows upon heat exposure at 30°C (14.64 kg/day) than at 20°C (16.96 kg/day). On the other hand, Kim et al. (2010) observed greater digestibility of dry matter at 30°C (68.2%) than 20°C (64.7%). The reason behind greater digestibility of dry matter might be lower DMI and sustained retention of feed in gastrointestinal tract which could easily be digested within a specific time duration. Another possible explanation could be lower gut motility, rumination and ruminal contraction (Attenberry and Johnson 1969; Beede and Collier 1986; Yadav et al. 2013; Park et al. 2019) leading to prolonged retention of feed, which might send inhibitory signals to hypothalamic appetite centre to decrease DMI during heat stress. In another study, West (2003) reported significant reduction in ruminal contractions in cattle grazing under direct sunlight during hot summer months than those cattle kept under shade. Moreover, reduction in rumination rate was also observed in dairy cows during exposure to elevated environmental temperature (Collier et al. 1982; Tapki and Sahin 2006). Park et al. (2019) investigated the impact of increase in THI on rumination time in lactating HF cows. They exposed cows to THI at 70–75 (T1), 76–81 (T2), and 82–87 (T3). They noted gradual reduction in rumination time from T1 to T3. Moreover, rumination time was recorded to be 473.10, 454.76 and 399.60 min/day in T1, T2 and T3 respectively. In an experiment conducted in growing HF bull calves, DMI was decreased by 12% when temperature inside psychometric chamber was increased from 29.4 to 40°C (O'Brien et al. 2010). In line with earlier studies, Bernabucci et al. (2010) observed reduction in DMI in dairy cattle exposed to higher environmental temperature. Similarly, Wheelock et al. (2010) reported a 30% drop in DMI in lactating HF cows on heat exposure at 38.9°C. According to Rhoads et al. (2013), DMI was reduced by 40% in lactating cows during exposure to ambient temperature at 40°C. Drop in DMI reported by O'Brien et al. (2010), Bernabucci et al. (2010), Wheelock et al. (2010) and Rhoads et al. (2013) might be due to the same reasons discussed earlier in this section. Again in another study, DMI was significantly decreased in Tharparkar (6.25 kg/day) and Karan Fries (KF) (6.31 kg/day) heifers during heat stress than control heifers (Tharparkar—6.92 kg/day; KF—7.52 kg/day) within TNZ (Banerjee and Ashutosh 2011). More reduction in DMI in KF heifers could be attributed to less thermotolerance ability of crossbred KF heifers. Valente et al. (2015) did not notice any change in DMI in Nellore bulls exposed to high heat stress (THI=81.5) than control (THI=72.6) but found a drop in DMI in Angus bulls by 15% per day during heat stress (31.6 g/kg) than control (36.2 g/kg). This could be due to high heat sensitiveness and low heat tolerance ability of Angus breed. Yadav et al. (2015) observed significant dip in DMI in crossbred cattle during heat exposure at 40°C (4.99 kg/day) than at 35°C (5.85 kg/day), 30°C (6.37 kg/day) and 25°C (6.18 kg/day). Furthermore, Yadav et al. (2016) documented significant reduction in DMI in crossbred cattle on heat exposure at 40°C (5.26 kg/day) than at 35°C (6.43 kg/day), 30°C (6.70 kg/day) and 25°C (5.94 kg/day). Marked decrease in DMI at 40°C could be due to the probable reasons described earlier. Again, these findings further validate the fact about significant reduction in DMI at 40°C given by NRC (1989). Additionally, Yadav et al. (2016) estimated significant increase in digestibility of dry matter on heat exposure at 35°C (66.34%) followed by heat exposure at 40°C (62.53%), 30°C (60.62%) and 25°C (59.68%). Significant increase in digestibility of dry matter at 35°C might be due to prolonged mean retention time of feed. Identically, De Andrade Ferrazza et al. (2017) observed significant reduction in DMI in heat-stressed cows (8.27±0.33kg/day) at 36.3°C in comparison with cows under TNZ at 25.9°C (14.03±0.29 kg/day). Subsequently, Garner et al. (2017) determined decline in DMI by 48% in dairy cows exposed to short-term heat stress in controlled climate chambers. Decline in DMI in heat-stressed cattle might be due to reasons provided earlier in this section. In addition, decline in DMI might lower the metabolic heat production to maintain thermal balance during heat stress. Additionally, DMI was reported to be lowered during acute heat stress and revived during chronic heat stress in crossbred cattle (Yadav et al. 2021), dairy heifers (Bernabucci et al. 1999), beef cattle (Brown-Brandl et al. 2003) and HF cattle (De Andrade Ferrazza et al. 2017). Several authors have aimed to find out the effect of shade and cooling on DMI in cattle during heat stress. Roman-Ponce et al. (1981) found 9.7% higher DMI in lactating dairy cows under shade than those without shade during summer season. In another study, DMI was significantly higher in cooled HF cows under close-sided barn with evaporative cooling system than non-cooled crossbred HF kept under open-sided barn with a tiled roof, at early (10.5 versus 8.4 kg/day), mid (10.9 versus 8.3 kg/day) and late (11.4 versus 8.1 kg/day) lactation (Chaiyabutr et al. 2008). Uniformly, Min et al. (2015) reported higher DMI in cool lactating HF cows under low THI at 53.4 (24.45 kg/day) than high THI at 81.7 (17.89 kg/day). Greater DMI in cool cows under shade could be attributed to low level of heat stress, which might have stimulated hypothalamic appetite centre to continue feeding.
Water intake
WI could be influenced by ambient temperature, types of feed, breed types (genotype), body weight and physiological parameters (Arias and Mader 2011). Generally, cattle tend to drink more water on exposure to higher environmental temperature to cope up with the adverse condition of heat stress (Bernabucci et al. 2010). WI was increased by 75% in cattle during summer than winter months (Mullick 1964). According to McDowell and Weldy (1967), WI was increased by two to four times in cattle during heat exposure at 32°C than at 2–10°C. Similarly, WI was found to be increased in lactating HF cows on exposure to high ambient temperature (El-Koja et al. 1980). On exposure to summer heat stress, WI was found to be 19% higher in no shade lactating dairy cows than shade cows (Roman-Ponce et al. 1981). Uniformly, WI was found to be increased by 35% in heat-stressed bulls compared to control bulls (Meyerhoeffer et al. 1985). Consistently, WI was elevated by 1.2 kg/°C in dairy cattle during heat stress (West 2003). Significant increase in WI was observed in beef cattle exposed to simulated heat waves (Brown-Brandl et al. 2005). Higher WI during heat stress reported in the aforementioned studies might be due to plasma hyperosmolarity resulted from evaporative heat loss via sweating (177%) (McDowell and Weldy 1967). Then, plasma hyperosmolarity might be sensed by central osmoreceptors followed by activation of hypothalamic thirst centre to render higher WI in heat-stressed cattle. Greater WI during summer heat stress could also be attributed to higher urine output (25%) and evaporation via respiratory tract (54%) (McDowell and Weldy 1967). In another study, WI was found to be significantly increased between days 8 to 11 Angus whereas between days 5 to 11 in Brahman cattle on exposure to heat stress at 32°C for 15 days (Beatty et al. 2006). In general, significant increase in WI in Angus breed should have started prior to Brahman breed but reverse trend was noticed by Beatty et al. (2006), which might be due to their difference in DMI described earlier. As there was no change in DMI in Brahman breed during heat stress periods, so WI might have begun earlier than Angus breed. In an interesting study undertaken by Chaiyabutr et al. (2008), WI was found to be lower in cooled crossbred lactating HF cattle under close-sided barn with evaporative cooling system than non-cooled cattle under open-sided barn at early (57.2 versus 93.6 L/day), mid (54.4 versus 84.4 L/day) and late (60.0 versus 75.3 L/day) lactation. Cooled cattle might have experienced lower heat stress thereby drink less water compared to non-cooled cattle. Pereira et al. (2008) found non-significant increase in WI by 6% in Mertolenga while significant rise in WI by 104, 93 and 88% in Limousine, Alentejana and HF respectively during afternoon than morning session on exposure to high THI at 85. Result found in Mertolenga breed might be due to their heat tolerance ability. Result found in the rest three breeds might be due to activation of thirst centre by the mechanism described earlier. Banerjee and Ashutosh 2011) reported higher WI in Tharparkar and KF heifers exposed to heat stress (Tharparkar—31.86 L/day; KF—31.86 L/day) than control (Tharparkar—21.71 L/day; KF—21.71 L/day). Arias and Mader (2011) detected increase in WI by 87.3% in feedlot cattle during summer heat stress than winter. In another intriguing study by Kamal et al. (2016), crossbred Vrindavani calves were kept under thatch shading roof (T1), agro-net shading roof (T2), asbestos with canvas shading roof (T3) and tree (T4) during summer season. Kamal et al. (2016) found significant increase in the duration of WI in T3 group (13.67 min) than those in T4 (11.21 min), T1 (10.29 min) and T2 (9.71 min). Furthermore, time spent near the water tank was significantly higher in T3 (25.90 min) and T4 (24.02 min) than those in T1 (14.42 min) and T2 (10.41 min). Higher values on duration of WI and time spent near the water tank in the T3 group suggest that the T3 group was more affected by heat stress. Based on the lowest values in the T2 group, Kamal et al. (2016) believed that agro-net shading roof (T2) might be very useful during summer heat stress. In crossbred cows, Yadav et al. (2015) noticed a sharp increase in WI on heat exposure at 40°C (23.83 L/day) and 35°C (21.85 L/day) than at 25°C (17.79 L/day) and 30°C (14.85 L/day). Later on, Yadav et al. (2016) reported a significant increase in WI in crossbred cattle on exposure to heat challenge at 40°C (25.01 L/day) than at 35°C (20.96 L/day), 30°C (16.65 L/day) and 25°C (16.48 L/day). Moreover, Yadav et al. (2021) found a significant increase in WI in crossbred cattle subjected to heat stress at 40°C (mean WI ~24 L/day) compared to 25°C (mean WI ~ 16.5 Lt/day). Results of Yadav et al. (2015), Yadav et al. (2016) and Yadav et al. (2021) suggest that heat exposure at 40°C and 35°C could have caused excessive sweating which might have triggered hypothalamic thirst centre resulting in higher WI. One more possible explanation is that heat exposure at 40°C and 35°C might have directly activated the preoptic area and anterior hypothalamus leading in higher WI. Similarly, Kim et al. (2018) found a striking increase in WI by 11.2 L/day in Korean beef calves on exposure to higher THI at 84.05 (31.8 L/day) than lower THI at 74.22 (20. 6 L/day). It has been seen that cattle spend more time around the water trough when shade is limited or not available (Mader et al. 1997; Coimbra et al. 2012). Moreover, percentage of outdoor or un-shaded beef cattle near water trough was 2 to 3 times higher than those kept under the shade during extreme heat stress (Mader et al. 1997). Inconsistent with all the previous findings, Valente et al. (2015) did not find any change in WI in Nellore and Angus bulls during heat exposure (THI=81.5) compared to control (THI=72.6), albeit Angus bulls had significantly higher WI than Nellore bulls. This finding indicates that Angus bulls might be more susceptible to heat stress than their counterpart Nellore bulls.
Lying down and standing behaviour
Lying down behaviour is considered an ideal indicator to ascertain dairy cattle health, welfare, reproduction and production performance (Solano et al. 2016; Tullo et al. 2019). Longer duration in lying down posture suggests that cattle are healthy and productive (Fregonesi and Leaver 2001). Normal duration in laying down posture in cattle is around 9 to 14 h/day (Tullo et al. 2019). However, environmental variables such as ambient temperature, relative humidity, solar radiation, wind velocity and rainfall affects laying down behaviour of cattle thereby declining their health and production (Tullo et al. 2019). Lying down duration was found to be highest during early morning and late night whereas lowest during late afternoon and evening (Overton et al. 2002). This could be due to less intensity of heat stress in early morning and later night compared to late afternoon and evening when intensity heat stress is more. Duration of standing time was significantly increased in lactating dairy cows when their body temperature exceeds 38.6°C during exposure to heat stress (Allen et al. 2015). Various authors also reported that, duration of standing time was increased with increase in heat load on dairy cows (Overton et al. 2002; Kamal et al. 2016; Polsky and Von Keyserlingk 2017). Similarly, duration of standing time was reported to be increased by 10% (13.8 to 15.3 h/day) in cattle when heat load was increased by 15% (Tucker et al. 2008). Increase in standing time might render more evaporative and convective heat loss (due to exposure of more body surface area to air or wind) resulting in more heat elimination to the surrounding. Standing also minimises heat gain via conduction and radiation from hot ground (Ansell 1981; Kamal et al. 2016). In another study, Kim et al. (2018) identified gradual reduction in laying duration in Korean native beef calves on heat exposure to THI at 71.7 to 87.72, with highest at 71.7 (388 min/day) and lowest at 87.72 (208 min/day). On the other hand, standing time was found to be highest on heat exposure to THI at 87.72 (392 min/day) and lowest on heat exposure to THI at 71.7 (212 min/day). As the severity of heat stress increases, cattle might feel discomfort and prefer to stand for more heat dissipation via evaporation and convection. This might be the reason behind gradual reduction in lying down duration with increase in THI reported by Kim et al. (2018). It has been seen that dairy cows prefer to stand instead of lying down under the shade during a hot environment even when they were prohibited of lying for the last 12 h (Schutz et al. 2008). This could be attributed to severe impact of heat stress that even though they were tired as they were restricted from lying down for the last 12 h, still they opted to stand to withstand the heat stress conditions by enhancing heat loss via evaporation and convection.
Shade seeking behaviour
Generally, shades were used as the major mitigation strategy to diminish the negative effects of heat stress in cattle during high heat and humidity stress (Her et al. 1988; Muller et al. 1994). Normally, cattle prefer to move towards either tree shade or roof shade during extreme environmental temperature. Cattle basically remain under shade during day time and prefer to graze during night to avoid the detrimental effects of heat stress (Shearer et al. 1991). Reports indicate that shade-seeking behaviour increases in cattle under extreme ambient temperature (Schutz et al. 2008; Polsky and Von Keyserlingk 2017). Fisher et al. (2002) noticed shade-seeking behaviour in dairy cattle when environmental temperature reached 20°C, which was more pronounced at 25°C. Finding of Kendall et al. (2006) was in line with Fisher et al. (2002), as they found prominent shade-seeking behaviour in lactating dairy cows with increase in ambient temperature and solar radiations. Kendall et al. (2006) finally suggested that shade-seeking behaviour in lactating dairy cows was positively correlated with ambient temperature and solar radiations. However, shade-seeking behaviour was found to be less pronounced when relative humidity exceeds 55% (Schutz et al. 2008). Normally, cattle prefer to stand under the shade rather than lying down to avoid any sorts of heat gain from the hot ground via conduction or radiation (Schutz et al. 2008). Gaughan et al. (1998) demonstrated that lactating HF cows prefer iron roof shade than shade cloth, choko vines and single trees, as iron roof prevents around 70% of solar radiation during extreme summer months. Even the colour of the paint used in shade materials could influence the amount of radiation emitted from different types of shade (Bond et al. 1954). Skin colour of dairy cattle also influence shade-seeking behaviour as dark-coloured cattle prefer more shade than light-coloured cattle (Tucker et al. 2008). Collectively, shade-seeking behaviour is exhibited by cattle to escape from adverse effects of heat stress during summer season. Moreover, use of several cooling systems like fans, sprinklers, high-pressure foggers and misters under the shade might increase evaporative cooling thereby making a favourable environment for cattle during heat stress.
Estrus behaviour
Heat stress markedly alters estrus behaviour in all farm animals including cattle. Duration of estrus was found to be 11 h in HF heifers on exposure to higher THI inside climatic chamber, 14 h during summer heat stress and 20 h inside cool house (Gangawar et al. 1965). In addition, poor expression of estrus was noticed when ambient temperature exceeds 20.5°C (Bulbul and Ataman 2009). Poor expression of estrus and lower duration of estrus could be due to lower E2 level that resulted from impaired GC steroidogenesis in heat-stressed HF heifers. Another possible explanation is that higher adreno-corticotropic hormone (ACTH) and cortisol during heat stress might preclude E2 production thereby repressing expression of estrus behaviour (Hein and Allrich 1992). In Japanese Black cows, duration of estrous cycle was noted to be 21.5 days in winter season while 23.4 days in summer season (Sakatani et al. 2012). Longer duration of estrous cycle during summer season could be attributed to delayed luteolysis (Wilson et al. 1998). Moreover, incidence of anestrus and silent ovulation were markedly increased in dairy cattle on exposure to summer heat load (Gwazdauskas et al. 1981; Nebel et al. 1997). Collectively, heat stress vitiates entire female reproductive cycle by attrition of various functional aspects such as follicular dynamics (Murphy et al. 1991; Wolfenson et al. 2000), expression of estrus (Gilad et al. 1993; De Rensis and Scaramuzzi 2003; Schuller et al. 2017), duration and intensity of estrus (Gwazdauskas et al. 1981; Younas et al. 1993; Khodaei-Motlagh et al. 2013; Das et al. 2016), oocyte competence (Al-Katanani et al. 2002; Torres-Junior et al. 2008; Paes et al. 2016), ovulation (Jonsson et al. 1997) and early embryonic development (Biggers et al. 1987; Hansen 2007; Gendelman et al. 2010).
Physiological responses of cattle against heat stress
Physiological responses in cattle are commenced after behavioural responses to counteract the harmful effects of heat stress. Different physiological responses (Fig. 1) manifested by cattle are rectal temperature, respiratory rate, heart rate, sweating rate and skin temperature (Ahmad et al. 2018). Various physiological responses shown by cattle under heat stress are highlighted in this section. In addition, different physiological responses exhibited by different breeds of cattle against heat stress are presented in Table 2.
