Abstract
Protodioscin poisoning of Brachiaria spp. has been a serious problem for lambs in grazing systems. The defoliation process can stimulate the appearance of new leaves and, the younger leaves have a lower concentration of protodioscin. Thus, it was aimed to assess the effect of different leaf offerings on the protodioscin content in forage and if protodioscin can interfere with the dry matter intake (DMI) and on metabolic and productive parameters of lambs. Twelve tester lambs (average weight 17.5 ± 3.48 kg) were divided into four groups of different levels of dry leafy matter (60, 75, 90, or 105 g/kg body weight). In addition to these, 33 regulator lambs were used as needed to adjust the leaf offerings of pasture. The animals were divided and kept in 12 paddocks, each with a tester lamb. The DMI was estimated using chromic oxide as an external marker. In vitro digestibility and degradation kinetics and ruminal, blood, and urinary parameters were measured in the forage sampled by the hand plucking method. The protodioscin concentration in forage was determined by high-performance liquid chromatography. The DMI increased linearly as a result of the supply of leaves. However, the protodioscin content and its consumption showed a quadratic behavior. There was no effect of leaf supply on in vitro digestibility, in vitro degradation kinetics of forage, and on ruminal, blood, and urinary parameters of lambs. However, a negative correlation was observed between the DMI and the concentration of protodioscin at the highest level of leaf supply. This is due to the fact that in the most intense grazing, there is a stimulus for greater regrowth; therefore, there was an increase in the concentration of protodioscin in forages in older forages. Protodioscin poisoning was confirmed by urinary and blood parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brazilian sheep flock was estimated at 18.433.810 animals, and about 503.821 are found in the Central-West region (IBGE 2016). The Brachiaria grass is a well-adapted forage in the Central-West region in Brazil (Castro et al. 2011; Costa et al. 2013), being an important feeding option in livestock. However, sheep poisoning by Brachiaria spp. is consistently reported as an important cause of economic loss in Brazil (Riet-Correa and Medeiros 2001).
A substantial number of sheep poisoning cases have been reported in Brazil due to Brachiaria grass intake, being protodioscin one of the main bioactive compounds presented in forages and considered to be responsible for this toxicity condition (Riet-Correa et al. 2011). Protodioscin degradation is carried out by the rumen microbiota and transported to the liver (Wina et al. 2005). Furthermore, because grasses of the genus Brachiaria display a slow rate of passage through the gastrointestinal tract and low fiber digestibility (Detmann et al. 2001), which can do protodioscin permanence in the rumen environment longer.
Some studies have demonstrated some factors that can influence the toxicity of protodioscin (Castro et al. 2011; Mustafa et al. 2012; Faccin et al. 2014). In fact, further investigation is needed, including systematic quantification of the toxic bioactive compound of Brachiaria grass under different stages of vegetative growth, pasture management, along with different climatic and regional soil conditions to help elucidate this issue (Mustafa et al. 2012). However, most studies provide only case reports of intoxicated animals and there is a lack of information about how the mechanisms of protodioscin poisoning occur in the rumen. Thus, it was aimed to assess the effect of different leaf offerings on the protodioscin content in forage and if protodioscin can interfere with the dry matter intake (DMI) and on metabolic and productive parameters of lambs raised in a mixed of Brachiaria grass pasture (B. decumbens and B. brizantha).
Materials and methods
The experiment was conducted in the Sheep Section at School-Farm of Federal University of Mato Grosso do Sul (UFMS), located in Terenos county, Mato Grosso do Sul state, Brazil.
Animals, diets, and experimental design
Twelve tester lambs (mixed breed, average weight 17.5 ± 3.48 kg) were divided into four groups of different levels of dry leafy matter (GLDM) (60, 75, 90, or 105 g/kg of body weight). In addition to the 12 test animals, 33 regulating lambs were used as needed to adjust the leaf supply. The animals were divided and kept in 12 paddocks, each with a tester lamb. For this adjustment, the lambs were weighed at the beginning of the experimental period and every 28 days to obtain weight. Only one test animal was used per paddock and there were tripled for each GLDM level.
The paddocks were composed of a mixed of Brachiaria grass (B. decumbens and B. brizantha) that was adjusted for each 28 days using the method of the total cut sample, close to the soil (McMeniman 1997). Six samples were evaluated per paddock, using a 0.5 × 0.5 m (0.25m2) metal square area, at random points representative of each paddock. After weighing and identification, the morphological separation was performed to obtain the percentage of leaf, stem + sheath, and senescent material (progressive yellowish leaves and stems, dark and visible dehydration, characterizing the senescence phase of the plant). To adjust the stocking rate, the amount of forage available in each paddock (which had different sizes) and the percentage of green leaves was calculated. From this calculation, it was obtained how many kilos it was possible to put per paddocks, according to each treatment, 60, 75, 90, and 105 g/kg of body weight (BW).