Rectal temperature
RT has been accepted as the most reliable indicator amongst all the physiological responses shown by cattle against heat stress (Koga 2004; Morais et al. 2008; Rhoads et al. 2009; Taylor et al. 2014; Falkenberg et al. 2014; Bharati et al. 2017). Generally, TNZ for cattle ranges between 38 and 38.5°C and RT beyond 42°C is assumed as fatal for bovine species (Findlay 1958). A myriad of research works has been conducted in different breeds of cattle to find out the impact of heat stress on RT. RT in young claves was found to be elevated after 24 h of heat exposure at 40.5°C and then gradually declined on prolonged heat exposure for 14 days (Singh and Newton 1978). RT was found to be increased from 38.2°C in control cows to 38.7°C in heat-stressed cows (Meyerhoeffer et al. 1985). Identically, an increase in RT was observed in lactating HF cows under heat stress than control cows under TNZ (McGuire et al. 1991). Increase in RT could be attributed to more heat accumulation; as a result, dairy cattle might be impuissant to eliminate body heat via evaporative heat loss at elevated environmental temperature. Similarly, RT was significantly increased in HF calves during summer stress in Egypt (Marai et al. 1995). In another experiment, RT was significantly increased in HF and Jersey cows and found to be 0.55°C higher in HF (39.05°C) than Jersey cows (38.5°C) at 1500 h when environmental temperature was 35°C (Muller and Botha 1993). This might be due to less thermotolerance ability of HF than Jersey under elevated temperature. In another study, RT of Angus heifers (40.4°C) and Hereford (40.2°C) was noted to be higher compared to that of Brahman (39.6°C), Senepol (39.2°C) and Romosinuano (39.5°C) heifers during summer heat stress (Hammond et al. 1996). This might be due to less heat tolerance ability of temperate Bos taurus breeds (Angus and Hereford) compared to tropically adapted Bos taurus breeds (Senepol and Romosinuano) and Bos indicus breed (Brahman). Hammond et al. (1996) also reckon that variation in RT between temperate and tropical Bos taurus breeds could be due to their difference in temperament to heat stress. Likewise, Itoh et al. (1998) observed significantly higher RT in lactating HF cows upon heat exposure at 28°C (40.6°C) compared to TNZ (38.7°C). In another study, Guzeloglu et al. (2001) found higher RT in heat-stressed dairy cows (39.28°C) than control cows (38.78°C). Likewise, Koubkova et al. (2002) noted a significant upregulation in RT from 37.3 to 39.3°C in HF cows on exposure to environmental temperature at 32°C. Consistently, RT was significantly escalated in ongole bulls on exposure to heat strain (Chakravarthi et al. 2004). Furthermore, Spiers et al. (2004) documented elevation in RT in HF cows exposed to heat stress (40.5°C) at THI between 76.4 and 78 compared to TNZ (39°C) at THI between 62.5 and 65. Consistent with earlier studies, Singh and Singh (2005) examined higher RT in KF and Sahiwal heifers exposed to direct solar radiations during summer heat stress. In another study, summer heat stress at 39.83°C induced an increase in RT in lactating cows than autumn at 38.30°C (Padilla et al. 2006). RT was increased in both Angus (41.2°C) and Brahman (40.4°C) cattle after prolonged heat exposure at 32°C for 15 days (Beatty et al. 2006). This might be due to longer duration of heat exposure which had increased RT in Brahman breed despite of its high heat tolerance ability. Nonaka et al. (2008) depicted a spike in RT by 0.2 and 1.2°C in pre-pubertal HF heifers during exposure to ambient temperatures at 28 and 33°C respectively. In an interesting study, Pereira et al. (2008) observed an increase in RT in HF (40.03°C), Alentejana (39.47°C), Limousine (39.77°C) and Mertolenga (38.76°C) breeds by 2.0%, 1.1%, 1.8% and 0.2% respectively at 1500 h on exposure to THI at 85. Highest RT in HF indicates that they are very heat sensitive while least RT in Mertolenga suggests that they are more heat-tolerant breed. Uniformly, RT was increased from 38.7 to 40.2°C in lactating HF cows when ambient temperature was increased from 29.7 to 39.2°C (Rhoads et al. 2009). In another study, increase in ambient temperature from 29.4 to 38.9°C resulted in significant elevation in RT from 38.6 to 40.4°C in lactating HF cows (Wheelock et al. 2010). Rise in RT was noticed in Romosinuano and Angus steers with higher RT in Angus steers (38.49°C) compared to Romosinuano steers (38.21°C) when they were exposed to ambient temperature at 36°C for 14 days (Scharf et al. 2010). Higher RT in Angus steers could be attributed to more susceptibility towards heat stress. Similarly in lactating HF cows, Kim et al. (2010) found an increase in morning RT by 0.6°C and evening RT by 0.9°C on exposure hot phase of environment at 30°C (morning—39.5°C; evening—39.7°C) compared to TNZ at 20°C (morning—38.9°C; evening—38.8°C). This could be explained by higher impact of heat waves during evening than heat waves during morning session of hot environment. Scharf et al. (2011) found higher RT in crossbred steers at 1500 h (40.5°C) than 0800 h (38°C) upon heat stress. In another study, Vaidya et al. (2011) detected elevation in RT by 1.0°C and 1.4°C in adult and growing KF cattle during summer, suggesting that growing KF cattle are more sensitive to summer heat stress. In concurrence, Banerjee and Ashutosh (2011) noticed higher RT in heat-exposed Tharparkar heifers (morning—38.47°C; evening—38.51°C) than TNZ (morning—38.23°C; evening—38.25°C) and KF heifers (morning—38.64°C; evening—38.86°C) than TNZ (morning—38.56°C; evening—38.59°C). Higher RT in KF heifers suggests their low heat tolerance ability than Tharparkar heifers. Moreover, higher RT in evening hours in both Tharparkar and KF heifers indicate more intensity of heat stress during evening than morning hours. Sakatani et al. (2012) investigated an up-surge in RT in Japanese Black cow exposed to summer heat strain compared to winter. Bhan et al. (2012) found significantly higher RT during afternoon hours of summer (growing—39.73°C; adult—39.55°C) than spring (growing—38.96°C; adult—38.67°C) months in growing and adult Sahiwal cattle. This could be due to more heat increment (internal metabolic heat and environment heat) in growing and adult Sahiwal cattle during summer months. Likewise, RT was increased by 0.87 and 0.77°C in growing KF cattle whereas 0.79 and 0.88°C in adult KF cattle during forenoon and afternoon of summer than spring season (Bhan et al. 2013). Subsequently, Cardoso et al. (2015) carried out an experiment to find out variations in RT amongst different cattle breeds against heat stress at 35.9°C and found higher RT in Gir (39.05°C) and Indubrasil (39.00°C) than Nellore (38.87°C), Sindhi (38.86°C) and Girolando (38.65°C). These findings suggest that Sindhi and Girolando have shown more adaptation while Gir and Indubrasil had shown less adaptation against heat stress at 35.9°C. Meanwhile, Min et al. (2015) reported pronounced increase in RT in lactating HF cows on exposure to moderate heat-stressed (39.31°C) compared to mild heat stress (38.70°C). Further, Mayengbam et al. (2015) detected significantly higher RT in Sahiwal and KF cattle during exposure to THI at 80.3 (Sahiwal—39.33°C; KF—39.25°C) than THI at 53.6 (Sahiwal—38.64; KF—38.64°C). High THI increases heat load than heat loss (via evaporation through skin and respiratory tract) leading to higher RT in both the cattle breeds. In a study conducted in beef cows, Boehmer et al. (2015) examined greater RT upon exposure to ambient temperature at 36.8°C (40.8°C) than at 28°C (38.1°C). In another study, Jian et al. (2015) noticed significant increase in RT with increase in THI and found highest RT at 1500 h (39.3°C) and lowest RT at 0600 h (37.8°C) but RT between Sahiwal and different crossbreds of HF cows did not change significantly, which could be due to the effect of interaction between breeds and time of heat exposure. Yadav et al. (2015) estimated highest RT in crossbred cattle on exposure to heat stress at 40°C (39.14°C) than at 35°C (38.41°C), 30°C (38.17°C) and 25°C (38.12°C). Subsequently, Yadav et al. (2016) determined highest RT in heat-stressed crossbred cattle at 40°C (39.14°C) than at 35°C (38.38°C), 30°C (38.16°C) and 25°C (38.18°C). Furthermore, Yadav et al. (2021) found significant increase in RT in crossbred cattle during heat exposure at 40°C (mean RT ~39°C) compared to 25°C (mean RT ~38.1°C). Reports indicate that heat stress is more severe at 40°C and 35°C, which might have induced elevation in RT in crossbred cattle. In corroboration with earlier results, Maibam et al. (2017) observed higher RT in KF than Tharparkar cattle during summer season (KF—39.47°C; Tharparkar—38.88°C) than TNZ (KF—38.58°C; Tharparkar—38.41°C), which indicates less heat tolerance ability of KF than Tharparkar. In a study conducted by Sailo et al. (2017), RT was found to be 39.186°C and 38.398°C in KF cows, while 38.810°C and 38.178°C in Sahiwal cows during summer and spring season respectively. Grewal and Aggarwal (2018) noticed an elevation in RT in periparturient Sahiwal and KF cows during hot humid season (THI=81.11) compared to winter season (THI=59.5). Moreover, RT was observed to be greater in KF (40.0°C) compared to Sahiwal cows (39.0°C) on day of parturition during hot humid season. These reports further confirm less heat tolerance ability of KF than Sahiwal cows. In another study, Kumar et al. (2017) reported significant upregulation in RT in Hariana and Sahiwal cows during summer (38.99°C and 39.04°C) than winter season (37.87°C and 37.92°C). Likewise, Katiyatiya et al. (2017) found greater RT in Boran (36°C) than Nguni (35.1°C) cows when subjected to heat stress for 3 h, which could be due to thicker skin and longer hairs of Boron (24.3 mm) compared to Naguni cows (20.2 mm). Further, Katiyatiya et al. (2017) reported that cows with white-red coat colour had highest RT (39.02°C) whereas cows with fawn coat colour had lowest RT (35.55°C), indicating the influence of coat colour on RT in heat-stressed cows. In addition, RT in dairy cows with black hair coat increased by 1.3°C/h while those with white hair coat increased by 0.8°C/h when exposed to THI at 81 (Hillman et al. 2001), which indicates that black hair coat renders more heat accumulation resulting in more increase in RT than white hair coat colour cows. Consistently in HF cows, De Andrade Ferrazza et al. (2017) reported an increase in RT from 38.56°C under TNZ at 25.9°C to 39.87°C during heat stress at 36.3°C. Comparably, RT was significantly elevated in Chinese HF dairy cows on exposure to THI at 80.5 (39.7°C) than THI at 66 (38.4°C) (Chen et al. 2018). Recently, Kim et al. (2018) noted an upregulation in the RT in Korean native beef claves exposed to THI at 87.72 (39.9°C) than THI at 70.01 (38.9°C). Additionally in HF bulls, Sayah et al. (2019) noticed highest RT during summer (35.26°C) compared to spring (34.03°C) season. Uniformly, Singh et al. (2019) evaluated highest RT in Hariana cattle during summer (102.14F°) followed by rainy (101.88F°) and winter season (100.13F°). Pires et al. (2019) documented an elevation in RT in Nelore (38.8°C) and Caracu cattle (39.2°C) on exposure to heat stress, suggesting that Nelore cattle are better thermotolerant than Caracu cattle. In a recent study, Park et al. (2019) sought to determine the effect of higher THI on RT in lactating HF cows. They exposed the cows to three THI ranges such as 70–75 (T1), 76–81 (T2) and 82–87 (T3). They found highest RT in cows exposed to T3 (39.05°C) followed by T2 (38.69°C) and T1 (38.41°C). This report suggests that RT might be positively correlated with THI. Anthetically, Sanap et al. (2018) neither observed any significant difference in RT in crossbred calves between seasons such as hot humid, hot dry season and spring season nor between different roofing materials like brick and mortar, brick and asbestos, hatch and mud, and under tree shade. This might be due to the differences in the temperament of crossbred calves and effect of interaction of different seasons with different housing systems. Additionally, Kumar et al. (2019) did not notice any variation in RT in lactating Hariana cattle under higher THI during summer months. They presume that homeostatic and homeorhetic mechanisms of Hariana cattle might have impeded noticeable increase in RT. There are some literatures regarding the effect of shade on RT of heat-stressed cattle. Roman-Ponce et al. (1977) found lower RT in cows kept under shade (38.9°C) compared to those under direct sunlight (39.4°C). Prasanpanich et al. (2002) noted higher RT in lactating HF-cross cows grazed outside without any shade (40.4°C) than those kept indoor (39°C). Chaiyabutr et al. (2008) observed higher RT (39.7°C) in non-cooled cattle compared to cooled cattle (38.7°C) during afternoon at 1400 h. Uniformly, RT was found to be higher in heat-stressed (39.4°C) than in cool (39.0°C) HF cows during afternoon session of summer months (Do Amaral et al. 2011). Similarly, Aengwanich et al. (2011) found lower RT in Thai Brahman cattle housed in artificial shade (38.57°C) than either tree shade (38.94°C) or direct sunlight (38.89°C). Comparatively lower RT in cooled cows under shade could be attributed to lower THI experienced by cooled cows than cows without shade. There are few studies on the influence of different cooling systems on RT in cattle during heat stress. Omar et al. (1996) found that cooling via forced ventilation and sprinkler reduce RT thereby increase milk yield by 15% in HF cows during summer heat stress. Chaiyabutr et al. (2011) found significant decline in RT in HF cows treated with mist-fan system (MF) compared to those under normal shade (NS) at 1300 h in early (MF=38.8°C and NS=39.4°C), mid (MF=38.2°C and NS=39.6°C) and late lactation (MF=38.4°C and NS=39.0°C) phase. One possible explanation is that mist-fan system (MF) could render more convective heat loss resulting in a greater reduction in RT than normal shade. In agreement, RT was found to be 39.3°C in heat-stressed and 39°C in cool dairy cows kept under sprinklers and fans (Tao et al. 2012). Interestingly, RT was found to be significantly lower in lactating Sahiwal cattle maintained under a combination of roof shade, fans and sprinklers (101F°) followed by those kept in roof shade plus fan and only roof shade during summer heat stress in sub-tropics (Ahmad et al. 2018). This could be attributed to lowest THI witnessed by lactating Sahiwal cattle offered with roof shade, fans and sprinklers (THI-77.7) than roof shade plus fan (THI-80.5) or only roof shade (THI-81.1). All the above mentioned findings suggest that Zebu cattle (Bos indicus) are more adapted to summer heat stress compared to their counterpart exotic cattle (Bos Taurus).
Respiration rate
Respiration rate (RR) is considered the most sensitive indicator amongst all the physiological responses shown by cattle under heat stress (Morais et al. 2008; Indu and Pareek 2015; Brown-Brandl et al. 2003; De Andrade Ferrazza et al. 2017; Bharati et al. 2017a). RR tends to increase with rise in ambient temperature and could be influenced by species, types of breed, age, sex, body condition, feeding time, feeding management, plane of nutrition, previous heat exposure, shelter management and cooling strategies (Gaughan et al. 2000). Singh and Newton (1978) found an increase in RR in young calves after 24 h followed by a gradual dip during chronic heat exposure at 40.5°C for 14 days, which could be due to thermal adaptation. Meyerhoeffer et al. (1985) observed higher RR in heat-stressed cows (54.2 breaths/min) than control cows (29.9 breaths/min). McGuire et al. (1991) reported higher RR in lactating HF cows exposed to heat stress compared TNZ. In another study, RR was significantly increased in both HF and Jersey cows with greater RR in HF (79.1 breaths/min) than Jersey cows (63.7 breaths/min) during afternoon at 1500 h when environmental temperature was 35°C (Muller and Botha 1993). Hike in RR might prevent rise in RT to maintain homeostasis during heat stress as described vividly in the previous section. Higher RR could eliminate excessive heat from skin and respiratory surface which might induce evaporative cooling to recuperate homeostasis. It has also been shown that higher RR might trigger evaporative heat loss by 30% in heat exposed Ayrshire calf (Mclean 1963). This explanation was further verified by Vaidya et al. (2011), where higher RR resulted in cutaneous (adult—76.8%; growing—73.9%) and pulmonary (adult—23.2%; growing—26.1%) heat loss in adult and growing KF cattle at 1400 h during summer season. Higher RR could also be due to more oxygen demand by cellular systems during heat stress. In other way, increase in RR might cause respiratory alkalosis due to excess removal of carbon dioxide into the environment thereby upsets acid base homeostasis during heat stress. In an interesting experiment, RR was found to be faster in Angus (69 breaths/min) and Hereford (64 breaths/min) heifers than Brahman (36 breaths/min), Romosinuano (55 breaths/min) and Senepol (57 breaths/min) heifers on hot summer days (Hammond et al. 1996). This might be due to least heat tolerance ability of Angus heifers and it has already been seen that Angus heifers had highest RT (described earlier) which might have induced RR much more than any other breeds. Moreover, Brahman heifers had shown promising heat tolerance ability to have lowest RR amongst all the breeds. Uniformly, Itoh et al. (1998) detected marked increase in RR in lactating dairy cows on heat exposure at 28°C (85.3 breaths/min) than TNZ (42.5 breaths/min). In another study, RR was elevated from 28 to 81 breaths/min (~2.6-fold) in HF cows on exposure to heat stress at 32°C (Koubkova et al. 2002). Similarly, Soley and Singh (2003) detected higher RR in crossbred calves during afternoon session than morning session in summer season. Elevation in RR as reported by different authors might improve evaporating heat loss thereby causes cooling during heat stress. In another study, Spiers et al. (2004) reported an upregulation in RR in HF cows upon heat exposure at THI between 76.4 and 78 (88.6 breaths/min) compared to TNZ at THI between 62.5 and 65 (59.6 breaths/min). Identically, Padilla et al. (2006) noted greater RR in lactating cows during summer (71.5 breaths/min) stress than winter (38.8 breaths/min). Similarly in Angus cows, Beatty et al. (2006) documented noticeable increase in RR on exposure to heat stress at 32°C (127 breaths/min) compared to 26°C (75 breaths/min). These reports suggest existence of positive relationship between RR with both ambient temperature and THI. Comparably, RR was found to be increased in Limousine (2.5-fold), Alentejana (2.7-fold), HF (2.8-fold) and Mertolenga (2.9-fold) during late afternoon under THI at 85 (Pereira et al. 2008). Highest RR in Mertolenga could be attributed to their larger body surface area-to-mass ratio, which provides efficient heat loss during heat stress. Moreover, highest RR with maximum heat loss resulted in least RT in Mertolenga breed during heat stress. Therefore, it is plausible to state that Mertolenga breeds have got the highest heat tolerance ability compared to the rest of the cattle breeds. In a study conducted in pre-pubertal HF heifers, RR was elevated by 23 and 58 breaths/min during exposure to ambient temperature at 28 and 33°C respectively (Nonaka et al. 2008). Consistently, increase in ambient temperature from 29.7 to 39.2°C escalated RR from 46 to 82 breaths/min in lactating HF cows (Rhoads et al. 2009). Identically, rise in environmental temperature from 29.4 to 38.9°C elevated RR from 44 to 89 breaths/min in lactating HF cows (Wheelock et al. 2010). Reports of these aforementioned studies further validate that RR is positively correlated with both ambient temperature and THI. Akin to previous studies, increase in RR was detected in both Romosinuano and Angus steers cattle with higher RR in Angus (61 breaths/min) compared to Romosinuano steers (42 breaths/min) upon heat exposure to 36°C for 14 days (Scharf et al. 2010). RR tends to be higher in order to increase evaporative heat loss to reduce RT thereby maintaining homeostasis during heat stress. Additionally, more RR in Angus steers indicates that they are less heat tolerant than Romosinuano steers. Later on, Scharf et al. (2011) identified greater RR in crossbred steers during 1500 h (150 breaths/min) than 0800 h (80 breaths/min) upon hyperthermia, which suggests more intensity of heat stress during afternoon session than morning session. Bhan et al. (2012) found significantly higher RR during afternoon session of summer (growing—29.83 breaths/min; adult—27.67 breaths/min) than spring (growing—25.33 breaths/min; adult—22.00 breaths/min) seasons in growing and adult Sahiwal cattle. Higher RR in growing Sahiwal cattle might be due to more heat accumulation which could be eliminated by enhancing evaporative heat loss during summer months than adult Sahiwal cattle. In another study, Banerjee and Ashutosh 2011) noticed higher RR in heat-exposed Tharparkar heifers at 38–39°C (morning—21.90 breaths/min; evening—24.19 breaths/min) than TNZ (morning—18.69 breaths/min; evening—19.83 breaths/min) as well as heat-exposed KF heifers at 38–39°C (morning—27.21 breaths/min; evening—34.26 breaths/min) than TNZ (morning—22.17 breaths/min; evening—23.40 breaths/min). Greater RR in both cattle breeds during evening hours could be attributed to higher magnitude of heat stress than morning hours. Moreover, higher RR in KF cattle suggests their low thermotolerance potential than Tharparkar cattle during summer season. Congruently, RR was elevated by 4.67 and 3.67 breaths/min in growing KF cattle whereas 2.83 and 6.67 breaths/min in adult KF cattle during forenoon and afternoon of summer than spring season (Bhan et al. 2013). Higher RR in growing KF cattle might owe to higher heat production within their body during summer season. Consistently, RR was found to be 54.00 and 31.00 breaths/min in Sahiwal and 57.00 and 31.00 breaths/min in KF cattle on exposure to heat stress (THI=80.3) and TNZ (THI=53.6) respectively (Mayengbam et al. 2015). Higher RR in both cattle breeds might be attributed to higher total heat production (body metabolic heat and environment heat). Further, higher RR promotes cutaneous and pulmonary evaporative heat loss to reduce heat load and to lower RT during higher THI. Identically, RR was found to be 18.158 and 29.818 breaths/min in Sahiwal cows whereas 22.979 and 47.299 breaths/min in KF cows during spring and summer respectively (Sailo et al. 2017). It could be speculated that KF cows are heat sensitive and possess low thermotolerance ability than Sahiwal cows. A significant upregulation in RR was observed in both Sahiwal and KF cattle during hot humid season than winter season, with significantly higher RR in KF (59.83 breaths/min) compared to Sahiwal cows (38.33 breaths/min) on the day of parturition during hot humid season (THI=81.11) (Grewal and Aggarwal 2018). This finding further confirms low heat tolerance ability of KF cows than Sahiwal cows. Later on, Cardoso et al. (2015) found highest RR in Nelore (41.00 breaths/min) and lowest in Indrabusil (33.75 breaths/min) while RR did not differ significantly in Gir, Sindhi and Girolando during heat stress. This might be attributed to difference in their physical characteristics and difference in temperament in response to heat stress. Min et al. (2015) evaluated highest RR in lactating HF dairy cows exposed to moderate heat stress (85 breaths/min) followed by mild (52.75 breaths/min) and no heat-stressed cows (43.58 breaths/min). In accord with earlier studies, RR was found to be 45.2 and 29.5 breaths/min in Nellore bulls and 104 and 86.3 breaths/min in Angus bulls on exposure to heat stress (THI=81.5) and TNZ (THI=72.6) respectively (Valente et al. 2015). Higher RR in Angus bulls might indicate that they are more heat sensitive and possess less heat tolerance ability than Nellore bulls. In beef cows, Boehmer et al. (2015) examined significantly higher RR during summer heat stress at 36.8°C (114 breaths/min) with respect to winter at 28°C (36 breaths/min). In an interesting study, Jian et al. (2015) reported higher RR in pure breed (HF100%=48 breaths/min) and crossbred HF cows (HF87.5%=54 breaths/min; HF50%=42 breaths/min) compared to Sahiwal (25 breaths/min) on exposure to high THI. This report suggests that percentage of crossbred does matter to evaluate magnitude of thermotolerance as HF87.5% had highest RR compared to HF50% and purebred HF100% cattle, with least thermotolerance ability of HF87.5%. In addition, purebred HF100% cattle also possess less heat tolerance ability than Sahiwal cows. Moreover, Jian et al. (2015) also found highest mean RR in both the breeds at 1500 h (74 breaths/min) than lowest at 0600 h (18 breaths/min). This might be due to high heat load during afternoon than early morning. Yadav et al. (2015) determined maximum RT in crossbred cattle exposed to heat stress at 40°C (71.20 breaths/min) than at 35°C (35.90 breaths/min), 30°C (24.10 breaths/min) and 25°C (21.70 breaths/min). Concurrently, RR reached zenith in heat-stressed crossbred cattle at 40°C (75.94 breaths/min) than at 35°C (35.04 breaths/min), 30°C (23.81 breaths/min) and 25°C (21.64 breaths/min) (Yadav et al. 2016). In another study, Yadav et al. (2021) recorded significant elevation in RR in crossbred cattle during heat exposure at 40°C (mean RR ~70 breaths/min) compared to 25°C (mean RR ~30 breaths/min). These findings suggest maximum heat load at 40°C followed by 35°C which had resulted in significant elevation in RR while rest ambient temperatures did not affect that much to alter RR in crossbred cattle. Again, Yadav et al. (2021) noticed increase in RR after day 1 followed by a sharp drop after day 11 until day 21 of heat exposure at 40°C. Reduction in RR could be attributed to heat adaptation in crossbred cattle. In line with earlier studies, Kumar et al. (2017) estimated an increase in RR Hariana and Sahiwal cows on exposure to high THI at 86.83 during summer (Hariana—28.71 breaths/min; Sahiwal—27.50 breaths/min) compared to low THI at 60.52 (Hariana—18.00 breaths/min; Sahiwal—20.52 breaths/min) during winter season. In HF cows, De Andrade Ferrazza et al. (2017) found an increase in RR from 39.70±0.71 breaths/min in control cows within TNZ-25.9°C to 76.02±1.70 breaths/min in heat-stressed cows at 36.3°C. In another study, Sanap et al. (2018) evaluated that RR was significantly elevated in crossbred calves in hot humid season followed by hot dry season and winter season. Similarly, Kumar et al. (2019) reported higher RR of 22 breaths/min in lactating Hariana cattle on exposure to THI between 78 and 80, but no significant increase in RR was noticed further with increase in THI which might be due thermal adaption against THI between 78 and 80. Likewise in Hariana cattle, Singh et al. (2019) noted higher RR in summer (27.84 breaths/min) than in winter season (16.23 breaths/min). In tune with other findings, Park et al. (2019) noticed the highest RR in lactating HF cows exposed to THI at 82–87 (T3=84.05 breaths/min) followed by THI at 76–81 (T2= 66.12 breaths/min) and THI at 70–75 (T1=58.60 breaths/min). This report suggests a positive relationship between RR and THI. There are also numerous studies on use of shade on RR in cattle during heat stress. Roman-Ponce et al. (1977) found significant reduction in RT in cows kept under shade (54 breaths/min) than the cows under direct sunlight (82 breaths/min). Likewise, Parihar et al. (1992) noticed significantly lower RR in cattle kept under shed than those placed in open environment. Similarly, Prasanpanich et al. (2002) reported greater RR in lactating HF-cross cows grazed outside without any shade (87.9 breaths/min) than those kept indoor (62.9 breaths/min). Further, Singh and Singh (2006) reported lower RR in cattle kept under shed compared to free ranged cattle. A greater RR (86 breaths/min) was noted in non-cooled cattle than cooled cattle (64 breaths/min) during afternoon at 1400 h (Chaiyabutr et al. 2008). In another study, RR was noted to be greater in heat-stressed HF cows (78 breaths/min) than cool cows (56 breaths/min) during afternoon hours of summer months (Do Amaral et al. 2011). Likewise, Aengwanich et al. (2011) found lowest RR in Thai Brahman cattle kept under artificial shade (16.11 breaths/min) followed by tree shade (19.62 breaths/min) and direct sunlight (23.42 breaths/min). Uniformly, RR was found to be 48 and 69 breaths/min in cool and heat-stressed dairy cows respectively (Tao et al. 2012). Furthermore, Sanap et al. (2018) detected lower RR in cows housed under brick walls and asbestos roofing than cows under roof made up of hatch and mud. Lower RR under shade with asbestos roofing could be attributed to low exposure to solar radiations than roof with hatch and mud. It has also been reported that shed reduces solar radiation around 30% which ultimately resulted in lower RR in cattle than those present outside under direct sunlight (Eigenberg et al. 2009). There are few studies on the influence of different cooling systems on RR in cattle during heat stress. Chaiyabutr et al. (2011) noted reduction in RR in HF cows cooled by mist-fan system (MF) than the noncooled cows under normal shade (NS) at 1300 h in early (MF=52 and NS=70 breaths/min), mid (MF=50 and NS=71 breaths/min) and late lactation (MF=49 and NS=69 breaths/min) phase. Mist-fan system might expedite heat loss via convection thereby reduce RR in heat-stressed cattle. Identically, RR was found to be lowest in lactating Sahiwal cattle housed in a combination of roof shade, fans and sprinklers (21.2 breaths/min) followed by those treated with either roof shade plus fan or roof shade alone during summer months in sub-tropics (Ahmad et al. 2018). This finding suggests that combined use of fans and sprinklers under shade might culminate in maximum heat loss during heat stress periods. Thus, Ahmad et al. (2018) indicated that the productive potential of Sahiwal cows could be enhanced by using a combination of roof shade, fan and sprinkler during summer heat stress. In an interesting study, Aritonang et al. (2017) observed alternation in physiological responses exhibited by Bali and ongole cattle where Bali depends mostly on RT whereas ongole relies preferebly on RR to maintain body homeostasis during elevated temperature. This might be due to the difference in expression of physiological responses to attenuate heat stress.