The animals received concentrated feed (calculated for an average consumption of 16 g/kg of BW) based on corn, soybean meal, and minerals, containing 220 g/kg of crude protein (CP) and 3.1 Mcal ME/kg of dry matter (DM). The experiment was structured in a completely randomized design, containing four levels of levels of dry matter of green leaves (60, 75, 90, and 105 g/kg BW in GLDM).
Data collection and analysis
The experiment consisted of 84 days subdivided into four 21-day periods, allowing the animals to get used to the external marker and obtain a homogeneous plateau of ingestion and excretion; five (5) days for fecal collection and manual grazing simulation (hand plucking method); and 1 day for ruminal fluid collection. Subsequently, the animals were subjected to an 8-day interval to eliminate the external marker from the digestive tract. Fecal samples were collected directly from the rectum once a day for 5 consecutive days, in portions of 5–10 g/animal. Always at the same time at 8 am. All samples were identified and stored in the freezer at −10°C. To determine forage consumption, the chromic oxide marker (4 g/animal/day) was used, offered orally, through pellets, made with kraft paper, for 12 days (7 days for adaptation and obtaining the plateau and 5 days for collection).
The hand plucking method used to evaluate the forage; based in the natural habit of grazing the animal, the type of material consumed was identified and a sample similar to the ingested food was collected. The sampler walked beside the animal in the paddock and observing what he selected, a similar sampling was done. That due to the natural habit of grazing sheep, basically the sample was made up of leaves.
Analysis procedures were conducted at Laboratory of Applied Nutrition of College of Veterinary Medicine and Animal Science at the Federal University of Mato Grosso do Sul, and at Laboratory of Applied Biotechnology of Animal Nutrition at the Catholic University Dom Bosco, both located in Campo Grande, MS, Brazil.
Fecal samples and manual grazing simulation material (hand plucking) were collected to obtain a sample made up of animal/experimental period and, subsequently, chemical analysis according to the National Institute of Science and Technology of Animal Science - INCT-CA (Detmann et al. 2012). The samples were dried in a force-air oven at 55 °C and milled in a Wiley mill with a 2- and 1-mm sieve to determine dry matter (DM, INCT-CA L-003/1), mineral matter (MM; INCT-CA M-001/1), and crude protein (CP; INCT-CA N-001/1) contents according to methods described by Detmann et al. (2012). Ether extract (EE) was determined using an Ankom XT10 extractor (ANKOM Technology, NY, USA) in XT4® bags; neutral detergent fiber (NDF; INCT-CA F-001/1) and acid detergent fiber (ADF; INCT-CA F-003/1) were performed according to methods described by Detmann et al. (2012). The total carbohydrates (TC) were estimated by equation TC = 100 − (CP + MM + EE), and the non-fibrous carbohydrates (NFC) were calculated using the equation proposed by Hall (2000): NFC = 100 − (CP + MM + EE + NDF) (Table 1).
Lignin content was followed by Klason methodology (INCT-CA F-007-1) after NDF and ADF analysis, using MA443 (Marconi®, Piracicaba, SP, Brazil) incubator jars for degradability tests (28 non-woven textile bags per jar + two blanks), shaken for 3 h with a 500 mL lignin solution (72% sulfuric acid) and washed in running water until neutral pH stabilization.
Chromic oxide (Cr2O3) content was determined using 0.2-g fecal samples and 5 mL of digestive solution and was packed into borosilicate microtubes, were placed in digesters block at 200 °C until orange color formation and final volume between 1 and 2 mL. A standard curve was prepared containing 0, 2, 4, 6, 8, and 10 mg of Cr2O3, and was read on spectrophotometer (Thermo Scientific AquaMate Spectrophotometer AQ8000-AquaMate) under 357.9-nm wavelength (Detmann et al. 2012).
Protodioscin concentration of forage samples was determined by extraction with acetonitrile and high-performance liquid chromatography (HPLC) analysis using an evaporative light scattering detector (ELSD) (Ganzera et al. 2001).
Fecal dry matter (FDM), dry matter digestibility (DMD), and dry matter intake (DMI) were estimated following the equations according to Lippke (2002):
FDM = ingested marker (g) / concentration of marker in feces
DMD = (DM ingested − DM excreted) / DM ingested
DMI = FDM / (1 – digestibility)
Ruminal, blood, and urinary parameters
Rumen fluid samples were collected using gastric tube and vacuum pump after fasting food period of 24 h. This fasting period was used since the gastric tube could not achieve the liquid fraction of rumen. Rumen samples were fractionated into 15-mL tubes. To one of the tubes, a 1:1 sulfuric acid solution (H2SO4) was added for ammonia nitrogen (N-NH3) analysis.