Heart rate and pulse rate
Cardio-pulmonary system seems to be regulated by ambient temperature, relative humidity, duration of day light and seasons (Mohr et al. 2002; Marai et al. 2007). Along with RT and RR, heart rate (HR) is also considered a valuable indicator to quantify the intensity of heat stress in cattle (Das et al. 2016). Earlier, Lefcourt et al. (1999) recorded HR by a noninvasive method to measure the impact of heat stress in cattle. Later on, certain noninvasive approaches were identified to monitor HRV to quantify the intensity of heat stress in cattle (Mohr et al. 2002; Von Borell et al. 2007). Bianca (1962) found higher HR in cattle during acute heat stress than chronic heat stress. This could be due to heat adaptation in cattle during chronic heat stress. Muller and Botha (1993) noticed significantly higher HR in primiparous HF cows (81.4 beats/min) than Jersey cows (78.2 beats/min) at 1500 h on heat exposure at 35°C, suggesting the fact that Jersey cows are better adapted to warmer regions of South Africa than HF cows. According to Muller and Botha (1993), better adaption during heat stress could be due to the smaller body size and higher body surface area per body weight in Jersey compared to HF cows. A significant hike in pulse rate (PR) from 64 to 81 beats/min was observed in HF cows on exposure to heat stress at 32°C (Koubkova et al. 2002). Increase in HR during heat stress could be to higher secretion of catecholamines which might activate adrenergic receptors in cardiac myocytes (Janzekovic et al. 2006). Additionally, higher HR in heat-exposed animals might enhance cardiac output thereby directing more blood flow towards peripheral circulation resulting in more evaporative heat loss to the environment. Similarly, Beatty et al. (2006) did not observe any change in HR in Brahman upon heat exposure at 32°C but found a reduction in HR in Angus cattle from 80 to 60 beats/min between day 5 and day 15 on heat exposure at 32°C indicating zebu cattle (Brahman) are more thermo-tolerant than exotic European cattle (Angus). However, reduction in PR in Angus cattle between days 5 to 15 of heat exposure at 32°C might be due to heat adaptation. Vaidya et al. (2011) reported highest PR in growing KF cattle during summer season (80 beats/min) and lowest in adult KF cattle during spring season (59 beats/min) at 1400 h. This report suggests growing KF might have experienced higher heat strain as they are more prone to heat stress (described earlier) resulting in higher PR than adult KF cattle. Later on, Banerjee and Ashutosh 2011) noticed higher HR in heat-exposed Tharparkar heifers at 38–39°C (morning—72.62 beats/min; evening—79.05 beats/min) than TNZ (morning—65.29 beats/min; evening—65.40 beats/min) as well as heat-exposed KF heifers at 38–39°C (morning—83.90 beats/min; evening—85.71 beats/min) than TNZ (morning—69.69 beats/min; evening—70.19 beats/min). Higher HR in both the breeds during evening might be due to higher heat load than morning hours. However, higher HR in KF heifers could be attributed to their higher sensitiveness for heat stress than Tharparkar heifers. Exactly, PR was upregulated in growing and adult KF cattle during forenoon and afternoon of hot humid and summer season than spring season (Bhan et al. 2013). This might also be attributed to higher intensity of heat stress in hot humid and summer seasons which might have induced sympathetic adrenal medullary (SAM) axis to secrete higher catecholamines culminating in higher PR in growing as well as adult KF cattle. In another experiment, HR was noticed to be 96 and 80 beats/min in Sahiwal and 96 and 88 beats/min in KF cattle on exposure to heat stress (THI=80.3) and TNZ (THI=53.6) respectively (Mayengbam et al. 2015). This report validates the fact that KF cattle have less thermotolerance ability than Sahiwal cattle. Likewise, PR was escalated in Sahiwal and KF cattle during hot humid season (THI=81.11) than winter season with higher PR in KF (84.16 beats/min) than Sahiwal cows (76.66 beats/min) on the day of calving during hot humid season (Grewal and Aggarwal 2018). This finding confirms the finding of Mayengbam et al. (2015) regarding lower heat tolerance ability of KF cows. Similarly, Bhan et al. (2012) reported greater PR during afternoon session of summer (growing—74.83 beats/min; adult—64.50 beats/min) than spring (growing—70.00 beats/min; adult—56.50 beats/min) seasons in growing and adult Sahiwal cattle. Growing Sahiwal cattle might have perceived higher heat load resulting in higher PR. Likewise, HR was registered highest in Gir (66.82 beats/min) followed by Nelore (64.11 beats/min) and lowest in Sindhi (56.53 beats/min) upon heat exposure at 35.9°C (Cardoso et al. 2015). This might be due to their physical characteristics and efficiency of heat adaptation during heat stress. Yadav et al. (2015) reported significant rise in PR in crossbred cattle exposed to heat stress at 35°C (60.65 beats/min) and 40°C (60.60 beats/min) than at 25°C (53.30 beats/min) and 30°C (52.55 beats/min), though there was no significant change in PR between heat stress at 35°C and 40°C as well as between 25°C and 30°C. Akin to their previous report, Yadav et al. (2016) found significant upregulation in PR in crossbred cattle during heat exposure at 40°C (61.64 beats/min) and 35°C (60.02 beats/min) than at 30°C (53.04 beats/min) and 25°C (52.29 beats/min), albeit there was no significant change in PR between heat stress at 40°C and 35°C as well as between 30 and 25°C like that of Yadav et al. (2015). Later on, Yadav et al. (2021) found a non-significant increase in PR in crossbred cattle exposed to heat stress at 40°C (mean PR ~59 beats/min) than 25°C (mean PR ~54 beats/min). Higher PR at 40°C and 35°C might be due to higher secretion of catecholamines and higher expression of adrenergic receptors on cardiomyocytes as compared to other heat exposure temperatures. Uniformly, Kumar et al. (2017) found striking elevation in PR in Hariana and Sahiwal cows on exposure to high THI at 86.83 during summer (Hariana—69.04 beats/min; Sahiwal—66.88 breaths/min) compared to low THI at 60.52 (Hariana—62.22 beats/min; Sahiwal—60.86 beats/min) during winter season. In another study conducted in Korean native calves, Kim et al. (2018) indicated that HR was increased by 12.7 beats/min during exposure to high THI at 87.72 (73 beats/min) than low THI at 70.01 (60.3 beats/min). The finding of Kim et al. (2018) provides a hint about positive correlation between HR and THI. Uniformly, Sanap et al. (2018) observed an increase in PR in crossbred calves in hot humid season than hot dry season. This could be explained by higher gravity of heat stress in hot humid season than hot dry season. Kumar et al. (2019) also found higher PR of 65 beats/min in lactating Hariana cattle on exposure to THI between 78 and 80 followed by a decline with further increase in THI, which could be due to heat resistance to THI at 80. Furthermore, Singh et al. (2019) noticed maximum PR in Hariana cattle during summer (71.23 beats/min) than during winter season (61.64 beats/min), which could be due to higher potency of heat stress in summer season resulting in higher PR in Hariana cattle. Aengwanich et al. (2011) aimed to unmack the efficacy of shade and cooling systems on alternations in PR in heat-stressed cattle and reported noticeable decrease in HR in Thai Brahman cattle kept under artificial shade (52.48 beats/min) and tree shade (46.74 beats/min) compared to those under direct sunlight (63.22 beats/min). On the contrary, HR was found to be reduced in young claves on exposure to heat stress at 40.5°C (Singh and Newton 1978). This could be due to different body physiological status of young claves or due to difference in diets or due to different environmental conditions in those particular geographical regions. In lactating dairy cows, Itoh et al. (1998) noticed lower HR on heat exposure at 28°C (64.8 beats/min) than TNZ (76.3 beats/min). This might be either due to reduction in catecholamine secretion or due to stronger heat-resistance ability of dairy cows during lactation. In addition, De Andrade Ferrazza et al. (2017) found significant reduction in HR in heat-stressed HF cows at 36.3°C (62.13 beats/min) than cows housed under TNZ at 25.9°C (66.23 beats/min). This might be either due to reduction in catecholamine secretion as higher RR might have already eliminated excess heat load via evaporation in heat-stressed HF cows. Valente et al. (2015) had seen lower PR in Angus bulls during heat exposure under THI at 81.5 (87 beats/min) compared to control under THI at 72.6 (93 beats/min). This could be due to lower body heat load as higher RR might have already taken care of restoring body heat via evaporation in heat-stressed HF cows.
Sweating rate
Sweating leads to evaporative heat loss and considered a vital process in cattle to counteract the harmful effects of heat stress (Gebremedhin et al. 2008). When environmental temperature emulates animal’s core body temperature, then cattle prefers to eliminate body heat via evaporation thereby experience better cooling (Gebremedhin and Wu 2001). Evaporative heat loss in cattle becomes more prominent when THI exceeds 90 (Jian et al. 2015). Evaporative heat loss occurs either via either cutaneous or via respiratory surfaces. Evaporative heat loss that occurs via cutaneous surface is known as cutaneous evaporation or sweating while through respiratory surface is known as panting (Maia et al. 2008; Da Silva and Maia 2011). Moreover, sweating contributes around 65% while panting contributes around 35% of the total evaporative heat loss in cattle under extreme heat stress (Maia et al. 2008; Da Silva and Maia 2011; Jian et al. 2015). In their review, Rashamol et al. (2018) described the influence of different environmental variables like ambient temperature, relative humidity, solar radiations, wind velocity and rainfall on sweating rate (SR) in livestock species. Finch (1985) indicated that SR tended to increase by 50% in Shorthorn steers when ambient temperature was increased from 28 to 45°C. Increase in SR could be attributed to increase in peripheral vasodilation caused by higher cardiac output to dissipate more heat waves to the external environment during heat stress, as it was already established that blood flow to cutaneous capillaries dominates over blood flow to systemic capillaries during heat stress. In addition, spike in core body temperature by only 0.5°C lead to 7-fold increase in cutaneous blood flow in cattle (Cunningham 2002). Moreover, increase SR tends to dissipate more heat to the surrounding environment to maintain thermal balance between animal’s body and hot environment (Johnson and Hales 1983). In an interesting study, Yeck and Kibler (1958) compared the ratio of evaporative heat loss between heat exposure at 26.7 to 10°C in six cattle breeds and found greatest thermo-tolerance ability in Brahman calves with a ratio of 2.75 followed by Santa Gertrudis, Brown Siwss, Jersey, HF and Shorthorn respectively. Similarly, SR was gradually increased in young calves following 48 h of heat exposure at 40.5°C until 14 days (Singh and Newton 1978). Moreover, shoulder SR was increased by more than 4-fold in Angus and Romosinuano steers with greater SR in Angus (292.6 g/m2/h) than Romosinuano (175.23 g/m2/h) steers during heat exposure at 36°C for the first 7 days and then declined up to 14 days (Scharf et al. 2010), indicating low heat tolerance ability of Angus steers than Romosinuano steers. In another study, Jian et al. (2015) documented higher SR in Sahiwal (595 g/m2/h) compared to HF cows (HF100%=227 g/m2/h; HF87.5%=299 g/m2/h; HF50%=335 g/m2/h) under high THI at 91.68. This might be explained by the fact that Sahiwal cows mostly rely on sweating while HF chiefly depends on panting to counteract heat stress (Koatdoke 2008). Moreover, mean SR in both the breeds was maximum at 1500 h (510 g/m2/h) and minimum at 0600 h (224 g/m2/h). This clearly indicates the severity of heat stress at 1500 h when THI was 93 than 0600 h when THI was 72. Apart from these observations, plenty of reports suggested that hair coat colour, density and thickness influence SR in heat-exposed cattle. Gebremedhin et al. (2008) suggested that efficiency of SR was not only influenced by length, density and thickness of hair coat, and physical and optical properties hair coat, but also by skin colour. Bernabucci et al. (2010) further indicated that efficiency of evaporative heat loss is modulated by sweat gland density and function, hair coat colour, length, density and thickness and skin colour including pattern of peripheral blood flow during heat stress. In another study, Hidalgo (2009) elucidated that types of hair coat influences heat transfer from animal’s skin to the environment thereby modifying core body temperature. They also indicated that slick hair coat HF are better thermotolerant than normal hair coat HF. In another study, Wang et al. (2012) also indicated that skin and its constituents could play an important role in thermoregulation in Thai cattle. Furthermore, Da Silva (1999) documented that cattle with smaller hair coat thickness (< 8 mm) are adapted to tropical climates while cattle with longer hair coat thickness (> 15 mm) are adapted to temperate climate. This could be explained by that smaller hair coat thickness in tropical cattle might be helpful for better heat dissipation during heat stress. Moreover, tropical cattle witness greater heat load compared to temperate cattle thereby tropical cattle might have thinner hair coat to eliminate more heat to external environment to withstand the heat rigours. In another study, SR was found to be declined by 17% when thickness of hair coat increases from 3 to 10 mm during exposure to environmental temperature at 20°C (Turnpenny et al. 2000). Moreover, Lucena and Olson (2000) revealed that cattle with short and sleek hair coat are better adapted to heat stress. In addition, Gaughan et al. (2010) further clarified the concept of ‘slick’ gene responsible for thermo-tolerance in cattle. According to Gaughan et al. (2010), ‘slick’ gene indicates shorter hair length and cattle with shorter hair coats, greater diameter and lighter coat colour are better adapted to hot environment compared to those with longer hair coats, smaller diameter and darker coat colours. Earlier, Finch et al. (1984) revealed that dark colour coats receive higher heat waves from solar radiation compared to light colour coats. Further, cattle with black or dark hair coat absorb more short-wave radiations (89%) than cattle with light or white hair coat (66%) and as a consequence black skinned cows witnessed greater SR than white skinned cows to maintain thermal balance (Hillman et al. 2001). Identically, Silva and Maia (2011) detected greater SR in black skin areas than white skin areas in HF cows in tropical climates. Several authors also reported the effect of shade and cooling systems on variations in SR during heat stress. Prasanpanich et al. (2002) found lower SR in lactating HF cows kept under shade (68.6 g/m2/h) than those grazed outdoor without any shade (559.7 g/m2/h). This could be explained by the fact that shed reduces solar radiation around 30% which ultimately resulted in lower SR in cattle kept under shade than those present outside under direct sunlight (Eigenberg et al. 2009). Likewise, Aengwanich et al. (2011) reported lower SR in Thai Brahman cattle housed under artificial shade (914.07 g/m2/h) and tree shade (887.79 g/m2/h) compared to those in direct sunlight without any shade (1774.77 g/m2/h).
Skin temperature
Exchange of thermal waves occurs between animal’s skin and surrounding environment during heat stress. Higher skin temperature (ST) during heat stress has detrimental effects as it precludes heat dissipation from the animal’s body to the external environment to maintain homeostasis. Normally, heat loss from the animal’s body occurs via four processes like conduction, convection, radiation and evaporation. The first three processes are considered sensible heat loss which depends on temperature gradient between the animal’s body and the environment and occurs efficiently when environmental temperature is below or within the TNZ, whereas evaporative heat loss predominates when the environmental temperature emulates TNZ (Maia et al. 2005). ST was markedly increased in young claves after 24 h and then gradually dropped on continued heat exposure at 40.5°C for 14 days (Singh and Newton 1978). Increase in ST might be attributed to total body heat production due to rise in environmental temperature. However, decline in ST during prolonged heat exposure at the same ambient temperature might be due to heat adaptation to the same temperature gradient. Similarly, on exposure to THI at 81, ST in dairy cows with black hair coat elevated by 4.8°C while those with white hair coat increased by meagre 0.7°C (Hillman et al. 2001). This might be due to higher absorption of solar radiation via black hair coat compared to white hair coat. Banerjee and Ashutosh (2011) noticed lower ST in Tharparkar at different sites in the body compared to KF heifers during exposure to high ambient temperature at 38–39°C. This could be explained by more reabsorption of solar radiations through the dark skin of KF cows which in turn accumulates more heat that might exceed the amount of heat loss to the surrounding during summer season. This could also be attributed to lower SR in KF heifers (as they mostly rely on panting) which might have impaired heat loss via sweating resulting in higher ST of KF heifers than Tharparkar heifers. Moreover, higher SR in KF heifers indicates lower thermal adaptability of KF heifers compared to Tharparkar heifers. Vaidya et al. (2011) found higher ST in growing KF cattle (39.30°C) than adult KF cattle (38.60°C) at 1400 h during summer season. This might owe to less heat loss in growing KF cattle (2298 kJ/h) than adult (2712 kJ/h) during summer season. In harmony, Bhan et al. (2013) found hike in ST by 3.81°C and 4.82°C in growing KF cattle whereas 2.93°C and 4.92°C in adult KF cattle during forenoon and afternoon of summer than spring season. Higher ST in growing and adult KF cattle might be attributed to higher heat storage over their amount of heat loss during afternoon hours of summer season. Comparably, Jian et al. (2015) detected higher ST in pure and crossbred HF (HF100%=38°C; HF87.5%=37.9°C; HF50%=35.5°C) with hair with respect to Sahiwal cows (34.1°C) on exposure to higher THI at 91.68. Under similar environmental condition, the authors found higher ST in pure and crossbred HF (HF100%=37.4°C; HF87.5%=37.1°C; HF50%=35.5°C) without hair compared to Sahiwal cows (34.8°C). Overall, ST in both with hair and without hair 87.5% HF and 100% HF was significantly higher than that in 50% HF and Sahiwal cattle, indicating lower thermal adaptability in crossbreds of 100% HF and 87.5% HF than 50% HF and Sahiwal cows. Even pure 100% HF has shown lower heat tolerance ability than Sahiwal cattle under higher THI. Similarly, Maibam et al. (2017) noticed higher ST in KF than Tharparkar cattle during summer season (KF—43.01°C; Tharparkar—39.07°C) than TNZ (KF—34.32°C; Tharparkar—33.83°C), which indicates less heat tolerance ability of KF than Tharparkar. This report again verified lower thermal adaptability of KF cattle than their counterpart Tharparkar cattle. In a study conducted by Bhan et al. (2012), ST was significantly upregulated during the afternoon session of summer (growing—39.47°C; adult—38.33°C) than spring (growing—31.69°C; adult—32.01°C) seasons in growing and adult Sahiwal cattle. This might be explained by higher heat generation in growing Sahiwal cattle leading to higher ST than adult Sahiwal cattle. Identically, Cardoso et al. (2015) determined highest ST in Gir during morning (34.24°C) and afternoon (31.96°C) hours of heat stress than Girolando, Nellore, Indrabusil and Sindhi suggesting the fact that Gir possesses the lowest thermotolerance ability amongst all the breeds. In another study, ST was found to be higher in Boran cows (36.0°C) than in Nguni cows (35.1°C) on exposure to summer heat stress (Katiyatiya et al. 2017). This could be explained by the fact that Boran cows have thicker skins with longer hairs (24.3 mm) than Nguni cows with smaller hairs (20.2 mm). Thicker skin in Boran cows might have prevented adequate heat loss resulting in higher ST than Nguni cows. In accordance with previous findings, ST was greater in Sahiwal and KF cows during hot humid than winter season with a nonsignificant change in Sahiwal (36.16°C) and KF (38.16°C) cows on the day of calving during hot humid season (Grewal and Aggarwal 2018). This could be explained by higher potency of heat stress in hot humid season. In a study conducted in lactating HF cows, Park et al. (2019) investigated gradual upregulation in ST during exposure to increase in THI at 70–75 (36.41°C), 76–81 (36.51) and 82–87 (37.39°C), suggesting positive correlation of ST with THI. Furthermore, Silva and Maia (2011) reported higher ST at black areas of hair coats than white areas in HF cows during heat stress in tropical climate and suggested that ST could be used to anticipate the rate of cutaneous evaporative heat loss in HF cows in tropical climate. Various studies in cattle have shown that ambient temperature influences ST at different body regions, i.e., head (Singh and Singh 2006), flank, neck and gluteus region (Silva and Maia 2011), eye (Church et al. 2014) and neck, lumbar and thigh region (Yadav et al. 2017). Moreover, Yadav et al. (2017) detected highest ST at neck region in crossbred cattle before and after heat exposure at 25°C, 35°C and 40°C. In another study, Prasanpanich et al. (2002) found significantly higher ST in lactating HF cows grazed outdoor without any shade (41.2°C) than those kept indoor (38.2°C), indicating the fact that shade restricts the absorption of solar radiations.
Neuro-endocrine responses of cattle against heat stress
Neuro-endocrine responses are exhibited following behavioural and physiological responses to attenuate the negative effects of heat stress. Neuro-endocrine responses are accomplished by alternation in secretion pattern of various hormones into systemic circulation and executing their specific functions on respective target cells or tissues upon heat stress. Heat stress evokes hypothalamo-pituitary adrenal axis (HPA) and SAM axis (Fig. 1) to produce and release various hormones into systemic circulation to modulate different body metabolisms thereby revive energy homeostasis (Spencer and Deak 2017). Synergistic action of HPA and SAM axis further succour livestock to get adapted to the heat stress rigours (Kumar et al. 2011; Mishra 2021). This section focuses on various neuro-endocrine responses (Table 3) displayed by different breeds of cattle under heat stress. Major neuro-endocrine hormones responsible for thermal adaptation are cortisol, catecholamines, thyroid hormone, growth hormone (GH), insulin, prolactin, aldosterone, anti-diuretic hormone (ADH), leptin and reproductive hormones (Mishra and Palai 2014b; Mishra 2021).
Cortisol
Heat stress excites hypothalamic PVN to secrete corticotropin-releasing hormone (CRH) which acts on corticotrophs of adenohypophysis to secrete ACTH which further activates zona fasciculata of adrenal cortex to secrete cortisol into systemic circulation. Innumerable reports indicate elevation in plasma cortisol level in heat-stressed cattle. Plasma cortisol concentration was significantly increased from 2.4 to 3.9 μg/dl after 2 h, reached maximum (5.4 μg/dl) after 4 h, followed by gradual reduction to basal level (2.4 μg/dl) after 48 h and remained at plateau during long-term heat stress at 35°C (Alvarez and Johnson 1973). Immediate increase in plasma cortisol level could be due to the activation of HPA axis which might have triggered the secretion of cascade of hormones like CRH (hypothalamic PVN), ACTH (adenohypophysis) and cortisol (adrenal cortex). However, gradual reduction and plateau stage of cortisol level could be due to thermal adaptation during long-term heat stress. In HF steers, plasma cortisol level was gradually increased from 9.7 ng/ml (TNZ) to 11.6, 17.9 and 22.6 ng/ml at 60 min, 80 min and 110 min, respectively, reduced to 16.7 ng/ml at 120 min and then increased to 28.5 ng/ml at 160 min and finally reduced to 20.3 ng/ml after 240 min of heat exposure at 42°C (Abilay et al. 1975). Initial increase in plasma cortisol level up to 110 min of heat exposure might be due to activation of hypothalamic PVN, then reduction at 120 min might be due to initial adaptation to acute heat stress, then elevation at 160 min might be due to recurrent activation of hypothalamic PVN in response to the 2nd phase of acute heat stress followed by dip at 240 min which might be due to the adaption to the 2nd phase of acute heat stress. Likewise, mean plasma cortisol concentration was found to be significantly higher in lactating dairy cows without shade (13.04 ng/ml) than under shade (8.72 ng/ml) during summer months (Roman-Ponce et al. 1981). This could be due to the fact that cows without any shade might have experienced more heat stress thereby releasing more cortisol to combat the situation. One possible explanation of higher secretion of cortisol during acute heat stress is that it might stimulate hepatic gluconeogenesis thereby restoring metabolic homeostasis. Hammond et al. (1996) found higher plasma cortisol level in Romosinuano (32.8 ng/ml) and Brahman (29.1 ng/ml) heifers than Angus (17.8 ng/ml), Hereford (17.4 ng/ml) and Senepol (21.8 ng/ml) heifers on hot summer days which could be due to the variations in RT (described above) and temperament amongst different breeds in response to heat stress. In another study, cortisol level was found to be increased from 11 to 29 ng/ml in HF calves exposed to solar radiations during hot summer season (Yousef et al. 1997). Likewise, cortisol level was significantly increased from 3.8 to 6.5 ng/ml when temperature inside the psychometric chamber was increased from 24 to 38°C for 9 h (Habeeb et al. 2001). Elevation in cortisol levels reported by Yousef et al. 1997) and Habeeb et al. (2001) could be due to the similar mechanisms clarified above. Additionally, cortisol might serve as vasodilator to enhance evaporative heat loss and could stimulate lipolysis along with proteolysis to restore metabolic homeostasis in cattle against acute heat stress. Later on, Pereira et al. (2008) reported highest blood cortisol level in Mertolenga (2.11μg/dl) followed by Limousine (1.59 μg/dl), Alentejana (1.06 μg/dl) and HF (0.76 μg/dl) during late afternoon under THI at 85. Breed-specific increase in cortisol levels could be due to the combination of behavioural, physiological and endocrine responses manifested under heat stress. In Sahiwal cattle, Bhan et al. (2012) reported significantly greater plasma cortisol level during summer (8.91 ng/ml) than spring season (1.92 ng/ml). Uniformly in growing KF cattle, Bhan et al. (2013) determined elevation in plasma cortisol level by 10.95, 10.97 and 15.25% during afternoon than morning session of winter, hot humid and summer season respectively. Concurrently in adult KF cattle, Bhan et al. (2013) found an increase in cortisol concentration by 32.17, 3.23 and 16.99% during afternoon compared to morning session of winter, hot humid and summer season respectively. Highest elevation in plasma cortisol level could be attributed to the magnitude of heat stress witnessed by growing and adult KF cows in different seasons. Additionally, growing KF cattle might be highly sensitive to summer-induced heat stress while adult KF cattle might be more susceptible to cold stress during winter season. Yadav et al. (2015) also reported elevation in serum cortisol level in crossbred cattle during heat stress at 40°C (32.23 nM/ml) and 35°C (29.23 nM/ml) than heat exposure at 30°C (15.75 nM/ml) and 25°C (15.70 nM/ml). Gradual increase in cortisol level could be due to gradual increase in temperature and highest temperature at 40°C could have induced maximum secretion cortisol indicating the fact that cortisol induction might be temperature sensitive. Akin to previous studies, Chen et al. (2018) found that plasma and milk cortisol levels were significantly elevated in Chinese HF dairy cows on exposure to THI at 80.5 (plasma=32 ng/ml and milk=29 ng/ml) than THI at 66 (plasma=14 ng/ml and milk=15 ng/ml). Concurrently, Kim et al. (2018) documented highest serum cortisol level in Korean native calves exposed to THI at 87.72 (17.1 ng/ml) and lowest on exposure to THI at 76.51 (4.6 ng/ml). Explanations for the findings of Chen et al. (2018) and Kim et al. (2018) could be very much similar to the explanations provided earlier. Moreover, findings of Chen et al. (2018) and Kim et al. (2018) indicate the fact that cortisol induction might be sensitive to THI as well. Identically, Sanap et al. (2018) found significantly greater plasma cortisol level in crossbred calves during hot humid and hot dry season than spring season, suggesting that hot humid and hot dry seasons might be more life threatening than spring season. In another study, Yadav et al. (2021) observed significantly greater serum cortisol level in crossbred cattle on day 1 upon heat exposure at 40°C (mean level ~ 32 nM/ml) than TNZ at 25°C (mean level ~15.5 nM/ml), followed by a gradual dip from day 6 up to days 21 with a significant dip on days 16 of heat exposure at 40°C. The initial peak in plasma cortisol level could be attributed to sudden activation of HPA axis as described before. However, gradual reduction in cortisol level from days 6 to 21 followed by significant reduction on days 16 might be due to heat acclimation in crossbred cattle. Identically, Kumar et al. (2019) documented higher plasma cortisol level in lactating zebu cattle exposed to THI at 82 (250 ng/ml) followed by significant reduction with exposure to higher THI. This could be due to the saturation of hypothalmic PVN to THI at 82 and then cortisol secretion might have downregulated on exposure to THI beyond 82. According to the report of Pires et al. (2019), heat stress had elevated plasma cortisol level in Nelore (18.5 ng/ml) and Caracu cattle (23.7 ng/ml). On the other hand, prolonged heat stress at 33.5°C dwindle cortisol secretion in Guernsey heifers (Abilay et al. 1975a). Later on, Kamal et al. (1989) found significant decline in cortisol level by 45% in HF claves on chronic heat exposure. Similarly, Ronchi et al. (2001) noticed lower cortisol levels in HF Heifers exposed to high air temperature. Decline in cortisol level during prolong heat exposure could be attributed to negative feedback effect of cortisol on HPA axis. Based on the aforementioned reports obtained by various authors, it could be summarised that acute heat stress escalates cortisol level whereas chronic heat stress declines cortisol level in cattle. Taken together, cortisol could be considered the major anti-stress hormone in cattle.
Catecholamines
Catecholamine group of hormones comprises of epinephrine, nor-epinephrine and dopamine. Heat stress stimulates SAM axis which further activates adrenal medulla to secrete catecholamines to mediate fight or flight reaction. Plasma epinephrine level was markedly increased by 45% and 91% while nor-epinephrine was strikingly increased by 42% and 70% in HF cows on exposure to acute and chronic heat stress respectively (Alvarez and Johnson 1973). Likewise, Lamp et al. (2015) reported significant increase in plasma epinephrine and nor-epinephrine levels in early lactating German HF cows during heat stress (THI=76) than TNZ (THI=59.7). In detail, plasma epinephrine level was found to be 104.79 pg/ml during heat stress and 64.29 pg/ml in TNZ. On the other hand, plasma nor-epinephrine level was found to be 255.4 pg/ml during heat stress and 228.4 pg/ml in TNZ. Increase in plasma catecholamine concentration could be attributed to activation of SAM axis in heat-stressed cows and as a result catecholamines might commence fight or flight response to counteract the negative effects of heat stress in cattle (Palkovits 2014). Moreover, higher concentration of catecholamines might evoke sweat gland activity during heat stress (Allen and Bligh 1969). In addition, catecholamines behave as positively chronotropic and ionotropic factor thereby help cattle to adapt against the lethal effects of heat stress (McMorris 2016).
Thyroid hormones and TSH
Thyroid hormones play profound role in thermoregulation and energy homeostasis thereby affecting the reproductive and productive performances of cattle (Huszenicza et al. 2002; Djokovic et al. 2010; Mishra 2021). Multiple stimuli activate hypothalamo-pituitary thyroid axis (HPT) axis to produce thyroid hormone which alters basal metabolic rate thereby regulating energy homeostasis. Hypothalamic PVN synthesises thyrotropin-releasing hormone (TRH) which activates thyrotrophs of adenohypophysis to produce thyroid-stimulating hormone (TSH) which finally triggers thyroid follicle to produce T3 and T4 (Fekete and Lechan 2013; Omidi et al. 2015). Thyroid gland is highly thermo-sensitive and influenced by heat stress imposed by high environmental temperature (Rasooli et al. 2004). It is well known that high temperature coupled with high relative humidity during summer months wane thyroid gland activity in cattle (Morais et al. 2008; Saber et al. 2009; Aggarwal and Upadhyay 2013). In general, plasma T3 and T4 levels were reduced up to 25% in lactating cows on exposure to heat stress (Magdub et al. 1982). Likewise, Johnson et al. (1988) reported significant reduction in plasma T3 level from 2.2 to 1.16 ng/ml in lactating dairy cows exposed to acute heat stress. McGuire et al. (1991) found lower plasma T3 level in lactating HF cows exposed to heat stress (0.51 ng/ml) compared TNZ (0.7 ng/ml). Significant drop in thyroid hormones levels in the aforementioned studies could be attributed to inhibition of HPT axis during heat stress. When cattle are exposed to high temperature stress, then it would become difficult for them to maintain thermal balance. Moreover, it is well known that thyroid hormones are the major metabolic and calorigenic hormones. Thus, during heat stress, HPT axis might get downregulated which subsequently reduce thyroid hormones levels. As a result, low thyroid hormones levels not only to decrease the body metabolism but also prevent further metabolic heat generation to maintain thermal equilibrium between animal’s body and surrounding environment. Similarly, plasma T3 concentration markedly declined from 151 to 126 ng/dl in HF calves exposed to direct solar radiation during summer season (Yousef et al. 1997). Similarly, Habeeb et al. (2001) reported lower circulating level of T3 and T4 in male HF calves after 9 h of heat exposure at 38°C in psychrometric chamber. In another study, Rasooli et al. (2004) recorded lower T3 and T4 levels in HF cows during heat exposure at 35°C. Consistently, plasma T3 and T4 levels were depressed in dairy cows during summer season (Morais et al. 2008). Dip in thyroid hormone levels in the studies conducted by Yousef et al. 1997), Habeeb et al. (2001), Rasooli et al. (2004) and Morais et al. (2008) could be due to the same reason explained earlier. It could also be attributed to inhibition of hypothalamic PVN resulted from inhibition of HPT axis during high temperature stress, which consequently reduces the secretion of TRH, TSH and thyroid hormones to maintain thermal balance in heat-stressed cattle. Pereira et al. (2008) documented that heat stress (THI=85) reduced blood T3 and T4 levels by 14.4 and 21.7%, 16 and 15%, 18.3 and 15.3%, and 22 and 23.8% in Mertolenga, HF, Alentejana and Limousine breed respectively. Pereira et al. (2008) indicated that Mertolenga had highest thermo-tolerance ability while HF had the least amongst the four investigated cattle breeds. Actually, the conclusion regarding the best and least thermo-tolerance ability had not been decided solely on the basic of percentage of reduction in thyroid hormones levels; rather, it was decided by taking into account of behavioural, physiological (described above) and endocrine parameters. Uniformly, Kohli et al. (2014) found significantly lower and higher plasma T3 and T4 levels in crossbred dairy cows during summer (T3=1.0 nmol/L and T4=20.0 nmol/L) and winter (T3=2.0 nmol/L and T4=61.0 nmol/L) season respectively. Kahl et al. (2015) documented decline in T3 and T4 levels by 25.9% and 45.4% respectively in steers exposed to heat stress between 32.2°C to 40°C than steers within TNZ at 19°C. Significant reduction in thyroid hormone levels as reported by Kohli et al. (2014) and Kahl et al. (2015) could be due to the same mechanisms stated above. Additionally, inhibition of hypothalamic PVN might fail to secrete optimum TRH thereby reducing thyroid hormone secretion during heat stress. Thereafter, lower thyroid hormones levels tend to minimise body metabolism and reduce heat production to cope up with heat stress. In crossbred cattle, Yadav et al. (2015) did not notice any significant change in serum T3 level on exposure to different temperature gradients while they found significant decline in serum T4 level during heat exposure at 35°C (55.63 nM/ml) and 40°C (56.94 nM/ml) compared to heat exposure at 30°C (61.17 nM/ml) and 25°C (69.38 nM/ml). Akin to their previous findings, Yadav et al. (2016) did not find any significant variation in serum T3 level in crossbred cattle on exposure to different temperature gradients while they found reduction in T4 level during heat exposure at 35°C (55.63 nM/ml) and 40°C (59.64 nM/ml) than at 30°C (61.17 nM/ml) and 25°C (69.38 nM/ml). Identical with their previous findings, Yadav et al. (2021) noted a significant dip in T4 level in crossbred cattle exposed to heat shock at 40°C (mean level ~ 73 nM/ml) compared to 25°C (mean level ~56 nM/ml). Significant reduction in T4 level obtained by Yadav et al. (2015), Yadav et al. (2016) and Yadav et al. (2021) could be explained in similar manner as above. However, no significant variation in T3 level in both studies could be attributed to sparse secretion of T3 compared to T4. Basically, thyroid gland produces around 95% of T4 and only 5% of T3 into systemic circulation. Thus, such less concentration of plasma T3 might not have produced a significant difference. In early lactating HF cows, Weitzel et al. (2017) reported that plasma T3 was significantly reduced from 2 nmol/L (TNZ at 15°C, THI=60) to 1.2 nmol/L (heat stress at 28°C, THI=76) while plasma T4 level declined from 65 nmol/L within TNZ to 35 nmol/L upon heat stress. In tune with earlier reports, Chen et al. (2018) found that plasma T3 and T4 levels significantly declined in Chinese HF dairy cows on exposure to THI at 80.5 (T3=2.0 ng/ml and T4=10.0 ng/ml) than THI at 66 (T3=3.2 ng/ml and T4=22.0 ng/ml). In the same study, Chen et al. (2018) also found that milk T3 and T4 levels were significantly dropped in Chinese HF dairy cows on exposure to THI at 80.5 (T3=1.9 ng/ml and T4=9.0 ng/ml) than THI at 66 (T3=2.9 ng/ml and T4=30.0 ng/ml). The possible explanation is that inhibition in HPT axis might have reduced both the plasma and milk thyroid hormones levels in heat-stressed cattle. Apart from reduction in thyroid hormones levels reported by numerous authors, plasma TSH level was also found to be reduced by 40% in heat-stressed steers (Kahl et al. 2015). This could be due to inhibition of HPT axis, resulting in low TSH level in heat-stressed steers. In contrast, Weitzel et al. (2017) did not find any change in plasma TSH level in lactating HF cows upon heat exposure at 28°C. However, Weitzel et al. (2017) documented lower level of thyroid hormones in this same experiment as stated above. This nonsignificant variation in TSH level could be due to the fact that inhibition in HPT axis which could have declined TRH secretion from hypothalamic PVN might not have been sufficient to decrease TSH secretion from thyrotrophs of adenohypophysis.
GH and IGF
GH is secreted from somatotroph cells of adenohypophysis under the influence of GHRH and responsible for thermoregulation in heat-stressed cattle. Finding of Mitra et al. (1972) indicated that plasma GH concentration significantly declined in Jersey cows exposed to heat stress at 35°C (13.5 ng/ml) than TNZ at 18°C (18.2 ng/ml). Likewise, McGuire et al. (1991) reported lower plasma GH level in lactating HF cows under heat stress (5.3 ng/ml) than TNZ (8.4 ng/ml). In another study, plasma GH concentration was found to be declined in heat-stressed lactating HF cows (Rhoads et al. 2009). In another study, Igono et al. (1988) noticed marked reduction in milk GH level in lactating HF on exposure to THI more than 70. This appreciable decline in plasma GH level during heat stress reported by the aforementioned authors could be attributed to the calorigenic potential of GH. As GH is one of the major calorigenic hormones, therefore drop in GH level might reduce body calorigenesis which seems to be redundant during heat stress to render thermal balance. It could also be due to negative impact of heat stress on hypothalamic ARC which might have resulted in lower GH level. Another possible explanation is that overproduction of CRH in response to heat stress could stimulate somatostatin to abate GH secretion in heat-stressed cattle (Riedel et al. 1998). Lower GH level in heat-stressed cattle could also be explained by the fact that combined reduction in GH and thyroid hormone (delineated earlier) might depress metabolic heat production thereby maintaining thermal balance in cattle during extreme heat stress. In another study, Tucker and Wetteman (1976) exposed heifers to 4.5, 21 and 32°C for 9 days inside a psychometric chamber after exposing them under ambient temperature at 21°C for 10 days. Authors found a nonsignificant increase in serum GH level at 4.5°C (4.0 ng/ml), 21°C (6.3 ng/ml) and 32°C (9.4 ng/ml). In addition, exogenous TRH treatment did not affect serum GH level at any temperature gradient inside psychometric chamber. Tucker and Wetteman (1976) concluded that both ambient temperature and exogenous TRH did not influence serum GH level in heifers. Findings of Tucker and Wetteman (1976) could be explained by the fact that neither exposure to different temperatures nor the concentration of exogenous TRH used by them might have triggered the hypothalamic ARC to secrete significant amount of GH. In contrast, plasma GH level was found to be greater in dairy cows kept under combination of shade, spray and fan compared to under shade only (Igono et al. 1987). This could be due to less impact of heat stress on dairy cows kept under shade and offered spray and fan than cows under shade only. Rhoads et al. (2009) observed decline in insulin-like growth factor I (IGF-I) concentration by 16% in heat-stressed lactating HF cows. Similarly, Aggarwal and Upadhyay (2013) found lower plasma IGF-I level in cattle exposed to summer heat stress. One possible explanation is that low GH during heat stress (described earlier) might not be sufficient to induce hepatic GH-IGF axis thereby reducing GH-dependent hepatic IGF-I production in heat-stressed cows. However, McGuire et al. (1991) did not notice any significant change in IGF-I level but found greater insulin-like growth factor II (IGF-II) level in lactating HF cows upon heat stress (469 ng/ml) compared to TNZ (368 ng/ml). Though McGuire et al. (1991) detected lower GH level (5.3 ng/ml) during heat stress (described earlier), still at this concentration GH might not had affected the concentration of IGF-I. However, increase in concentration of IGF-II could be due to some different mechanism as IGF-II secretion is less dependent on GH than IGF-I.
Insulin
Impact of heat stress on plasma insulin level in cattle is quite baffling. It was reported that high environmental temperature declines insulin level by 54% in HF heifers (Sejrsen et al. 1980), 30% in HF calves (Habeeb 1987) and 33% in HF cows (Abdel-Samee et al. 1989). Reduction in plasma insulin level in heat-stressed HF heifers, HF calves, and HF cows could be attributed to the negative energy balance resulted from lower dry matter intake. O’Callagan and Boland (1999) also suggested that insulin is imperative for follicular development and production of competent oocytes; thus, lower insulin level might avert reproductive efficiency in dairy cows during summer stress. Cool dairy cows kept under sprinklers and fans had lower plasma insulin level than heat-stressed dairy cows exposed to THI at 78 (Tao et al. 2012). Lower insulin level in cool cows could be due to lower blood glucose level as a consequence to lower cortisol level. In contrast, several authors reported higher insulin level in cattle upon heat exposure (Itoh et al. 1998; Wheelock et al. 2010; O'Brien et al. 2010). In a study undertaken by Itoh et al. (1998), exogenous treatment of arginine and butyrate had upregulated plasma insulin level in lactating HF cows upon heat exposure at 28°C. Uniformly, rise in environmental temperature from 29.4 to 38.9°C had increased plasma insulin level by 30% in lactating HF cows (Wheelock et al. 2010). It has been well established that insulin is one of the major hormones involved in the regulation of lactogenesis and galactopoiesis in lactating cows. This may be the reason for higher insulin level in heat-stressed lactating HF cows. Similarly, plasma insulin level was elevated by 30% in growing HF bull calves when ambient temperature was elevated from 29.4 to 40°C (O'Brien et al., 2010). Higher insulin level in growing HF bull calves could be attributed to higher plasma glucose level resulted from higher cortisol level during high temperature stress. In another study, Min et al. (2015) did not find any significant change in serum insulin concentrations between heat-stressed (THI 81.7) and cool lactating HF cows (THI 53.4), which might be due to variation in agro-climatic zone, variation in severity of heat stress, variation in breed types and temperament of a particular breed under heat stress.
Prolactin
Downregulation of prolactin-inhibiting hormone or dopamine stimulates prolactin secretion from lactotroph cells of adenohypophysis (Alamer 2011). Acute heat stress depresses the activity of dopaminergic neurons in dairy calves thereby inducing more prolactin secretion (Tucker et al. 1991). In another study, Wettemann and Tucker 1974) found that rise in ambient temperature from 21 to 27°C had increased serum prolactin level from 8 to 22 ng/ml at 3-h interval in heifers. Moreover, serum prolactin level was escalated by 2-fold in heifers subjected to chronic heat exposure at 27°C for 5 days than control at 21°C. On the other hand, decrease in ambient temperature from 21 to 10°C reduced serum prolactin level from 13 to 4 ng/ml at 4-h interval. In the same study, serum prolactin level declined by 38% when heifers exposed to 10°C for 5 days compared to control at 21°C. In their next study, Tucker and Wetteman (1976) exposed heifers at 4.5, 21 and 32°C for 9 days inside a psychometric chamber following exposure to ambient temperature at 21°C for 10 days. The authors observed that serum prolactin level was linearly elevated by 1.17 ng/ml/°C within the first 24 h when the temperature inside psychometric chamber was increased from 21 to 32°C. Further, serum prolactin was linearly decreased by 0.6 ng/ml/°C within the first 24 h when the temperature inside psychometric chamber was reduced from 21 to 4.5°C. Interestingly, serum prolactin level was gradually upregulated at 4.5°C (2.6 ng/ml), 21°C (13.0 ng/ml) and 32°C (27.7 ng/ml) between days 2 to 9. The results obtained from the above studies clearly indicate that low ambient temperature might stimulate hypothalamic dopaminergic neurons of ARC to decrease prolactin secretion while high temperature might inhibit the same to induce more prolactin secretion. In another study, prolactin level was elevated by 6 to 7-fold in Hereford steers exposed to summer than winter season (Smith et al. 1977). In another experiment of the same study, Smith et al. (1977) observed 4-fold increase in prolactin level in Hereford steers exposed to ambient temperature 40°C compared to TNZ. Furthermore, Smith et al. (1977) reported a decline in prolactin level by 55–80% when ambient temperature was reduced from 20–21 to 4–7°C. The reports obtained by Smith et al. (1977) are in accordance with Wettemann and Tucker 1974) and Tucker and Wetteman (1976) and further substantiates the proposed mechanism behind alternations in secretion pattern of prolactin during low (decrease prolactin level) and high ambient temperature (increase prolactin level). Uniformly, serum prolactin was significantly upregulated in HF or Angus bulls under hyperthermia at 35°C (Schams et al. 1980). In another study in lactating cows, plasma prolactin level was increased from 38 to 86 ng/ml during exposure to direct sunlight for 20 weeks (Roman-Ponce et al. 1981a). Identically, serum prolactin level was found to be gradually increased after 5 days of heat exposure at 7°C (9.0 ng/ml), 21°C (20.9 ng/ml) and 31°C (29.5 ng/ml) (Wettermann et al., 1982). Similarly, prolactin level was upregulated by more than 3-fold in HF heifers when environmental temperature increased from 18 to 32°C (Ronchi et al. 2001). Identically, prolactin level was upregulated in Angus cattle during heat exposure at 36°C for 14 days (Scharf et al. 2010). In another study, Do Amaral et al. (2011) found significantly higher prolactin in heat-stressed HF cows (150 ng/ml) than cool cows (93 ng/ml). These findings are also in concurrence with earlier findings pertaining to elevation in prolactin secretion with increase severity of heat stress. Another possible mechanism is that higher prolactin level could activate sweat gland stimulating evaporative heat loss thereby helping in thermal adaptation in heat-stressed cattle (Beede and Collier 1986). Apart from accumulated evidences on elevation in serum prolactin level, Igono et al. (1988) investigated higher milk prolactin level in lactating HF cows during summer heat stress. Several authors have tried to find out the effect of TRH on plasma prolactin levels during different ambient temperatures (Wettemann and Tucker 1974; Tucker and Wetteman 1976; Wettemann et al. 1982). Wettemann and Tucker (1974) noticed an increase in serum prolactin from 8 to 70 ng/ml and 20 to 140 ng/ml within 5 min of TRH treatment at 10°C and 27°C respectively. But, Tucker and Wetteman (1976) did not notice any significant change in serum prolactin level within 5 mins of TRH treatment at 4.5°C. However, Tucker and Wetteman (1976) reported an increase in serum prolactin level from 15.7 to 62.8 ng/ml and 20.4 to 109.8 ng/ml within 5 mins of TRH treatment at 21°C and 32°C respectively. Uniformly, Wettemann et al. 1982) determined an increase in serum prolactin level from 7.0 to 45.7 ng/ml, 13.1 to 97.2 ng/ml and 18.2 to 96.2 ng/ml in dairy heifers within 5 min of TRH treatment at 7°C, 21°C and 31°C respectively. Finally, Wettemann and Tucker 1974), Tucker and Wetteman (1976) and Wettemann et al. 1982) concluded that ambient temperature could be the predominant zeitgeber which influences basal and TRH-stimulated prolactin secretion in different cattle breeds. In another study, Igono et al. (1987) reported significant dip in plasma prolactin level in dairy cows kept under combined treatment of shade, spray and fan than cows kept under only shade, suggesting that combined treatment alleviates the harmful effects of heat stress thereby reducing prolactin level. Finally, Alamer (2011) revealed that positive correlation exists between environmental temperature and prolactin secretion in domestic ruminants including cattle. According to Scharf et al. (2010), prolactin could be used an indicator of heat stress like RT as they reckon rise in RT might have induced prolactin secretion in cattle during exposure to high environmental temperature.
Aldosterone
Aldosterone is basically secreted from zona glomerulosa of adrenal cortex in response to hyperkalemia and considered the major mineralocorticoid hormone in domestic mammals as it controls mineral homeostasis in the animal’s body. Plasma aldosterone level did not vary during first 8 h and then significantly declined after 24 h of heat exposure at 35°C (El-Nouty et al. 1980). The reduction in plasma aldosterone after 24 h of heat stress could be attributed to loss of potassium ions via excessive sweating. Likewise, plasma aldosterone level was decreased in dairy cows on exposure to high environmental temperature (Collier et al. 1982), which could be due to excretion of potassium ions via sweating under high-temperature stress.
ADH
ADH is secreted from magnocellular neurons of PVN and supra-optic nuclei (SON) of hypothalamus in response to hyper-osmolarity and regulates osmolarity of the extra cellular fluid. Plasma ADH concentration was upregulated in dairy cattle upon exposure to heat stress (El-Nouty et al. 1980). The elevation in plasma ADH concentration could be due to increase in plasma osmolarity as the dairy cattle might have undergone dehydration resulted from excessive sweating during heat stress. Moreover, severe dehydration during summer heat stress due to excess evaporative heat loss via respiratory tract and higher sweat gland activity could provoke PVN and SON nuclei to synthesise ADH which then stimulates neurohypophysis to release ADH into systemic circulation to restore plasma osmolarity thereby maintaining water homeostasis (Kumar et al. 2011; Stricker and Verbalis 2013).
Leptin
Leptin is predominantly secreted from white adipocytes including other cellular systems and regulates food intake and energy homeostasis in the animal’s body (Mishra and Palai 2014b; Mishra et al. 2016; Reshma et al. 2016). Leptin serves as a metabolic messenger as it signals metabolic status to the brain and acts on different hypothalamic nuclei to regulate feed intake thereby maintaining energy homeostasis in the animal’s body (Singh et al. 2012). In addition, leptin acts on its receptors localised in ARC and PVN of hypothalamus thereby influencing reproductive behaviour in domestic species including cattle (Mishra and Palai 2014b; De la Hoya et al. 2015). Cyclical change of season from early winter to summer had increased serum leptin level by 34% in dairy cattle and then remained constant throughout the year (Garcia et al. 2002). Uniformly, plasma leptin levels were found to be higher in lactating cows under warm climate than cold climate (Kokkonen et al. 2002). In addition, plasma leptin level was elevated in peri-parturient dairy cows exposed to hot season (Bernabucci et al. 2006). The upregulation of leptin in the aforementioned studies could be a reason for the reduction of feed intake in dairy cows under summer months. Furthermore, leptin might have stimulated POMC and CART neurons in the ARC, sending an anorexigenic signal to PVN, resulting in decline in feed intake and subsequently reduce metabolic heat production to cope up with hot summer months. Similarly, serum leptin level was significantly upregulated in both Angus and Romosinuano cattle exposed to controlled heat stress at 36°C inside psychometric chamber, with a greater leptin level in heat-susceptible Angus than heat-tolerant Romosinuano steers (Scharf et al. 2010). Comparatively lower level of leptin in Romosinuano steer could be due to their light and lean body size compared to Angus steers. Identically, plasma leptin levels in Hariana cattle were found to be 4.39 and 6.73 ng/ml whereas in Sahiwal cattle were found to be 5.06 and 6.89 ng/ml, during winter and summer season respectively (Kumar et al. 2017). Increase in plasma leptin level in both the cattle breeds during summer season suggests the role of leptin in reduction of apetite by stimulating the satiety centre in the hypothalamus. Plasma leptin level was found to be increased by 1.4-fold in Israeli-HF dairy cows offered eight cooling sessions per day than those offered five cooling sessions per day, suggesting the fact that the dairy cows offered eight cooling sessions might have improved nutritional status which could have enhanced plasma leptin levels to maintain energy homeostasis (Kleinjan-Elazary et al. 2020). In contrast, Kumar et al. (2019) determined lower plasma leptin level in lactating Hariana cattle on exposure to THI at 81 and then they did not find any change in leptin level with further increase in THI. This deviation in plasma leptin level could be due to the lactating stage of Hariana cattle. As during lactation, Hariana cattle might be under negative energy balance due to conversion of much of the blood glucose to milk lactose; therefore, plasma leptin level could have declined to allow the Hariana cattle to continue feeding as per standard requirement during lactation stage. In another study, serum leptin concentrations did not alter significantly between heat-stressed (THI 81.7) and cool lactating HF cows (THI 53.4) (Min et al. 2015), which might be due to variation in agro-climatic zone, variation in severity of heat stress, variation in breed types and temperament of a particular breed in response to heat stress.
Reproductive hormones
Heat stress triggers HPA axis to induce higher secretion of cortisol into systemic circulation which subsequently downregulate HPG axis (Salles et al. 2017). In particular, high cortisol level might inhibit hypothalamic gonadotropin-releasing hormone (GnRH) neurons thereby depressing the frequency and amplitude of GnRH, which ultimately decline luteinizing hormone (LH) secretion from adenohypophysis, resulting in marked reduction in reproductive efficiency in dairy cows (Gilad et al. 1993; Breen and Karsch 2006; Naqvi et al. 2012; Salles et al. 2017). Additionally, it has also been shown that summer heat stress tends to reduce ovarian sensitivity to gonadotropins thereby dwindling follicular steroidogenesis and as a result negatively affects the ovarian follicular dynamics (Wolfenson et al. 1997). Impact of heat stress on plasma LH concentrations in dairy cows has been quite perplexing. Roman-Ponce et al. (1981) reported higher plasma LH level in lactating dairy cows kept without any shade during hot summer months in subtropical environment, suggesting the incidence of recurring estrous cycles in heat-stressed lactating dairy cows. Various authors detected lower LH levels (Wise et al. 1988; Gilad et al. 1993; Lee 1993; De Rensis and Scaramuzzi 2003; Roth and Wolfenson 2016), which could be due to inhibition of hypothalamic GnRH neurons in heat-stressed cattle. Apart from reports like increased and decreased levels of LH in heat-stressed cattle, some authors did not notice any significant change in plasma LH concentrations in dairy cows during heat stress (Gwazdauskas et al. 1975; Howell et al. 1994; Guzeloglu et al. 2001), which could be due to the difference in breed or severity of heat stress exposure. Subsequently, meagre plasma LH level could lead to decline in 17β-estradiol secretion from dominant follicle of dairy cattle (Gilad et al. 1993; De Rensis and Scaramuzzi 2003). In another study, Wilson et al. (1998) detected lower serum E2 concentration between days 11 to 21 of estrous cycle in HF heifers exposed to heat stress. Similarly, Bridges et al. (2005) found significantly lower E2 secretion from cultured bovine follicles exposed to heat stress at 41°C. Recently, Boni (2019) observed lower E2 level in follicular fluid of dominant follicles of dairy cattle during summer and autumn seasons compared winter season. Lower E2 levels in the abovementioned studies could be attributed to lower LH level upon heat stress, as optimum level of LH is required to secrete E2 from the granulosa cells (GCs) of dominant follicle. Reduction in E2 could also be due to sparse expression of CYP19A1 in GCs of dominant follicles in heat-stressed cattle. In addition, reduction in E2 could be due to less production of thecal androgen (androstenedione) due to lower expression of 17α-hydroxylase on theca cells of dominant follicles of heat-stressed cattle (Wilson et al. 1998; Roth et al. 2000). Lower E2 during heat stress might also be attributed to higher reactive oxygen species (ROS) production in GCs which preclude GC steroidogenesis. Recently, Khan et al. (2020) further confirmed the fact that E2 secretion was significantly reduced in heat-stressed culture bovine GCs at 41°C (1.00 ng/ml) compared to control at 38°C (3.25 ng/ml). In contrast, Guzeloglu et al. (2001) did not find any significant change in E2 level in the follicular fluid of dominant follicles of control (1662 ng/ml) and heat-exposed dairy cows (1493 ng/ml), which might be due to the constant LH level in both control and heat-stressed dairy cows mentioned earlier. Overall, heat stress inhibits granulosa and thecal steroidogenesis of dominant or pre-ovulatory follicle in cattle. According to Jolly et al. (1995), hypoglycemia resulted from lower DMI during heat stress could depress pulsatile LH release thereby averting ovulation as well.
Several studies indicated about the reduction in potency of dominant follicle and increase in number of subordinate follicles in the follicular environment of heat-stressed dairy cows (Badinga et al. 1993; Wilson et al. 1998; Roth et al. 2000). More population of subordinate follicles could be due to lower secretion of inhibin from GCs of dominant follicle of heat-stressed cows and could lead to follicular atresia of antral and pre-antral follicle in heat-stressed cows. On the other hand, lower inhibin level might result in higher plasma follicle-stimulating hormone (FSH) level in HF cows during summer heat stress (Ingraham et al. 1974). Paradoxically, GnRH-induced FSH secretion significantly declined on day 12 of the estrous cycle in cyclic cows exposed to heat stress at 40°C (Gilad et al. 1993). These observations indicate that plasma FSH levels are also inconsistent in heat-stressed cattle like plasma LH levels described above. Nevertheless, higher FSH concentration could not counter lower LH level, which might decline thecal androgen production resulting in the reduction in E2 synthesis from GCs of dominant follicle in heat-exposed dairy cows (Wilson et al. 1998; Roth et al. 2000). Consequently, lower levels of E2 could not provide sufficient positive feedback on hypothalamic pre-optic nuclei to commence pre-ovulatory LH surge thereby averting ovulation leading to summer infertility in dairy cattle (Mihm et al. 1994; Wolfenson et al. 2000; Benyei et al. 2001; Amundson et al. 2006; Hansen 2007). In another study, ovulation failure in dairy cows was found to be more in warm periods (12.4%) than in cool periods (3.4%) (López-Gatius et al. 2005). Moreover, risk of ovulation failure was 3.9 times greater in dairy cows inseminated during warm period than cool period (López-Gatius et al. 2005). Ovulation failure during warm period might be due to lack of optimum pre-ovulatory LH surge in heat-stressed cows. Ovulation failure could also be due to longer duration of dominance of pre-ovulatory follicle in heat-stressed heifer (Mihm et al. 1994). Additionally, it has been investigated that lower plasma LH level reduces conception rate by 10–20% in heat-stressed dairy cows (Cavestany et al. 1985; Collier et al. 2006; Schuller et al. 2017), which could possibly due to failure in ovulation.
Luteal cell steroidogenesis culminates in the production of P4 which is the key for establishment and maintenance of pregnancy in bovine species (Mishra and Palai 2014a). Significant variations in plasma P4 concentrations were noticed in heat-stressed cattle. Plasma P4 level did not differ in heat-stressed cattle (Abilay et al. 1975; Wilson et al. 1998; Roth et al. 2000; Guzeloglu et al. 2001). No change in plasma P4 level in heat-stressed cattle could be due to multiple factors such as variation in breed types, variations in intensity of heat stress and difference in the physiological stage of cattle at the time of heat exposure. On the contrary, plasma P4 level was increased in cattle during heat stress (Abilay et al. 1975a; Vaught et al. 1977; Trout et al. 1998). For the report by Abilay et al., 1975a), it could be speculated that lower plasma cortisol level in Guernsey cows during chronic heat stress at 33.5°C might have abolished the negative feedback of cortisol on hypothalamic nuclei resulting in higher P4 level. In the study by Vaught et al. (1977), higher P4 level could induce alveolar growth and development in the mammary gland of heat-stressed lactating HF cows in Arizona. Same explanation could be applicable for higher P4 level in lactating HF cows subjected to heat stress at 38.3°C (Trout et al. 1998). Several authors also reported lower plasma P4 levels in dairy cows subjected to summer heat stress (Younas et al. 1993; Howell et al. 1994; Ronchi et al. 2001; Schuller et al. 2017). Just recently, Khan et al. (2020) found documented significant decline in P4 secretion in heat-stressed culture bovine GCs at 41°C (1.25 ng/ml) compared to control at 38°C (4.25 ng/ml). The best possible explanation could be lower LH levels during heat stress which might have reduced P4 secretion from corpus luteum. In addition, heat stress might reduce the luteal steroidogenesis by downregulating the expression of key steroidogenic enzymes like StAR, CYP11A1 and 3βHSD resulting in lower P4 secretion in heat-stressed cattle. Furthermore, Al-Katanani et al. (1999) reported that summer infertility was prevalent in high yielding lactating HF cows of Florida which could be attributed to lower P4 level during summer heat stress. Report of Bridges et al. (2005) had shown that elevated temperature reduces pulsatile secretion of LH resulting in premature luteinization thereby truncate fertility in dairy cows. Comparably, high environmental temperature inhibits corpus luteum activity and functions thereby reducing P4 production which negatively affects embryonic growth and development leading to early embryonic mortality (EEM) and pregnancy failure (Hansen 2009). Dearth in plasma P4 concentrations during luteal phase of estrous cycle might disable implantation (Mann et al. 1999) resulting in EEM in heat-stressed dairy cattle (Ahmad et al. 1995). It has been reported that heat stress shrinks peri-implantation period thereby increase EEM by 7.8% between days 34 to 45 of gestation in dairy cattle (Garcia-Ispierto et al. 2006). Taken together, low P4 might diminish endometrial function, implantation and embryonic growth and development culminating in EEM in heat-stressed dairy cows. Moreover, it could be inferred that lower secretion of interferon tau from embryonic trophoblasts might fail to send signal for MRP in heat-stressed cows resulting in EEM. In another study, pregnancy rate noticeably declined during summer (21%) than winter (36%) season, after 25 to 35 days of insemination (Ryan et al. 1993). Moreover, pregnancy rate reduced by 3.2% for each unit increase in THI beyond 70 while reduced by 3.5% for each degree increase in environmental temperature above 23.4°C (Amundson et al. 2005). Further, in beef cattle, pregnancy rate was noticed to be reduced by 62% on exposure to THI beyond 72.9 (Amundson et al. 2006). Reduction in pregnancy rate in beef cows could be due to premature luteolysis caused by increased level of uterine PGF2α during heat stress. In another study, PGF2α secretion was increased from cultured bovine endometrium when subjected to heat stress at 43°C for 18 h (Putney et al. 1988). It has been shown that, heat stress upregulates endometrial PGF2α secretion which might induce premature luteolysis resulting in pregnancy failure (Putney et al. 1989; Aggarwal and Upadhyay 2013). Gestation period was found to be 4 days shorter in heat-stressed cows compared to those cows kept under cool environment (Tao et al. 2012). Tao et al. (2012) also observed lower body weight in calves born from heat-stressed cows than those born from cool cows. This could be explained by the fact that heat stress might decline the secretion of pregnant oestrogen from the conceptus leading to diminish placental function resulting in lower birth weight of newborn calves. This might be due to different repertoire of various hormones in heat-stressed dam. It is plausible to presume that reduction in uterine blood flow in heat-stressed dam might have some role in lowering of body weight of newborn calves. It has been shown that rise in uterine temperature by 0.5°C than normal reduces fertility rate in dairy cattle (Gwazdauskas et al. 1973). Heat stress tends to upregulate intra-uterine temperature which could reduce uterine blood flow (Gwazdauskas et al. 1975; Roman-Ponce et al. 1978) resulting in impaired uterine functions (Wolfenson et al. 2000) thereby depressing embryonic growth and development (Ealy et al. 1995; Rivera and Hansen 2001; Gendelman et al. 2010) leading to EEM (Biggers et al. 1987; Ryan et al. 1992). Collectively, lower plasma gonadotropin and gonadal steroid level declines oocyte as well as embryo competence in heat-stressed dairy cows. Lower gonadotropin levels could be attributed to higher prolactin which might suppress follicular development thereby failing to secrete required gonadal steroids leading to summer anestrus in dairy cows (Lupoli et al. 2001). Moreover, Alamer (2011) reviewed that higher prolactin during heat stress may lead to summer infertility in domestic ruminants. Different authors had also observed the detrimental effect of ROS on oocyte competence. It has been seen that heat stress elevates ROS production in oocytes (Nabenishi et al. 2012; Cavallari de Castro et al. 2019) and embryos (Sakatani et al. 2008; Ortega et al. 2016) thereby negatively affecting their growth and development (Putney et al. 1989; Roth 2018). Latest research work by Khan et al. (2020) revealed that ROS production was significantly increased in heat-stressed bovine GCs at 40°C than control at 38°C. Accumulation of ROS in heat-stressed bovine GCs might induce GC apoptosis which ultimately ceases GC steroidogenesis. It has been noticed that lactating cattle are more prone to the harmful effects of heat stress than heifers (Badinga et al. 1985). This could be due to the fact that lactation generates more metabolic heat production which makes it more strenuous for lactating cows to maintain homeostasis during heat stress making them more susceptible to heat stress than non-lactating heifers (Sartori et al. 2002).
Like in females, heat stress alters male steroidogenesis, spermatogenic cycle and spermatogenesis (Rahman et al. 2018). There are mixed views regarding plasma testosterone level across different seasons. In Hereford bulls, plasma testosterone level was significantly reduced during the first 2 weeks and restored to normal after the 7th week of heat stress at 35.5°C (Rhynes and Ewing 1973), which could be due to acclimation of Hereford bulls to chronic heat stress. Hansen (2009) found lower LH secretion in bulls during summer heat stress, which might be due to lower LH during heat stress and consequently lower LH could have depressed testosterone secretion from Leydig cells. In another study, plasma testosterone level was increased from 5.7 ng/ml during fall to 8.0 ng/ml during spring season in HF bulls (Foote et al. 1976). Slight cold stress during fall might have depressed hypothalamic GnRH neurons thereby reduce testosterone level. Antithetical to previous findings, testosterone secretion was significantly increased in summer and spring seasons compared to that in winter season in bulls treated with bovine LH (Jiménez-Severiano et al. 2003). This could be attributed to the stimulatory effect of exogenous LH on Leydig cells which might have augmented testosterone production. Moreover, Sayah et al. (2019) observed the highest serum testosterone level in HF bulls exposed to summer season (2.45 ng/dl) while lowest during winter season (1.41 ng/dl). This might be due to difference in temperament of HF bulls in response to summer heat stress. On the contrary, plasma FSH and LH levels did not differ in HF or Angus bulls during exposure to hyperthermia at 35°C (Schams et al. 1980). In another study, serum testosterone level did not change significantly in Angus bulls exposed to ambient temperature at 34°C (Minton et al. 1981). It could be assumed that the intensity of heat stress upon exposure to ambient temperature at 34°C might not have affected the hypothalamic GnRH neurons to alter the gonadotropin level as well as the testosterone level in Angus bulls. In addition, variations in plasma testosterone level could be due to difference in breeds, magnitude of heat stress and difference in the thermal adaptation strategies in response to heat stress. Seminal parameters such as ejaculation volume, sperm concentration, mass motility, progressive motility and live sperm percent were decreased while sperm abnormalities were increased in bulls during summer season (Nichi et al. 2006; Sayah et al. 2019). Meyerhoeffer et al. (1985) detected higher numbers of abnormal and aged spermatozoa during summer stress compared to winter and spring. In addition, Mishra et al. (2013) observed lower live sperm count, acrosome integrity and HOST in different breeds of cattle exposed to extreme ambient temperature and concluded that elevated environmental temperature severely affects membrane integrity of spermatozoa. Taken together, summer heat stress downregulates testosterone production thereby impeding production of competent spermatozoa resulting in impaired spermatogenesis (Vogler et al. 1993; Kastelic et al. 1996; Rahman et al. 2018).
Molecular responses of cattle against heat stress
Molecular responses serve as the most dominant mechanism to resist the menace of heat stress in cattle. It has been well established that molecular responses are manifested via expression pattern of conserved family of proteins known as heat shock proteins (HSPs) (Mishra and Palai 2014; Bharati et al. 2017a). Almost all cellular systems such as skeletal myocytes, hepatocytes, lung cells, kidney cells, adipocytes, cardio-myocytes, skin fibroblast cells, aortic endothelial cells, mammary epithelial cells and granulosa cells express plethora of HSPs to counteract the negative effects of heat stress in cattle (Shandilya et al. 2020; Mishra 2020). However, PBMCs have shown tremendous expression of HSPs and therefore considered the standard model to evaluate the differential expression of various HSPs during heat stress (Mishra 2021). All the HSPs are subdivided according to their molecular weight like small HSPs and large HSPs (Sahu et al. 2019). HSPs whose molecular weight are less than 40 kDa are considered small HSPs whereas HSPs whose molecular weight are more than 40 kDa are considered large HSPs (Mishra 2021). Heat shock factors (HSFs) play a major role in the synthesis of HSPs in different cellular systems in heat-stressed cattle. Upon heat stress, HSF forms trimer and then HSF trimer translocates into the nucleus where it binds with heat shock response elements of the DNA to generate HSPs. It had been shown that the transcription of different HSFs was escalated in cattle during heat stress (Kolli et al. 2014; Kumar et al. 2015; Khan et al. 2020). Reports from various studies indicated that the mRNA expression of small HSPs such as HSP10 (Kumar et al. 2015) and HSP27 (Baek et al. 2019; Kim et al. 2020) was significantly upregulated in multiple cellular systems of heat-stressed cattle. Identically, multiple studies have also shown hyper-transcription of large HSPs like HSP40 (Shandilya et al. 2020), HSP60 (Pires et al. 2019), HSP70 (Khan et al. 2020), HSP72 (Zhang et al. 2014) and HSP90 (Kim et al. 2020) in different cellular systems in heat-shocked cattle. More details regarding differential expression pattern of the aforementioned HSPs are presented in Table 4. In addition, significant increase in the concentrations of various HSPs across different cattle breeds under heat stress is presented in Table 5. HSPs act as molecular chaperone to restore proteostasis thereby alleviating the harmful effects of heat stress in cattle. Furthermore, HSPs play a vital role as a cyto-protective molecule by inhibiting caspase-3 expression during heat stress (Palai and Mishra 2015). Therefore, HSPs are thought to be involved in the maintenance of cellular integrity and homeostasis in cattle during heat stress. Moreover, zebu cattle have stronger thermo-tolerance ability than crossbred and exotic cattle as they display lower expression of HSPs compared to their counterparts during heat stress.
Conclusion
Livestock growth and production have been jeopardised by perils of heat stress across the world. Sudden reduction in DMI and increase in WI along with spike in rectal temperature, respiration rate, heart rate and sweating rate in combination with higher plasma concentrations of various anti-stress hormones like cortisol and catecholamines could be considered an ideal indicator to confirm heat stress in cattle. Thus, behavioural, physiological and neuro-endocrine responses serve as the immediate weapons to counteract the hostile effects of heat stress. Moreover, overexpression of HSP27, HSP70 and HSP90 further validates heat stress in cattle. Therefore, HSPs are considered the molecular marker to evaluate the magnitude of heat stress in cattle. Moreover, molecular responses act synergistically with the earlier responses to ameliorate the detrimental effects of heat stress in cattle. Therefore, further research investigations are warranted to explore the neuro-endocrine signalling pathways and the possible interactions amongst various neuro-endocrine hormones in vivo along with the nexus amongst various HSPs during heat stress to have a deep insight into the mechanisms of thermal adaptation in cattle.
Data availability
Not applicable
Abbreviations
- 3βHSD:
-
3-Beta-hydroxysteroid dehydrogenase
- ACTH:
-
Adreno-corticotropic hormone
- ADH:
-
Anti-diuretic hormone
- ARC:
-
Arcuate nuclei
- BGTHI:
-
Black globe temperature humidity index
- CART:
-
Cocaine and amphetamine-regulated transcript
- CBG:
-
Corticosteroid-binding globulin
- CYP11A1:
-
Cholesterol side-chain cleavage enzyme
- CYP19A1:
-
Aromatase enzyme
- CRH:
-
Corticotropin-releasing hormone
- DMI:
-
Dry matter intake
- E2 :
-
17β-estradiol
- EEM:
-
Early embryonic mortality
- ETI:
-
Equivalent temperature index
- FSH:
-
Follicle-stimulating hormone
- GCs:
-
Granulosa cells
- GH:
-
Growth hormone
- GHRH:
-
Growth hormone–releasing hormone
- GnRH:
-
Gonadotropin-releasing hormone
- HF:
-
Holstein Friesian
- HLI:
-
Heat load index
- HOST:
-
Hypo-osmotic swelling test
- HPA:
-
Hypothalamo-pituitary adrenal axis
- HPG:
-
Hypothalamo-pituitary gonadal axis
- HPT:
-
Hypothalamo-pituitary thyroid axis
- HR:
-
Heart rate
- HRV:
-
Heart rate variability
- HSFs:
-
Heat shock factors
- HSPs:
-
Heat shock proteins
- IGF-I:
-
Insulin-like growth factor I
- IGF-II:
-
Insulin-like growth factor II
- IPCC:
-
Inter-governmental panel on climate change
- KF:
-
Karan Fries
- LH:
-
Luteinizing hormone
- LHA:
-
Lateral hypothalamic area
- MRP:
-
Maternal recognition of pregnancy
- PBMCs:
-
Peripheral blood mononuclear cells
- POMC:
-
Proopiomelanocortin
- P4 :
-
Progesterone
- PGF2α:
-
Prostaglandin F2α
- PR:
-
Pulse rate
- PVN:
-
Para-ventricular nuclei
- ROS:
-
Reactive oxygen species
- RR:
-
Respiration rate
- RT:
-
Rectal temperature
- SAM:
-
Sympathetic adrenal medullary axis
- SON:
-
Supra-optic nuclei
- SR:
-
Sweating rate
- ST:
-
Skin temperature
- StAR:
-
Steroidogenic acute regulatory protein
- T3 :
-
Tri-iodo thyronine
- T4 :
-
Thyroxine
- THI:
-
Temperature humidity index
- TNZ:
-
Thermo-neutral zone
- TRH:
-
Thyrotropin-releasing hormone
- TSH:
-
Thyroid-stimulating hormone
- UCT:
-
Upper critical temperature
- WI:
-
Water intake
References
Abdel-Samee, A.M., Habeeb, A.A.M., Kamal, T.H., Abdel-Razik, M.A., 1989. The role of urea and mineral mixture supplementation in improving productivity of heat-stressed Friesian calves in the sub-tropics. Proceedings of 3rd Egyptian-British Conference on Animal, Fish and Poultry Production, Alexandria University, Egypt, 2, 637-641.
Abilay, T.A., Mitra, R., Johnson, H.D., 1975. Plasma cortisol and total progestin levels in Holstein steers during acute exposure to high environmental temperature (42°C) conditions, Journal of Animal Science, 41, 113-118.
Abilay, T.A., Johnson, H.D., Madam, L.M., 1975a. Influence of environmental heat on peripheral plasma progesterone and cortisol during the bovine oestrus cycle, Journal of Dairy Science, 58, 1836-1840.
Aengwanich, W., Kongbuntad, W., Boonsorn, T., 2011. Effects of shade on physiological changes, oxidative stress, and total antioxidant power in Thai Brahman cattle, International Journal of Biometeorology, 55, 741-748.
Afsal, A., Sejian, V., Bagath, M., Krishnan, G., Devaraj, C., Bhatta, R., 2018. Heat Stress and Livestock Adaptation: Neuro-endocrine Regulation, International Journal of Veterinary and Animal Medicine, 1, 108.
Aggarwal, A., Upadhyay, R., 2013. Heat Stress and Hormones (eds) Aggarwal A, Upadhyay R In: Heat Stress and Animal Productivity, 27-51.
Ahmad, N., Schrick, F.N., Butcher, R.L., Inskeep, E.K., 1995. Effect of persistent follicles on early embryonic losses in beef cows, Biology of Reproduction, 52, 1129-1135.
Ahmad, M., Bhatti, J.A., Abdullah, M., Javed, K., Ali, M., Rashid, G., Uddin, R., Badini, A.H., Jehan, M., 2018. Effect of ambient management interventions on the production and physiological performance of lactating Sahiwal cattle during hot dry summer, Tropical Animal Health and Production, 50, 1249-1254.
Ahmed, M.M.M., El-Amin, A.I., 1997. Effect of hot dry summer tropical climate on forage intake and milk yield in Holstein-Friesian and indigenous Zebu cows in Sudan, Journal of Arid Environments, 35, 737-745.
Alamer, M., 2011. The role of prolactin in thermoregulation and water balance during heat stress in domestic ruminants, Asian Journal of Animal and Veterinary Advances, 6, 1153-1169.
Al-Katanani, Y.M., Webb, D.W., Hansen, P.J., 1999. Factors affecting seasonal variation in 90-day non return rate to first service in lactating Holstein cows in a hot climate, Journal of Dairy Science, 82, 2611-2616.
Al-Katanani, Y.M., Paula-Lopes, F.F., Hansen, P.J., 2002. Effect of Season and Exposure to Heat Stress on Oocyte Competence in Holstein Cows, Journal of Dairy Science, 85, 390-396.
Allen, T.E., Bligh, J., 1969. A comparative study of the temporal patterns of cutaneous water vapor loss from some domesticated mammals with epithelial sweat glands, Comparative Biochemistry and Physiology, 31, 347-363.
Allen, J.D., Hall, L.W., Collier, R.J., Smith, J.F., 2015. Effect of core body temperature, time of day, and climate conditions on behavioral patterns of lactating dairy cows experiencing mild to moderate heat stress, Journal of Dairy Science, 98, 118-127.
Alvarez, M.B., Johnson, J.D., 1973. Environmental heat exposure on cattle plasma catecholamine and glucocorticoids, Journal of Dairy Science, 56, 189-194.
Amundson, J.L., Mader, T.L., Rasby, R.J., Hu, Q.S., 2005. Temperature and temperature–humidity index effects on pregnancy rate in beef cattle. In: Proceedings of 17th International Congress on Biometeorology, Deutscher Wetterdienst, Offenbach, Germany.
Amundson, J.L., Mader, T.L., Rasby, R.J., Hu, Q.S., 2006. Environmental effects on pregnancy rate in beef cattle, Journal of Animal Science, 84:3415-3420.
Ansell, R.H., 1981. Extreme Heat Stress in Dairy Cattle and its Alleviation: A Case Report. In: Environmental Aspects of Housing for Animal Protection, Clark, J.A. (Ed.), Butterworths, London, UK, 285-306.
Arias, R.A., Mader, T.L., 2011. Environmental factors affecting daily water intake on cattle finished in feedlots, Journal of Animal Science, 89, 245-251.
Aritonang, S.B., Yuniati, R., Abinawanto, Imron M., Bowolaksono A., 2017. Physiology Response of the Indigenous Cattle Breeds to the Environment in West Sumbawa, Indonesia, AIP Conference Proceedings 1862, 030098. doi: https://doi.org/10.1063/1.4991202.
Attenberry, J.T., Johnson, H.D., 1969. Effect of environmental temperature, controlled feeding and fasting on rumen motility, Journal of Animal Science, 29, 734-737.
Badinga, L., Collier, R.J., Thatcher, W.W., Wilcox, C.J., 1985 Effects of climatic and management factors on conception rate of dairy cattle in subtropical environment, Journal of Dairy Science, 68, 78-85.
Badinga, L., Thatcher, W.W., Diaz, T., Drost, M., Wolfenson, D., 1993. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows, Theriogenology, 39, 797-810.
Baek, Y.C., Kim, M., Jeong, J.Y., Oh, Y.K., Lee, S.D., Lee, Y.K., Ji, S.Y., Choi, H., 2019. Effects of short term acute heat stress on physiological responses and heat shock proteins of Hanwoo steer (Korean cattle), Journal of Animal Reproduction and Biotechnology, 34, 173-182.
Banerjee, D., Ashutosh., 2011. Effect of thermal exposure on diurnal rhythms of physiological parameters and feed, water intake in Tharparkar and Karan Fries heifers, Biological Rhythm Research, 42, 39-51.
Baumgard, L.H., Rhoads, R.P., 2013. Effects of heat stress on post absorptive metabolism and energetic, Annual Review of Animal Biosciences, 1, 311-337.
Beatty, D.T., Barnes, A., Taylor, E., Pethick, D., McCarthy, M., Maloney, S.K., 2006. Physiological responses of Bos taurus and Bos indicus cattle to prolonged, continuous heat and humidity, Journal of Animal Science, 84, 972-985.
Beede, D.K., Collier, R.J., 1986. Potential nutritional strategies for intensively managed cattle during thermal stress, Journal of Animal Science, 62, 543-554.
Benyei, B., Gaspard, A., Barros, C.W.C., 2001. Changes in embryo production results and ovarian recrudescence during the acclimation to the semiarid tropics of embryo donor Holstein-Frisian cows raised in a temperate climate, Animal Reproduction Science, 68, 57-68.
Bernabucci, U., Bani, P., Ronchi, B., Lacetera, N., Nardone, A., 1999. Influence of short- and long-term exposure to hot environment on rumen passage rate and diet digestibility by Friesian heifers, Journal of Dairy Science, 82, 967-973.
Bernabucci, U., Lacetera, N., Basirico, L., Ronchi, B., Morera, P., Seren, E., Nardone, A., 2006. Hot season and BCS affect leptin secretion in periparturient dairy cows, Journal of Dairy Science, 89, 348.
Bernabucci, U., Lacetera, N., Baumgard, L.H., Rhoads, R.P., Ronchi, B., Nardone, A., 2010. Metabolic and hormonal acclimation to heat stress in domesticated ruminants, Animal, 4, 1167-1183.
Bhan, C., Singh, S.V., Hooda, O.K., Upadhyay, R.C., Mangesh, V., 2012. Influence of temperature variability on physiological, hematological and biochemical profile of growing and adult Sahiwal cattle, Journal of Environmental Research and Development, 7, 986-994.
Bhan, C, Singh, S.V., Hooda, O.K., Upadhyay, R.C., Beenam, B., 2013. Influence of temperature variability on physiological, haematological and biochemical profiles of growing and adult Karan Fries cattle, Indian Journal of Animal Science, 83, 1090-1096.
Bharati, J., Dangi, S.S., Mishra, S.R., Chouhan, V.S., Verma, V., Shankar, O., Bharti, M.K., Paul, A., Mahato, D.K., Rajesh, G., Singh, G., Maurya, V.P., Bag, S., Kumar, P., Sarkar, M., 2017. Expression analysis of Toll like receptors and interleukins in Tharparkar cattle during acclimation to heat stress exposure, Journal of Thermal Biology, 65, 48-56.
Bharati, J., Dangi, S.S., Chouhan, V.S., Mishra, S.R., Bharti, M.K., Verma, V., Shankar, O., Yadav, V.P., Das, K., Paul, A., Bag, S., Maurya, V.P., Singh, G., Kumar, P., Sarkar, M., 2017a. Expression dynamics of HSP70 during chronic heat stress in Tharparkar cattle, International Journal of Biometeorology, 61, 1017-1027.
Bianca, W., 1962. Relative importance of dry and wet bulb temperatures in causing heat stress in cattle, Nature, 195, 251-252.
Biggers, B.G., Buchanan, D.S., Geisert, R.D., Wetteman, R.P., 1987. Effects of Heat Stress on Early Embryonic Development in the Beef Cow, Journal of Animal Science, 64, 1512-1518.
Boehmer, B.H., Pye, T.A., Wettemann, R.P., 2015. Ruminal temperature as a measure of body temperature of beef cows and relationship with ambient temperature, The Professional Animal Scientist, 31, 387-393.
Bond, T.E., Kelly, C.F., Ittner, N.R., 1954. Radiation studies of painted shade materials, Agricultural Engineering, 36, 389-392.
Boni, R., 2019. Heat stress, a serious threat to reproductive function in animals and humans, Molecular Reproduction and Development, 86, 1307-1323.
Breen, K.M., Karsch, F.J., 2006. New insights regarding glucocorticoids, stress and gonadotropin suppression, Frontiers in Neuroendocrinology, 27, 233-245.
Bridges, P.J., Brusie, M.A., Fortune, J.E., 2005. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles, Domestic Animal Endocrinology, 29, 508-522.
Brown-Brandl, T.M., Nienaber, J.A., Eigenberg, R.A., Hahn, G.L., Freetly, H., 2003. Thermoregulatory responses of feeder cattle, Journal of Thermal Biology, 28, 149-157.
Brown-Brandl, T.M., Eigenberg, R.A., Hahn, G.L., Nienaber, J.A., Mader, T.L., Spiers, D.E., Parkhurst, A.M., 2005. Analyses of thermoregulatory responses of feeder cattle exposed to simulated heat waves, International Journal of Biometeorology, 49, 285-296.
Bulbul, B., Ataman, M.B., 2009. The effect of some seasonal conditions on estrus occurrence in cows, Archiv Tierzucht, 52, 459-465.
Cardoso, C., Peripolli, V., Amador, S., Brandao, E., Esteves, G., Sousa, C., Franca, M., Gonçalves, F., Barbosa, F., Montalvao, T., 2015. Physiological and thermographic response to heat stress in zebu cattle, Livestock Science, 182, 83-92.
Cavallari de Castro, F., Leal, C.L.V., Roth, Z., Hansen, P.J., 2019. Effects of melatonin on production of reactive oxygen species and developmental competence of bovine oocytes exposed to heat shock and oxidative stress during in vitro maturation, Zygote, 27, 180-186.
Cavestany, D., El-Whishy, A.B., Foot, R.H., 1985. Effect of season and high environmental temperature on fertility of Holstein cattle, Journal of Dairy Science, 68, 1471-1478.
Chaiyabutr, N., Chanpongsang, S., Suadsong, S., 2008. Effects of evaporative cooling on the regulation of body water and milk production in crossbred Holstein cattle in a tropical environment, International Journal of Biometeorology, 52, 575-585.
Chaiyabutr, N., Chanchai, W., Boonsanit, D., Sitprija, S., Chan-pongsang, S., 2011. Different responses of oxidative stress index in the plasma of crossbred Holstein cattle during cooling and supplemental recombinant bovine somatotropin, Journal of Animal and Veterinary Advances, 10, 1045-1053.
Chakravarthi, K., Bidarkar, D.K., Ramesh Gupta, B., Rao, G.N., Sudhakar, K., Babu Rao, K., 2004. Drought performance of Ongole bulls under thermal stress conditions, Indian Journal of Animal Science, 74, 119-121.
Chen, S., Wang, J., Peng, D., Li, G., Chen, J., Gu, X., 2018. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows, Scientific Reports, 8, 14606.
Church, J.S., Hegadoren, P.R., Paetkau, M.J., Miller, C.C., Regev-Shoshani, G., Schaefer, A.L., Schwartzkopf-Genswein, K.S., 2014. Influence of environmental factors on infrared eye temperature measurements in cattle, Research in Veterinary Science, 96, 220-226.
Coimbra, P.A.D., Filho, L.C.P.M., Hotzel, M.J., 2012. Effects of social dominance, water trough location and shade availability on drinking behaviour of cows on pasture, Applied Animal Behaviour Science, 139, 175-182.
Collier, R.J., Gebremedhin, K.G., 2015. Thermal biology of domestic animals, Annual Review of Animal Biosciences, 3, 513-532.
Collier, R.J., Beede, D.K., Thatcher, W.W., Israel, L.A., Wilcox, C.J., 1982. Influences of environment and its modification on dairy animal health and production, Journal of Dairy Science, 65, 2213-2227.
Collier, R.J., Dahl, G.E., Vanbaale, M.J., 2006. Major advances associated with environmental effects in dairy cattle, Journal of Dairy Science, 89, 1244-1253.
Collier, R.J., Renquist, B.J., Xiao, Y., 2017. A 100-Year Review: Stress physiology including heat stress, Journal of Dairy Science, 100, 10367-10380.
Collier, R.J., Baumgard, L.H., Zimbelman, R.B., Xiao, Y., 2019. Heat stress: physiology of acclimation and adaptation, Animal Frontiers, 9, 12-19.
Cunningham, J.G., 2002. Textbook of Veterinary Physiology. 3rd Ed. W. B. Saunders; Philadelphia, PA, USA.
Da Silva, R.G., 1999. Estimate of radiation heat balance of Holstein cows in the sun and under the shade in a tropical environment, Revista Brasileira de Zootecnia, 28, 1403-1411.
Da Silva, R.G., Maia, A.S.C., 2011. Evaporative cooling and cutaneous surface temperature of Holstein cows in tropical conditions, Revista Brasileira de Zootecnia, 40, 1143-1147.
Das, S., Palai, T.K., Mishra, S.R., Das, D., Jena, B., 2011. Nutrition in relation to diseases and heat stress in poultry, Veterinary World, 4, 429-432.
Das, S., Mishra, S.K., Swain, R.K., Mohanty, D.N., Mishra, S.R., 2012. Comparative study of certain serum biochemical parameters in anoestrus and repeat breeding cows of Bhadrak district of Orissa, Indian Journal of Field Veterinarians, 7, 71.
Das, R., Sailo, L., Verma, N., Bharti, P., Saikia, J., Mtiwati, I., Kumar, R., 2016. Impact of heat stress on health and performance of dairy animals: A review, Veterinary World, 9, 260-268.
De Andrade Ferrazza, R., Garcia, H.D.M, Aristizabal, V.H.V., de Souza Nogueira, C., Verissimo, C.J., Sartori, J.R., Sartori, R., Ferreira, J.C.P., 2017. Thermoregulatory responses of Holstein cows exposed to experimentally induced heat stress, Journal of Thermal Biology, 66, 68-80.
De la Hoya, M.P.G., Toca Ramirez, J.A., Contreras, P.R., Diaz, C.E.P, Saucedo, F.O.R., Saucedo, J.S.Q., 2015. Role of leptin gene in cattle production: review, Journal of Animal and Veterinary Advances, 14, 81-90.
De Rensis, F., Scaramuzzi, R.J., 2003. Heat stress and seasonal effects on reproduction in the dairy cow - a review, Theriogenology, 60, 1139-1151.
Djokovic, R., Samanc, H., Bojkovski, J., Fratric, N., 2010. Blood concentrations of thyroid hormones and lipids of dairy cows in transitional period, Lucrări Ştiinłifice Medicină Veterinară, 43, 34-40.
Do Amaral, B.C., Connor, E.E., Tao, S., Hayen, M.J., Bubolz, J.W., Dahl G.E., 2011. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows, Journal of Dairy Science, 94, 86-96.
Ealy, A.D., Arechiga, C.F., Howell, J.L., Hansen, P.J., Monterroso, V.H., 1995. Developmental changes in sensitivity of bovine embryos to heat shock and use of antioxidants as thermoprotectants, Journal of Animal Science, 73, 1401-1407.
Eigenberg, R.A., Brown-Brandl, T.M., Nienaber, J.A., 2009. Shade material evaluation using a cattle response model and meteorological instrumentation, International Journal of Biometeorology, 53, 501-507.
El-Koja, M.N., Belyea, R.L., Johnson, H.D., Marts, F.A., 1980. Effect of environmental temperature and deity fiber level upon intake, milk production and nutrient utilization of lactating dairy cows, American Journal of Dairy Science, 5, 63-85.
El-Nouty, F.D., Elbanna, I.M., Davis, T.P., Johnson, H.D., 1980. Aldosterone and ADH response to heat and dehydration in cattle, Journal of Applied Physiology, 48, 249-255.
Falkenberg, S.M., Ridpath, J., Vander Ley, B., Bauermann, F.V., Sanchez, N.B., Carroll, J.A., 2014. Comparison of temperature fluctuations at multiple anatomical locations in cattle during exposure to bovine viral diarrhea virus, Livestock Science, 164:159-167.
Fekete, C., Lechan, R.M., 2013. Central regulation of hypothalamic pituitary-thyroid axis under physiological and pathophysiological conditions, Endocrine Reviews, 35, 159-194.
Finch, V.A., 1985. Comparison of non-evaporative heat transfer in different cattle breeds, Australian Journal of Agricultural Research, 36, 497-508.
Finch, V.A., Bennett, I.L, Holmes, C.R., 1984. Coat color in cattle: effect of thermal balance, behaviour, and growth and relationship with coat type, The Journal of Agricultural Science, 102, 141-147.
Findlay, J.D., 1958. Physiological Reactions of Cattle to Climatic Stress, Proceedings of Nutrition Society, 17, 186-190.
Fisher, A.D., Roberts, N., Matthews, L.R., 2002. Shade: Its use by livestock and effectiveness at alleviating heat challenge, Report to MAF Policy, New Zealand.
Foote, R.H., Munkenbeck, N., Greene, W.A., 1976. Testosterone and libido in holstein bulls of various ages, Journal of Dairy Science, 59, 2011-2013.
Fregonesi, J.A., Leaver, J.D., 2001. Behaviour, performance and health indicators of welfare for dairy cows housed in strawyard or cubicle systems, Livestock Production Science, 68, 205-216.
Gangawar, P.C., Branton, C., Evans, D.L., 1965. Reproductive and physiological responses Holstein heifers to control and natural climate, Journal of Dairy Science, 48, 222-227.
Gantner, V., Mijic, P., Kuteroval, K., Solic, D., Gantner, R., 2011. Thermal humidity index values and their significance on daily production of dairy cattle, Mljekarstvo, 61, 56-63.
Garcia, M.R., Amstalden, M., Williams, S.W., Stanko, R.L., Morrison, C.D., Keisler, D.H., Nizielski, S.E., Williams, G.L., 2002. Serum leptin and its adipose gene expression during pubertal development, the estrous cycle, and different seasons in cattle, Journal of Animal Science, 80, 2158-2167.
Garcia-Ispierto, I., Lopez-Gatius, F., Santolaria, P., Yaniz, J.L., Nogareda, C., Lopez-Bejar, M., De Rensis, F., 2006. Relationship between heat stress during the peri-implantation period and early fetal loss in dairy cattle, Theriogenology, 65, 799-807.
Garner, J.B., Douglas, M., Williams, S.R.O., Wales, W.J., Marett, L.C., DiGiacomo, K., Leury, B.J., Hayes, B.J., 2017. Responses of dairy cows to short-term heat stress in controlled-climate chambers, Animal Production Science, 57, 1233-1241.
Gaughan, J.B., Goodwin, P.J., Schoorl, T.A., Young, B.A., Imbeah, M., Mader, T.L., Hall, A., 1998. Shade preferences of lactating Holstein-Friesian cows, Australian Journal of Experimental Agriculture, 38, 17-21.
Gaughan, J.B., Holt, S.M., Hahn, G.L., Mader, T.L., Eigenberg, R., 2000. Respiration rate-is it a good measure of heat stress in cattle?, Asian-Australasian Journal of Animal Science, 13, 329-332.
Gaughan, J.B., Mader, T.L., Holt, S.M., Sullivan, M.L., Hahn, G.L., 2010. Assessing the heat tolerance of 17 beef cattle genotypes, International Journal of Biometeorology, 54, 617-627.
Gaughan, J., Bonner, S., Loxton, I., Mader, T.L., 2013. Effects of chronic heat stress on plasma concentration of secreted heat shock protein 70 in growing feedlot cattle, Journal of Animal Science, 91, 120-129.
Gebremedhin, K.G., Wu, B., 2001. A model of evaporative cooling of wet skin surface and fur layer, Journal of Thermal Biology, 26, 537-545.
Gebremedhin, K.G., Hillman, P.E., Lee, C.N., Collier, R.J., Willard, S.T., Arthington, J.D., Brown-Brandl, T.M., 2008. Sweating rates of dairy cows and beef heifers in hot conditions, American Society of Agricultural and Biological Engineers, 51, 2167-2178.
Gendelman, M., Aroyo, A., Yavin, S., Roth, Z., 2010. Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos, Reproduction, 140, 73-82.
Gilad, E., Meidan, R., Berman, A., Graber, Y., Wolfenson, D., 1993. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows, Journal of Reproduction and Fertility, 99, 315-321.
Grewal, S., Aggarwal, A., 2018. Physiological response of periparturient sahiwal and karan fries cows during hot humid and winter seasons, International Journal of Chemical Studies, 6, 2258-2262.
Guzeloglu, A., Ambrose, J.D., Kassa, T., Diaz, T., Thatcher, M.J., Thatcher, W.W., 2001. Long term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress, Animal Reproduction Science, 66, 15-34.
Gwazdauskas, F.C., Thatcher, W.W., Wilcox, C.J., 1973. Physiological, environmental, and hormonal factors at insemination which may affect conception, Journal of Dairy Science, 56, 873-877.
Gwazdauskas, F.C., Wilcox, C.J., Thatcher, W.W., 1975. Environmental and management factors affecting conception rate in a subtropical climate, Journal of Dairy Science, 58, 88-92.
Gwazdauskas, F.C., Thatcher, W.W., Kiddy, C.A., Pape, M.J., Wilcox, C.J., 1981. Hormonal pattern during heat stress following PGF2alpha-tham salt induced luteal regression in heifers, Theriogenology, 16, 271-285.
Habeeb, A.A.M., 1987. The role of insulin in improving productivity of heat stressed farm animals with different techniques, Ph.D. Thesis, Faculty of Agriculture, Zagazig University, Zagazig, Egypt.
Habeeb, A.A.M., Aboulnaga, A.J., Kamal, T.H., 2001. Heat-induced changes in body water concentration, Ts, cortisol, glucose and cholesterol levels and their relationships with thermo neutral bodyweight gain in Friesian calves, Proceedings of the 2nd International Conference on Animal Production and Health in Semi-arid Areas, 29-31, El-Arish, North Sinai, Egypt, pp. 97-108.
Hammond, A.C., Olson, T.A., Chase. C.C.Jr., Bowers, E.J., Randel, R.D., Murphy, C.N., Vogt, D.W., Tewolde, A., 1996. Heat tolerance in two tropically adapted Bos taurus breeds, Senepol and Romosinuano, compared with Brahman, Angus, and Hereford cattle in Florida, Journal of Animal Science, 74, 295-303.
Hansen, P.J., 2007. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress, Theriogenology, 68, 242-249.
Hansen, P.J., 2009. Effects of heat stress on mammalian reproduction, Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 3341-3350.
Hein, K.G., Allrich, R.D., 1992. Influence of exogenous adrenocorticotropic hormone on estrous behavior in cattle, Journal of Animal Science, 70, 243-247.
Her, E., Wolfenson, D., Flamenbaum, I., Folman, Y., Kaim, M., Berman, A., 1988. Thermal, productive and reproductive responses of high yielding cows exposed to short-term cooling in summer, Journal of Dairy Science, 71, 1085-1092.
Hidalgo, E.I.A., 2009. Responses to heat stress in slick vs. Normal-haired holstein cows, A thesis presented to the graduate school of University of Florida, Florida, USA.
Hillman, P.E., Lee, C.N., Carpenter, J.R., Baek, K.S., Parkhurst, A., 2001. Impact of hair color on thermoregulation of dairy cows to direct sunlight. In: ASAE Annual International Meeting, Sacramento, CA, ASAE. Paper No. 014031.
Howell, J.L, Fuquay, J.W., Smith, A.E., 1994. Corpus luteum growth and function in lactating Holstein cows during spring and summer, Journal of Dairy Science, 77, 735-739.
Hu, H., Zhang, Y., Zheng, N., Cheng, J., Wang, J., 2016. The effect of heat stress on gene expression and synthesis of heat-shock and milk proteins in bovine mammary epithelial cells, Animal Science Journal, 87, 84-91.
Huszenicza, G., Kulcsar, M., Rudas, P., 2002. Clinical endocrinology of thyroid gland function in ruminants, Veterinary Medicine Czech, 47, 199-210.
Igono, M.O., Johnson, H.D., Steevens, B.J., Krause, G.F., Shanklin, M.D., 1987. Physiological, productive, and economic benefits of shade, spray, and fan system versus shade for Holstein cows during summer heat, Journal of Dairy Science, 70, 1069-1079.
Igono, M.O., Johnson, H.D., Steevens, B.J., Hainen, W.A., Shanklin, M.D., 1988. Effect of season on milk temperature, milk growth hormone, prolactin and somatic cell counts of lactating cattle, International Journal of Biometeorology, 32:194-200.
Indu, S., Pareek, A., 2015. A Review: Growth and Physiological Adaptability of Sheep to Heat Stress under Semi-Arid Environment, International Journal of Emerging Trends in Science and Technology, 2, 3188-3198.
Ingraham, R.H., Gillette, D.D., Wagner, W.D., 1974. Relationship of temperature and humidity to conception rate of Holstein cows in subtropical climate, Journal of Dairy Science, 57, 476-481.
Itoh, F., Obara, Y., Rose, M.T., Fuse, H., Hashimoto, H., 1998. Insulin and glucagons secretion in lactating cows during heat exposure, Journal of Animal Science, 76, 2182-2189.
Janzekovic, M., Mursec, B., Janzekovic, I., 2006. Techniques of measuring heart rate in cattle. Tehnicki Vjesnik, 13, 31-37.
Jian, W., Ke, Y., Cheng, L., 2015. Physiological responses and lactation to cutaneous evaporative heat loss in Bos indicus, Bos taurus, and their crossbreds, Asian-Australasian Journal of Animal Science, 28, 1558-1564.
Jiménez-Severiano, H., Quintal-Franco, J., Vega-Murillo, V., Zanella, E., Wehrman, M.E., Lindsey, B.R., Melvin, E.J., Kinder, J.E., 2003. Season of the year influences testosterone secretion in bulls administered luteinizing hormone, Journal of Animal Science, 81, 1023-1029.
Johnson, K.G., Hales, J.R.S., 1983. The microcirculation and sweating in isolated perfused horse and ox skin, Journal of Thermal Biology, 8, 273-277.
Johnson, H.D., Katti, P.S., Hahn, L., Shanklin, M.D., 1988. Short-term heat acclimation effects on hormonal profile of lactating cows, Univ of Missouri Rsch. Bull. No. 1061. Columbia. USA.
Jolly, P.D., McDougall, S., Fitzpatrick, L.A., Macmillan, K.L., Entwhitsle, K., 1995. Physiological effects of under nutrition on postpartum anoestrous in cows, Journal of Reproduction and Fertility. Supplement, 49, 477-492.
Jonsson, N.N., McGowan, M.R, McGuigan, K., Davison, T.M., Hussain, A.M., Kafi, M., Matschoss, A., 1997. Relationship among calving season, heat load, energy balance and postpartum ovulation of dairy cows in a subtropical environment, Animal Reproduction Science, 47, 315-326.
Kadzere, C.T., Murphy, M.R., Silanikove, N., Maltz E., 2002. Heat stress in lactating dairy cows: A review, Livestock Production Science, 77, 59-91.
Kahl, S., Elsasser, T.H., Rhoads, R.P., Collier, R.J., Baumgard, L.H., 2015. Environmental heat stress modulates thyroid status and its response to repeated endotoxin challenge in steers, Domestic Animal Endocrinology, 52, 43-50.
Kamal, T.H., Habeeb, A.A., Abdel-Samee, A.M., Abdel-Razik, M.A., 1989. Supplementation of heat-stressed Friesian cows with urea and mineral mixture and its effect on milk production in the subtropics, In: Proceedings of Symposium on Ruminant Production in the Dry Subtropics: Constraints and Potentials. Cairo, Egypt. EAAP Publ. No.38, Pudoc Sci. Publ., Wageningen. 183-185.
Kamal, R., Dutt, T., Patel, M., Dey, A., Chandran, P.C., Bharti, P.K., Barari, S.K., 2016. Behavioural, biochemical and hormonal responses of heat-stressed crossbred calves to different shade materials, Journal of Applied Animal Research, 44, 347-354.
Kastelic, J.P., Cook, R.B., Coulter, G.H., Saacke, R.G., 1996. Insulating the scrotal neck affects semen quality and scrotal/testicular temperatures in the bull, Theriogenology, 45, 935-942.
Katiyatiya, C.L.F., Bradley, G., Muchenje, V., 2017. Thermotolerance, health profile and cellular expression of HSP90AB1 in Nguni and Boran cows raised on natural pastures under tropical conditions, Journal of Thermal Biology, 69, 85-94.
Kendall, P.E., Nielsen, P.P., Webster J.R., Verkerk G.A., Littlejohn R.P., Matthews L.R., 2006. The effects of providing shade to lactating dairy cows in a temperate climate, Livestock Science, 103, 148-157.
Khan, A., Dou, J., Wang, Y., Jiang, X., Khan, M.Z., Luo, H., Usman, T., Zhu, H., 2020. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells, Journal of Animal Science and Biotechnology, 11, 25.
Khodaei-Motlagh, M., Zare Shahneh, A., Masoumi, R., Derensis, F., 2013. Alterations in reproductive hormones during heat stress in dairy cattle, African Journal of Biotechnology, 10, 5552-5558.
Kim, K.H., Kim, D.H., Oh, Y.K., Lee, S.S., Lee, H.J., Kim, D.W., Seol, Y.J., Kimura, N., 2010. Productivity and energy partition of late lactation dairy cows during heat exposure, Animal Science Journal, 81, 58-62.
Kim, W.S., Lee, J.S., Jeon, S.W., Peng, D.Q., Kim, Y.S., Bae, M.H., Jo, Y.H., Lee, H.G., 2018. Correlation between blood, physiological and behavioral parameters in beef calves under heat stress, Asian-Australasian Journal of Animal Science, 31, 919-925.
Kim, W.S., Nejad, J.G., Peng, D.Q., Jung, U.S., Kim, M.J., Jo, Y.H., Jo, J.H., Lee, J.S., Lee, H.G., 2020. Identification of heat shock protein gene expression in hair follicles as a novel indicator of heat stress in beef calves, Animal, 14, 1502-1508.
Kim, W.S., Nejad, J.G., Roh, S.G., Lee, H.G., 2020a. Heat-shock proteins gene expression in peripheral blood mononuclear cells as an indicator of heat stress in beef calves, Animals, 10, 895.
Kishore, A., Sodhi, M., Kumari, P., Mohanty, A.K., Sadana, D.K., Kapila, N., Khate, K., Shandilya, U., Kataria, R.S., Mukesh, M., 2014. Peripheral blood mononuclear cells: a potential cellular system to understand differential heat shock response across native cattle (Bos indicus), exotic cattle (Bos taurus), and riverine buffaloes (Bubalus bubalis) of India, Cell Stress and Chaperones, 19, 613-621.
Kishore, A., Sodhi, M., Sharma, A., Shandilya, U.K., Mohanty A., Verma, P., Mann. S., Mukesh, M., 2016. Transcriptional stability of heat shock protein genes and cell proliferation rate provides an evidence of superior cellular tolerance of Sahiwal (Bos indicus) cow PBMCs to summer stress, Journal Veterinary Science, 2, 34-40.
Kleinjan-Elazary, A., Ben-Meir, Y., Gacitua, H., Levit, H., Fridman, A., Shinder, D., Jacoby, S., Miron, J., Halachmi, I., Gershon, E., 2020. Cooling management effects on dry matter intake, metabolic hormones levels and welfare parameters in dairy cows during heat stress, Journal of Dairy Science, 87, 64-69.
Koatdoke, U., PhD Thesis. Khon Kaen University; Khon Kaen, Thailand: 2008. Comparative study on physiological mechanism and cellular responses related to heat tolerance between Bos indicus and Bos taurus.
Koga, A., 2004. Comparison of the thermoregulatory response of buffaloes and tropical cattle, using fluctuations in rectal temperature, skin temperature and haematocrit as an index, The Journal of Agricultural Science, 142, 351-355.
Kohli, S., Atheya, U.K., Thapliyal, A., 2014. Assessment of optimum thermal humidity index for crossbred dairy cows in Dehradun district, Uttarakhand, India, Veterinary World, 7, 916-921.
Kokkonen, T., Taponen, J., Alasuutari, S., Nousiainen, M., Anttila, T., Syrjala-Qvist, L., 2002. Plasma leptin in transition dairy cows. Effects of body fatness, ambient temperature and dietary factors, In: Proc. British Society of Animal Science, York, UK, 92.
Kolli, V., Upadhyay, R.C., Singh, D., 2014. Peripheral blood leukocytes transcriptomic signature highlights the altered metabolic pathways by heat stress in zebu cattle, Research in Veterinary Science, 96, 102-110.
Koubkova, M., Kuizkova, I., Kunc, P., Hartlova, H., Flusser, J., Dolezal, O., 2002. Influence of high environmental temperature and evaporative cooling on some physiological, haematological and biochemical parameters in high yielding dairy cows, Journal of Animal Science, 47, 309-318.
Kumar, S.B.V., Kumar, A., Kataria, M., 2011. Effect of heat stress in tropical livestock and different strategies for its amelioration, Journal of Stress Physiology and Biochemistry, 7, 46-54.
Kumar, A., Ashraf, S., Goud, T.S., Grewal, A., Singh, S.V., Yadav, B.R., Upadhyay, R.C., 2015. Expression profiling of major heat shock protein genes during different seasons in cattle (Bos indicus) and buffalo (Bubalus bubalis) under tropical climatic condition, Journal of Thermal Biology, 51, 55-64.
Kumar, J., Kumar, M., Madan, A.K., Singh, Y., Yadav, B., Anand, M., 2017. Effect of season on physiological parameters and production profile of hariana and sahiwal cattle, Haryana Vetenarian, 56, 69-71.
Kumar, J, Yadav, B, Madan, AK, Kumar, M, Sirohi, R, Reddy, A.V., 2019. Dynamics of heat-shock proteins, metabolic and endocrine responses during increasing temperature humidity index (THI) in lactating Hariana (Zebu) cattle, Biological Rhythm Research, 1-17. doi:https://doi.org/10.1080/09291016.2019.1566986.
Lamp, O., Derno, M., Otten, W., Mielenz, M., Nürnberg, G., Kuhla, B., 2015. Metabolic Heat Stress Adaption in Transition Cows: Differences in Macronutrient Oxidation between Late-Gestating and Early-Lactating German Holstein Dairy Cows, PloS one, 10, e0125264.
Lee, C.N., 1993. Environmental stress effect on bovine reproduction, Veterinary Clinics of North America: Food Animal Practice, 9, 263-273.
Lees, A.M., Sejian, V., Wallage, A.L., Steel, C.C., Mader, T.L., Lees, J.C., Gaughan, J.B., 2019. The impact of heat load on cattle, Animals, 9, 322.
Lefcourt, A.M., Erez, B., Varner, M.A., Barfield, R., Tasch, U., 1999. A non invasive radiotelemetry system to monitor heart rate for assessing stress responses of bovines, Journal of Dairy Science, 82, 1179-1187.
Lenis Sanin, Y., Zuluaga Cabrera, A.M., Tarazona Morales, A.M., 2015. Adaptive responses to thermal stress in mammals, Revista de Medicina Veterinaria, 5, 121-135.
López-Gatius, F., López-Béjar, M., Fenech, M., Hunter, R.H., 2005. Ovulation failure and double ovulation in dairy cattle: risk factors and effects, Theriogenology, 63, 1298-1307.
Lucena, C., Olson, T.A., 2000. Effect of hair coat type on rectal temperatures, milk production and calving interval in Holstein X Carora crossbred cows, In: Proceedings of 10th Congreso Venezolano de Zootecnia, Guanare, Venezuela 84.
Lupoli, B., Johansson, B., Uvnas-Moberg, K., Svennersten-Sjaunja, K., 2001. Effect of suckling on the release of oxytocin, prolactin, cortisol, gastrin, cholecystokinin, somatostatin and insulin in dairy cows and their calves, The Journal of Dairy Research, 68, 175-187.
Mader, T.L., Fell, L.R., McPhee, M.J., 1997. Behavior response of non-Brahman cattle to shade in commercial feedlots, In Proceedings of 5th International Livestock Environment Symposium American Society of Agriculture Engineering St. Joseph, 5, 795-802.
Magdub, A., Johnson, H., Belyea, R., 1982. Effect of environmental heat and dietary fiber on thyroid physiology of lactating cows, Journal of Dairy Science, 65, 2323-2331.
Maia, A.S., Da Silva, R.G., Battiston Loureiro, C.M., 2005. Sensible and latent heat loss from the body surface of Holstein cows in a tropical environment, International Journal of Biometeorology, 50, 17-22.
Maia, A.S.C., Da Silva, R.G., Loureiro, C.M.B., 2008. Latent heat loss of Holstein cows in a tropical environment: a prediction model, Revista Brasileira de Zootecnia, 37, 1837-1843.
Maibam, U., Hooda, O.K., Sharma, P.S., Singh, S.V., Upadhyay, R.C., 2017. Seasonal Change in Oxidative Stress Markers in Blood Plasma of Tharparkar (Bos indicus) and Karan Fries (Bos indicus x Bos taurus) Cattle under Tropical Climate, International Journal of Current Microbiology and Applied Science, 6, 1720-1730.
Maibam, U., Hooda, O., Sharma, P., Mohanty, A.K., Singh, S.V., Upadhyay, R.C., 2017a. Expression of HSP70 genes in skin of zebu (Tharparkar) and crossbred (Karan Fries) cattle during different seasons under tropical climatic conditions. Journal of Thermal Biology, 63, 58-64.
Mann, G.E., Lamming, G.E., Robinson, R.S., Wathes, D.C., 1999. The regulation of interferon-tau production and uterine hormone receptors during early pregnancy. Journal of Reproduction and Fertility, Supplement, 54, 317-328.
Marai, I.F.M., Habeeb, A.A.M., Daader, A.H., Yousef, H.M., 1995. Effects of Egyptian sub-tropical conditions and the heat stress alleviation techniques of water spray and diaphoretics on the growth and physiological functions of Friesian calves, Journal of Arid Environments, 30, 219-225.
Marai, I.F.M., El-Darawany, A.A., Fadiel, A., Abdel-Hafez, M.A.M., 2007. Physiological traits as affected by heat stress in sheep - a review, Small Ruminant Research, 71, 1-12.
Mayengbam, P., Tolenkhomba, T.C., Upadhyay, R.C., 2015. Mn-SOD and Cu, Zn-SOD mRNA expression in relation to physiological indices of Sahiwal and Karan Fries heifers during different temperature humidity indices, Journal of Agrometeorology, 17, 172-178.
McDowell, R.E., Weldy, J.R., 1967. Water Exhcange of cattle under heat stress, In Proceedings of the 3rd International Biometeorological Congress, London, UK, 414-424.
McGuire, M.A., Beede, D.K., Collier, R.J., Buonomo, F.C., Delorenzo, M.A., Wilcox, C.J., Huntington, G.B., Reynolds, C.K., 1991. Effect of acute thermal stress and amount of feed intake on concentrations of somatotropin, insulin-like growth factor I (IGF-I) and IGF-II and thyroid hormones in plasma of lactating dairy cows, Journal of Animal Science, 69, 2050-2056.
Mclean, J.A., 1963. The regional distribution of cutaneous moisture vaporization in the Ayrshire calf, The Journal of Agricultural Science, 61, 275-280.
McMorris, T., 2016. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: lessons from animal studies, Physiology and Behavior, 165, 291-299.
Meyerhoeffer, D.C., Wettemann, R.P., Coleman, S.W., Wells, M.E., 1985. Reproductive criteria of beef bulls during and after exposure to increased ambient temperature, Journal of Animal Science, 60, 352-357.
Mihm, M., Baguisi, A., Boland, M.P., Roche, J.F., 1994. Association between the duration of dominance of the ovulatory follicle and pregnancy rate in beef heifers, Journal of Reproduction and Fertility, 102, 123-130.
Min, L., Cheng, J., Shi, B., Yang, H., Zheng, N., Wang, J., 2015. Effects of heat stress on serum insulin, adipokines AMP-activated protein kinase, heat shock signal molecules in dairy cows, Journal of Zhejiang University Science B, 16, 541-548.
Minton, J.E., Wettemann, R.P., Meyerhoeffer, D.C., Hintz, R.L., Turman, E.J., 1981. Serum luteinizing hormone and testosterone in bulls during exposure to elevated ambient temperature, Journal of Animal Science, 53, 1551-1558.
Mishra, S.R., 2020. Significance of molecular chaperones and micro RNAs in acquisition of thermo-tolerance in dairy cattle, Animal Biotechnology, https://doi.org/10.1080/10495398.2020.1830788.
Mishra, S.R., 2021. Thermoregulatory responses in riverine buffaloes against heat stress: An updated review, Journal of Thermal Biology, 102844.
Mishra, S.R., Palai, T.K., 2014. Importance of HSP70 in Livestock - at cellular level, Journal of Molecular Pathophysiology, 3, 30-32.
Mishra, S.R., Palai, T.K., 2014a. Steroidogenesis in luteal cell: A critical pathway for progesterone production, American Journal of Physiology, Biochemistry and Pharmacology, 3, 170-172.
Mishra, S.R., Palai, T.K., 2014b. Leptin: A Metabolic Regulator of Reproduction in Livestock, Livestock Research International, 2, 75-80.
Mishra, S.R., Kundu, A.K., Mahapatra, A.P.K., 2013. Effect of ambient temperature on membrane integrity of spermatozoa in different breeds of bulls, The Bioscan, 8, 181-183.
Mishra, S.R., Bharati, J., Bharti, M.K., Kar, D., Sahoo, P.R., 2016. Adipokines as metabolic modulators of ovarian functions in livestock: A mini-review, Journal of Advanced Veterinary and Animal Research, 3, 206-213.
Mitra, R., Christison, G.I., Johnson, H.D., 1972. Effect of prolonged thermal exposure on growth hormone (GH) secretion in cattle, Journal of Animal Science, 34, 776-779.
Mohr, E, Langbein, J, Nurnberg, G., 2002. Heart rate variability-A non invasive approach to measure stress in calves and cows, Physiology and Behavior, 75, 251-259.
Morais, D.A.E.F., Maia, A.S.C., Silva, R.G., Vasconcelos, A.M., Lima, P.O., Guilhermino, M.M., 2008. Annual thyroid hormone variation and thermoregulators traits of milk cows in hot environment, Revista Brasileira de Zootecnia, 37, 538-545.
Moretti, R., Biffani, S., Chessa, S., Bozzi, R., 2017. Heat stress effects on Holstein dairy cows’ rumination, Animal, 11, 1-6.
Muller, C.J.C., Botha, J.A., 1993. Effect of summer climatic conditions on different heat tolerance indicators in primiparous Friesian and Jersey cows, South African Journal of Animal Science, 23, 98-103.
Muller, C.J.C., Botha, J.A., Smith, W.A., 1994. Effect of shade on various parameters of Friesian cows in a Mediterranean climate in South Africa. 1. Feed and water intake, milk production and milk composition, South African Journal of Animal Science, 24, 49-55.
Mullick, D.N., 1964. A study on the metabolism of food nutrients in cattle and buffaloes under climatic stress, Arid Zone Research, 14, 137.
Murphy, M.G., Enright, W.J., Crowe, M.A., McConnell, K., Spicer, L.J., Boland, M.P., Roche, J.F., 1991. Effect of dietary intake on pattern of growth of dominant follicles during the oestrus cycle in beef heifers, Journal of Reproduction and Fertility, 92, 333-338.
Nabenishi, H., Ohta, H., Nishimoto, T., Morita, T., Ashizawa, K., Tsuzuki, Y., 2012. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress, Zygote, 20, 249-259.
Naqvi, S.M.K., Kumar, D., Paul, R.K., Sejian, V., 2012. Environmental Stresses and Livestock Reproduction, In: Environmental Stress and Amelioration in Livestock Production. Springer-Verlag, GMbH Publisher, Germany, 97-128.
Nardone, A., Ronchi, B., Lacetera, N., Ranieri, M.S., Bernabucci, U., 2010. Effects of climate changes on animal production and sustainability of livestock systems, Livestock Science, 130, 57-69.
Nebel, R.L., Jobst, S.M., Dransfield, M.B.G., Pandolfi, S.M., Balley, T.L., 1997. Use of radio frequency data communication system Heat Watch, to describe behavioral estrus in dairy cattle, Journal of Dairy Science, 80, 179.
Nichi, M., Bols, P.E.J., Zuge, R.M., Barnabe, V.H., Goovaerts, I.G.F., Barnabe, R.C., Cordata, C.N.M., 2006. Seasonal variation in semen quality in Bos indicus and Bos Taurus bulls raised under tropical conditions, Theriogenology, 66, 822-828.
Nonaka, I., Takusari, N., Tajima, K., Suzuki, H.T., Kurihara, K.M., 2008. Effect of high environmental temperatures on physiological and nutritional status of prepubertal Holstein heifers, Livestock Science, 113, 14-23.
NRC (National Research Council). 1989. Nutrient Requirements of Dairy Cattle. 6th revised edn. National Academy Press, Washington DC, USA.
O’Callagan, D., Boland, M.P., 1999. Nutritional effects on ovulation, embryo development and the establishment of pregnancy in ruminants, Animal Science, 68, 299-314.
O'Brien, M.D., Rhoads, R.P., Sanders, S.R., Duff, G.C., Baumgard, L.H., 2010. Metabolic adaptations to heat stress in growing cattle, Domestic Animal Endocrinology, 38, 86-94.
Omar, E.A., Kirrella, A.K., Fawzy, S.A., El-Keraby, F., 1996. Effect of water spray followed by forced ventilation on some physiological status and milk production of post-calving Friesian cows, Alexander Journal of Agricultural Research, 41, 71-81.
Omidi, A., Kheirie, M., Sarir, H., 2015. Impact of vitamin C on concentrations of thyroid stimulating hormone and thyroid hormones in lambs under short-term acute heat stress, Veterinary Science Development, 5, 103-106.
Ominski, K.H., Kennedy A.D., Wittenberg, K.M., Moshtaghi Nia, S.A., 2002. Physiological and production responses to feeding schedule in lactating dairy cows exposed to short-term, moderate heat stress, Journal of Dairy Science, 85, 730-737.
Ortega, M.S., Rocha-Frigoni, N.A.S., Mingoti, G.Z., Roth, Z., Hansen, P.J., 2016. Modification of embryonic resistance to heat shock in cattle by melatonin and genetic variation in HSPA1L, Journal of Dairy Science, 99, 9152-9164.
Overton, M.W., Sischo, W.M., Temple, G.D., Moore, D.A., 2002. Using time-lapse video photography to assess dairy cattle lying behavior in a free-stall barn. Journal of Dairy Science, 85, 2407-2413.
Padilla, L., Matsui, T., Kamiya, Y., Tanaka, M., Yano, H. 2006. Heat stress decreases plasma vitamin C concentration in lactating cows, Livestock Science, 101, 300-304.
Paes, V.M., Vieira, L.A., Correia, H.H.V., Sa, N.A.R., Moura, A.A.A., Sales, A.D., Rodrigues, A.P.R., Magalhaes-Padilha, D.M., Santos, F.W., Apgar, G.A., Campello, C.C., Camargo, L.S.A., Figueiredo, J.R., 2016. Effect of heat stress on the survival and development of in vitro cultured bovine preantral follicles and on in vitro maturation of cumulus–oocyte complex, Theriogenology, 86, 994-1003.
Palai, T.K., Mishra, S.R., 2015. Caspases: an apoptosis mediator, Journal of Advanced Veterinary and Animal Research, 2, 18-22.
Palkovits, M., 2014. Catecholamines and stress, Ideggyogyaszati Szemle, 67, 89-93.
Parihar, A.S., Jain, P.K., Singh, H.S., Singh, Y.P., Singh, N.P., 1992. Seasonal variations in physiological responses of crossbred calves under different housing system, Indian Journal of Animal Science, 62, 686-688.
Park, J.H., Choi, H.C., Lee, H.J., Kim, E.T., Son, J.K., Kim, D.H., 2019. A Study on the Effect of Temperature-Humidity Index on the Respiration Rate Rectal Temperature and Rumination Time of Lactating Holstein Cow in Summer Season, Journal of the Korea Academia Industrial Cooperation Society, 20, 136-143.
Pereira, A.M.F., Baccari, Jr.F., Titto, E.A.L., Almeida, J.A.A., 2008. Effect of thermal stress on physiological parameters, feed intake and plasma thyroid hormones concentration in Alentejana Mertolenga, Frisian and Limousine cattle breeds, International Journal of Biometeorology, 52, 199-208.
Pires, B.V., Stafuzza, N.B., Lima, S., Lima, S.B.G.P.N.P., Negrão, J.A., Paz, C.C.P., 2019. Differential expression of heat shock protein genes associated with heat stress in Nelore and Caracu beef cattle, Livestock Science, 230, 103839.
Polsky, L., Von Keyserlingk, M.A.G., 2017. Invited review: Effects of heat stress on dairy cattle welfare, Journal of Dairy Science, 100, 8645-8657.
Prasanpanich, S., Semicha, S., Tunsaringkarn, K., Thwaites, C.J., Vajnabukka, C., 2002. Physiological responses of lactating cows under grazing and indoor feeding conditions in the tropics, Journal of Agricultural Science, 138, 341-344.
Putney, D.J., Malayer, J.R., Gross, T.S., Thatcher, W.W., Hansen, P.J., Drost, M., 1988, Heat stress-induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium, Biology of Reproduction, 39, 717-728.
Putney, D.J., Drost, M., Thatcher, W.W., 1989. Influence of summer heat stress on pregnancy rates of lactating dairy cattle following embryo transfer or artificial insemination, Theriogenology, 31, 765-778.
Rahman, M.B., Schellander, K., Luceño, N.L., Van Soom, A., 2018. Heat stress responses in spermatozoa: Mechanisms and consequences for cattle fertility, Theriogenology, 113, 102-112.
Rashamol, V.P., Sejian, V., Bagath, M., Krishnan, G., Archana, P.R., Bhatta, R., 2018. Physiological adaptability of livestock to heat stress: an updated review, Journal of Animal Behaviour and Biometeorology, 6, 62-71.
Rasooli, A., Nouri, M., Khadjeh, G.H., Rasekh, A., 2004. The influence of seasonal variations on thyroid activity and some biochemical parameters of cattle, Iranian Journal of Veterinary Research, 5, 1383-1391.
Reshma, R., Mishra, S.R., Thakur, N., Parmar, M.S., Somal, A., Bharti, M.K., Pandey, S., Chouhan, V.S., Verma, M.R., Singh, G., Sharma, G.T., Maurya, V.P., Sarkar, M., 2016. Modulatory Role of Leptin on Ovarian Functions in Water Buffalo (Bubalus Bubalis), Theriogenology, 86, 1720-1739.
Rhoads, M.L., Rhoads, R.P., VanBaale, M.J., Collier, R.J., Sanders, S.R., Weber, W.J., Crooker, B.A., Baumgard, L.H., 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism and aspects of circulating somatotropin, Journal of Dairy Science, 92, 1986-1997.
Rhoads, R.P., Baumgard L.H., Suagee, J.K., Sanders, S.R., 2013. Nutritional interventions to alleviate the negative consequences of heat stress, Advances in Nutrition, 4, 267-276.
Rhynes, W.E., Ewing, L.L., 1973. Testicular endocrine function in Hereford bulls exposed to high ambient temperature. Endocrinology, 92, 509-515.
Riedel, W., Layka, H., Neeck, G., 1998. Secretory pattern of GH, TSH, thyroid hormones, ACTH, cortisol, FSH, and LH in patients with fibromyalgia syndrome following systemic injection of the relevant hypothalamic-releasing hormones, Zeitschrift für Rheumatologie, 57, 81-87.
Rivera, R.M., Hansen, P.J., 2001. Development of cultured bovine embryos after exposure to high temperatures in the physiological range, Reproduction, 121, 107-115.
Roman-Ponce, H., Thatcher, W.W., Buffington, D.E., Wilcox, C.J., Van Horn, H.H., 1977. Physiological and production responses ofdairy cattle to a shade structure in a subtropical environment, Journal of Dairy Science, 60, 424-430.
Roman-Ponce, H., Thatcher, W.W., Canton, D., Barron, D.H., Wilcox, C.J., 1978. Thermal stress effects on uterine blood flow in dairy cows, Journal of Animal Science, 46, 175-180.
Roman-Ponce, H., Thatcher, W.W., Wilcox, C.J., 1981. Hormonal interrelationships and physiological responses of lactating dairy cows to shade management system in a subtropical environment, Theriogenology, 16, 139-154.
Roman-Ponce, H., Thatcher, W.W., Collier, R.J., Wilcox, C.J., 1981a. Hormonal responses of lactating dairy cattle to TRH and ACTH in a shade management system within a subtropical environment, Theriogenology, 16, 131-138.
Ronchi, B., Stradaioli, G., Verini Supplizi, A., Bernabucci, U., Lacetera, N., Accorsi, P.A., Nardone, A., Seren, E., 2001. Influence of heat stress and feed restriction on plasma progesterone, estradiol-17β LH, FSH, prolactin and cortisol in Holstein heifers, Livestock Production Science, 68, 231-241.
Roth, Z., 2018. Stress-induced alterations in oocyte transcripts are further expressed in the developing blastocyst, Molecular Reproduction and Development, 85, 821-835.
Roth, Z., Wolfenson, D., 2016. Comparing the effects of heat stress and mastitis on ovarian function in lactating cows: Basic and applied aspects, Domestic Animal Endocrinology, 56, S218-S227.
Roth, Z., Meidan, R., Braw-Tal, R., Wolfenson, D., 2000. Immediate and delayed effect of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows, Journal of Reproduction and Fertility, 120, 83-90.
Ryan, D.P., Blakewood, E.G., Munyakazi, L., Godke, R.A., Lynn, J.W., 1992. Effect of heat-stress on bovine embryo development in vitro, Journal of Animal Science, 70, 3490-3497.
Ryan, D.P., Prichard, J.F., Kopel, E., Godke, R.A., 1993. Comparing early mortality in dairy cows during hot and cold seasons of the year, Theriogenology, 39, 719-737.
Saadeldin, I.M., Swelum, A.A., Zakri, A.M., Tukur, H.A., Alowaimer, A.N., 2020. Effects of acute hyperthermia on the thermotolerance of cow and sheep skin-derived fibroblasts, Animals, 10, 545.
Saber, A.P.R., Jalali, M.T., Mohjeri, D., Akhoole, A.A., Teymuourluei, H.Z.N., Nouri, M., Garachorlo, S., 2009. The effect of ambient temperature on thyroid hormones concentration and histopathological changes of thyroid gland in cattle in Tabriz Iran, Asian Journal of Animal and Veterinary Advances, 4, 28-33.
Sahu, S., Mishra, S.R., Kundu, A.K., 2019. Impact of thermal stress on expression dynamics of HSP60 in cardiac fibroblast cells of goat, Animal Biotechnology, 28, 1-7. doi: https://doi.org/10.1080/10495398.2019.1696353.
Sailo, L., Gupta, I.D., Das, R., Chaudhari, M.V., 2017. Physiological Response to Thermal Stress in Sahiwal and Karan Fries Cows, International Journal of Livestock Research, 7, 275-283.
Sakatani, M., Yamanaka, K., Kobayashi, S., Takahashi, M., 2008. Heat shock-derived reactive oxygen species induce embryonic mortality in in vitro early stage bovine embryos, Journal of Reproduction and Development, 54, 496-501.
Sakatani, M., Balboula, A.Z., Yamanaka, K., Takahashi, M., 2012. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black Cow, Animal Science Journal, 83, 394-402.
Salles, A.Y.F.L., Batista, L.F., Souza, B.B., Silva, A.F., Correia, E.L.B., 2017. Growth and reproduction hormones of ruminants subjected to heat stress, Journal of Animal Behaviour and Biometeorology, 5, 7-12.
Sanap, V.N., Ludri, A., Mir, N.A., Kumar, B., Mittal, K.K., 2018. Physiological Performance of Crossbred Cattle Calves (Karan Fries) under Different Housing Conditions during Different Seasons, International Journal of Current Microbiology and Applied Science, 7, 2738-2748.
Sartori, R., Sartor-Bergfelt, R., Mertens, S.A., Guenther, J.N., Parrish, J.J., Wiltbank, M.C., 2002. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter, Journal of Dairy Science, 85, 2803-2812.
Sayah, M.S., Abu El-Hamd, M.A., El-Diahy, Y.M., Nagadi, S.A., 2019. Effect of Season and Housing on Physiological Reactions Testosterone and Semen Quality of Friesian Bulls, International Journal of Engineering Research and Technology, 08, 424-430.
Schams, D., Stephan, E., Hooley, R.D., 1980. The effects of heat exposure on blood serum of anterior pituitary hormones in calves, heifers and bulls, Acta Endocrinologica, 94, 309-314.
Scharf, B., Carroll, J.A., Riley, D.G., Chase, Jr. C.C., Coleman, S.W., Keisler, D.H., Weaber, R.L., Spiers, D.E., 2010. Evaluation of physiological and blood serum differences in heat-tolerant (Romosinuano) and heat-susceptible (Angus) Bos taurus cattle during controlled heat challenge, Journal of Animal Science, 88, 2321-2336.
Scharf, B., Leonard, M.J., Weaber, R.L., Mader, T.L., Hahn, G.L., Spiers, D.E., 2011. Determinants of bovine thermal response to heat and solar radiation exposures in a field environment, International Journal of Biometeorology, 55, 469-480.
Schuller, L.K., Michaelis, I., Heuwieser, W., 2017. Impact of heat stress on estrus expression and follicle size in estrus under field conditions in dairy cows, Theriogenology, 102, 48-53.
Schutz, K.E., Cox, N.R., Matthews, L.R., 2008. How important is shade to dairy cattle? Choice between shade or lying following different levels of lying deprivation Applied Animal Behaviour Science, 114, 307-318.
Sejrsen, K., Fitzgerald, E.M., Tucker, H.A., Huber, J.T., 1980. Effect of plane of nutrition on serum prolactin and insulin in pre-and post-pubertal heifers, Journal of Dairy Science, 53, 326-327.
Sengar, G.S., Deb, R., Singh, U., Raja, T.V., Kant, R., Sajjanar, B., Alex, R., Alyethodi, R.R., Kumar, A., Kumar, S., Singh, R., Jakhesara, S.J., Joshi, C.G., 2018. Differential expression of microRNAs associated with thermal stress in Frieswal (Bos taurus x Bos indicus) crossbred dairy cattle, Cell Stress and Chaperones, 23, 155-170.
Shandilya, U.K., Sharma, A., Sodhi, M., Mukesh, M., 2020. Heat stress modulates differential response in skin fibroblast cells of native cattle (Bos indicus) and riverine buffaloes (Bubalus bubalis), Bioscience Reports, 40, BSR20191544.
Shearer, J.K., Beede, D.K., Bucklin, R.A., Bray, D.R., 1991. Environmental modifications to reduce heat stress in dairy cattle, Agri-Practice, 12, 7-18.
Sheikh, A.A., Aggarwal, A., Indu, B., Aarif, O., 2017. Inorganic zinc supplementation modulates heat shock and immune response in heat stressed peripheral blood mononuclear cells of periparturient dairy cows, Theriogenology, 95, 75-82.
Silva, R.G.D., Maia, A.S.C., 2011. Evaporative cooling and cutaneous surface temperature of Holstein cows in tropical conditions, Revista Brasileira de Zootecnia, 40:1143-1147.
Silva, D.C., Passini, R., 2017. Physiological responses of dairy cows as a function of environment in holding pen, Engenharia Agrícola, 37, 206-214.
Singh, S.P., Newton, W.M., 1978. Acclimatization of young calves to high temperatures: physiological responses, American Journal of Veterinary Research, 39, 795-797.
Singh, R., Singh, S.V., 2005. Variations in cutaneous temperature, physiological responses and blood biochemical in Karan Fries and Sahiwal heifers during solar exposure in summer, Indian Journal of Dairy Science, 58, 415-419.
Singh, R., Singh, S.V., 2006. Circadian changes in peripheral temperature and physiological responses under solar exposure and shed during summer in Karan Fries heifers, Indian Journal of Animal Science, 76, 605-608.
Singh, S.V., Upadhyay, R.C., Kundu, S.S., Vaidya, M.M., Singh, A.K., 2012. Leptin as a Metabolic and Energy Homeostatic Hormone in Dairy Animals: A Review, Indian Journal of Animal Nutrition, 29, 109-116.
Singh, M., Lathwal, S.S., Kotresh, P.C., Choudhary, S., Barman, D., Keshri, A., Kumar, R., 2019. Health status of Hariana cattle (Bos indicus) in different seasons in its breeding tract of Haryana, India, Biological Rhythm Research, https://doi.org/10.1080/09291016.2019.1608729.
Smith, V.G., Hacker, R.R., Brown, R.G., 1977. Effect of alterations in ambient temperature on serum prolactin concentration in steers, Journal of Animal Science, 44, 645-649.
Solano, L., Barkema, H.W., Pajor, E.A., Mason, S., LeBlanc, S.J., Nash, C.G.R., Haley, D.B., Pellerin, D., Rushen, J., de Passillé, A.M., Vasseur, E., Orsel, K., 2016. Associations between lying behavior and lameness in Canadian Holstein-Friesian cows housed in freestall barns, Journal of Dairy Science, 99, 2086-2101.
Soley, M.J., Singh, S.V., 2003. Physiological, haematological and growth responses of crossbred male calves under two housing conditions, Indian Journal of Animal Science, 73, 202-203.
Somparn, P., Gibb, M.J., Markvichitr, K., Chaiyabutr, N., Thummabood, S., Vajrabukka, C., 2004. Analysis of climatic risk for cattle and buffalo production in northeast Thailand, International Journal of Biometeorology, 49, 59-64.
Spencer, R.L., Deak, T., 2017. A Users Guide to HPA Axis Research, Physiology and Behavior, 178, 43-65.
Spiers, DE, Spain, JN, Sampson, JD, Rhoads, R.P., 2004. Use of physiological parameters to predict milk yield and feed intake in heat-stressed dairy cows, Journal of Thermal Biology, 29, 759-764.
Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., 2013. IPCC: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 1535.
Stricker, E.M., Verbalis, J.G., 2013. Water and salt intake and body fluid homeostasis, In Fundamental Neuroscience (4th Edition), 783-797.
Tao, S., Thompson, I.M., Monteiro, A.P.A., Hayen, M.J., Young, L.J., Dahl, G.E., 2012. Effect of cooling heat-stressed dairy cows during the dry period on insulin response, Journal of Dairy Science, 95, 5035-5046.
Tapki, D., Sahin, A., 2006. Comparison of the thermoregulatory behaviours of low and high producing dairy cows in a hot environment, Applied Animal Behaviour Science, 99, 1-11.
Taylor, N.A., Tipton, M.J., Kenny, G.P., 2014. Considerations for the measurement of core, skin and mean body temperatures, Journal of Thermal Biology, 46, 72-101.
Thornton, P.K., 2010. Livestock production: recent trends, future prospects. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 2853-2867.
Torres-Junior, J.R.D.S., Pires, M.D.F.A., de Sa, W.F., Ferreira, A.D.M., Viana, J.H.M., Camargo, L.S.A., Ramos, A.A., Folhadella, I.M., Polisseni, J., de Freitas, C., Clemente, C.A.A., de Sa Filho, M.F., Paula-Lopes, F.F., Baruselli, P.S., 2008. Effect of maternal heat-stress on follicular growth and oocyte competence in Bos indicus cattle, Theriogenology, 69, 155-166.
Trout, J.P., McDowell, L.R., Hansen, P.J., 1998. Characteristics of the oestrous cycle and antioxidant status of lactating Holstein cows exposed to stress, Journal of Dairy Science, 81, 1244-1250.
Tucker, H.A., Wetteman, R.P., 1976. Effects of ambient temperature and relative humidity on serum prolactin and growth in heifers, Proceedings of the Society for Experimental Biology and Medicine, 151, 623-626.
Tucker, H.A., Chapin, L.T., Lookingland, K.J., Moore, K.E., Dahl, G.E., Evers, J.M., 1991. Temperature effects on serum prolactin concentrations and activity of dopaminergic neurons in the infundibulum/pituitary stalk of calves, Proceedings of the Society for Experimental Biology and Medicine, 197, 74-76.
Tucker, C.B., Rogers, A.R., Schutz, K.E. 2008. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Applied Animal Behaviour Science, 109, 141-154.
Tullo, E., Mattachini, G., Riva, E, Finzi, A, Provolo, G., Guarino, M., 2019. Effects of Climatic Conditions on the Lying Behavior of a Group of Primiparous Dairy Cows, Animals, 9, 869.
Turnpenny, J.R., Wathes, C.M., Clark, J.A., McArthur, A.J., 2000. Thermal balance of livestock, 2. Applications of a parsimonious model. Agricultural and Forest Meteorology, 101, 29-52.
Vaidya, M.M., Kumar, P., Singh, S.V., 2011. Circadian changes in heat storage and heat loss through sweating and panting in Karan Fries cattle during different seasons, Biological Rhythm Research, 43, 137-146.
Valente, E.E., Chizzotti, M.L., de Oliveira, C.V., Galvao, M.C., Domingues, S.S., de Castro Rodrigues, A., Ladeira, M.M., 2015. Intake, physiological parameters and behavior of Angus and Nellore bulls subjected to heat stress, Semina Ciencias Agrarias, 16, 4565-4574.
Vaught, L.W., Monty, D.W., Foote, W.C., 1977. Effect of summer heat stress on serum LH and progesterone values in Holstein-Frisian cows in Arizona, American Journal of Veterinary Research, 38, 1027-1032.
Vogler, C.J., Bame, J.H., DeJarnette, J.M., McGilliard, M.L., Saacke, R.G., 1993. Effects of elevated testicular temperature on morphology characteristics of ejaculated spermatozoa in the bovine, Theriogenology, 40, 1207-1219.
Von Borell, E.V., Langbein, J., Despres, G., Hansen, S., Leterrier, C., Marchant-Forde, J., Marchant, F.R., Minero, M., Mohr, E., Prunier, A., Valance, D., Veissier, I., 2007. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals - A review, Physiology and Behavior, 92, 293-316.
Wang, J., Katawatin, S., Suklerd, S., Pongthaisong, P., 2012. Skin morphology of Thai native cattle, Khon Kaen Agriculture Journal, 40, 392-394.
Weitzel, J.M., Viergutz, T., Albrecht, D., Bruckmaier, R., Schmicke, M., Tuchscherer, A., Koch, F., Kuhla, B., 2017. Hepatic thyroid signaling of heat-stressed late pregnant and early lactating cows, Journal of Endocrinology, 234, 129-141.
West, J.W., 2003. Effects of Heat-Stress on Production in Dairy Cattle, Journal of Dairy Science, 86, 2131-2144.
Wettemann, R.P., Tucker, H.A., 1974. Relationship of Ambient Temperature to Serum Prolactin in Heifers, Proceedings of the Society for Experimental Biology and Medicine, 146, 908-911.
Wettemann, R.P., Tucker, H.A., Beck, T.W., Meyerhoeffer D.C., 1982. Influence of ambient temperature on prolactin concentrations in serum of Holstein and Brahman x Hereford heifers, Journal of Animal Science, 55, 391-394.
Wheelock, J.B., Rhoads, R.P., Vanbaale, M.J., Sanders, S.R., Baumgard, L.H., 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows, Journal of Dairy Science, 93, 644-655.
Wilson, S.J., Marion, R.S., Spain, J.N., Spiers, D.E., Keisler, D.H., Lucy, M.C., 1998. Effects of controlled heat stress on ovarian function of dairy cattle, 1. Lactating Cows. Journal of Dairy Science, 81, 2124-2131.
Wise, M.E., Armstrong, D.V., Huber, J.T., Hunter, R., Wiersma, F., 1988. Hormonal alterations in the lactating dairy cow in response to thermal stress, Journal of Dairy Science, 71, 2480-2485.
Wolfenson, D., Lew, B.J., Thatcher, W.W., Graber, Y., Meidan, R., 1997. Seasonal and acute heat stress effects on steroid production by dominant follicles in cow, Animal Reproduction Science, 47, 9-19.
Wolfenson, D., Roth, Z., Meidan, R., 2000. Impaired reproduction in heat stressed cattle: Basic and applied aspects, Animal Reproduction Science, 60-61, 535-547.
Yadav, B., Singh, G., Verma, A.K., Dutta, N., Sejian, V., 2013. Impact of heat stress on rumen functions, Veterinary World, 6, 992-996.
Yadav, B., Singh, G., Wankar, A., 2015. Adaptive capability as indicated by redox status and endocrine responses in crossbred cattle exposed to thermal stress, Journal of Animal Science, 5, 67-73.
Yadav, B., Singh, G., Wankar, A., Dutta, N., Chaturvedi, V.B., Verma, M.R., 2016. Effect of simulated heat stress on digestibility, methane emission and metabolic adaptability in crossbred cattle, Asian-Australasian Journal of Animal Science, 29, 1585-1592.
Yadav, B., Singh, G., Wankar, A., 2017. The use of infrared skin temperature measurements for monitoring heat stress and welfare of crossbred cattle, Indian Journal of Dairy Science, 70, 127-131.
Yadav, B., Singh, G., Wankar, A., 2021. Acclimatization dynamics to extreme heat stress in crossbred cattle. Biological Rhythm Research, 52, 524-534. https://doi.org/10.1080/09291016.2019.16127.
Yeck, R.G., Kibler, H.H., 1958. Predicting heat tolerance from calf vaporization rates, Journal of Animal Science, 17, 1228-1229.
Younas, M., Fuquay, J.W., Smith, A.E., Moore, A.B., 1993. Estrus and endocrine responses of lactating Holsteins to forced ventilation during summer, Journal of Dairy Science, 76, 430-434.
Yousef, J.L.M., Habeeb, A.A., EL-Kousey, H., 1997. Body weight gain and some physiological changes in Friesian calves protected with wood or reinforced concrete sheds during hot summer season of Egypt, Egyptian Journal of Animal Production, 34, 89-101.
Zhang, F.J., Weng, G., Wang, J.F., Zhou, D., Zhang, W., Zhai, C.C., Hou, Y.X., Zhu, Y.H., 2014. Temperature humidity index and chromium supplementation on antioxidant activity, heat shock protein 72, and cytokine response of lactating cows, Journal of Animal Science, 92, 3026-3034.
Code availability
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
S. R. Mishra had written and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The manuscript does not contain clinical studies or patient data and thus no ethics approval is required.
Consent to participate
Not applicable
Consent for publication
Yes
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, S. Behavioural, physiological, neuro-endocrine and molecular responses of cattle against heat stress: an updated review. Trop Anim Health Prod 53, 400 (2021). https://doi.org/10.1007/s11250-021-02790-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-02790-4