The determination of NH3-N contents was performed according to the INCT-CA N-007/1 method, described by Detmann et al. (2012). The concentration of ammonia in the rumen fluid was estimated by the micro-Kjeldahl system, without acid digestion and using potassium hydroxide (2N) as base for distillation, after previous centrifugation of the sample at 1000×g, for 15 min.
The ruminal fluid temperature and pH were measured at the time of liquid sampling, using a digital potentiometer Sentron pH system meter model 1001 (Sentron Europe BV, Roden, Netherlands).
The spot urine collections were made in spontaneous urination of the animals. The samples were filtered through gauze, and a 10-mL aliquot was separated and diluted in 40 mL of sulfuric acid (0.036 N) to prevent bacterial destruction of purine derivatives and precipitation with uric acid Valadares et al. (1999).
Blood samples were obtained by puncture of the jugular vein in a BD Vacutainer® SST® II 1449 Advance® tube with clot activator and separating gel to obtain serum for biochemical analyzes. Analyses of alanine aminotransferase (kit ref. 10745138), aspartate aminotransferase (kit ref. 10745120), albumin (kit ref. 04657357), creatinine (kit ref. 10886874), total protein (kit ref. 04657586), urea (kit ref. 11200666), and triglycerides (kit ref. 04657594) were carried out.
In vitro digestibility and degradation kinetics
The in vitro digestibility, of forage samples (hand plucking), was performed according to Tilley and Terry (1963). A 0.5-g sample of dry samples is put in TNT bags (100g/cm2), sealed, and inoculated for 72 h in 1600 mL buffer solution (McDougall 1948; Camacho et al. 2019) and 400 mL of rumen inoculum was purged with CO2 to maintain anaerobic conditions. After 48 h of incubation, 8 g of the pepsin enzyme and 40 mL of HCl were added and incubated for 24 h.
Cumulative in vitro gas production was determined using the Ankom RF Gas Production System (ANKOM Technology, NY, USA). Vial contained 0.5 g of sample, 100 mL of a buffer solution preheated at 39°C, and 25 mL of rumen inoculum purged with CO2 to maintain anaerobic conditions. The flasks fitted with the pressure sensors remained at a constant temperature of 39°C and the pressure (psi) of each flask was determined and recorded every 5 min for 48 h and then processed for cumulative gas production in mL of gas/100 mg of incubated DM. Gas production kinetics parameters were obtained by the two-compartmental logistic model proposed by Schofield et al. (1994), as follows:
Y = A/{1+exp[2+ 4*B*(Lag-t)]}+D /{1+exp[2+4*E*(Lag-t)]},
in which Y = total gas volume at time t (extent of degradation); A = volume of gas (mL) of rapid degradation; D = volume of gas (mL) of slow degradation; B = degradation rate of the rapid fraction (/h); E = degradation rate of the slow fraction (/h); and Lag = bacterial colonization time (h).
Ruminal inoculum to determine in vitro digestibility and gas production was obtained from three castrated, mixed-breed, rumen-cannulated rams (weight average: 50 kg) which were kept in extensive grazing system of Brachiaria grass.
Statistical analysis
The data were analyzed by one-way ANOVA in a completely random design using the general linear model procedure of Statistical Analysis System Institute – SAS (2002). Significance was declared at P <0.05. When a significant F test was detected, it was analyzed by regression equations. The linear quadratic models were tested and the model was selected according to the significance of the regression coefficients, adopting the 5% probability level and the determination coefficient. Pearson’s correlation was used to determine the strength of the correlations between the variables analyzed using the SAS CORR procedure (Statistical Analysis System Institute – SAS 2002). The data analyzed using the following model:
Yij = μ + Li + eij, where:
Yij = Dependent variable; μ = General mean; Li = Effect of levels of dry matter of green leaves i (being i = 1,...,4); eij = Random error, assumption NID (0, σ2e).
The Gauss-Newton procedure was used in cumulative production gas to estimate the fermentation parameters. Data were analyzed as completely randomized design using the MIXED procedure of Statistical Analysis System Institute – SAS 2002; SAS Institute).
Results
Dry matter intake and protodioscin concentration
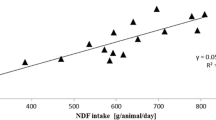
The estimated DMI (Table 2) increased linearly and according to the dry matter levels of the green leaves, with an increase in intake of 8 g/day for each increase in the leaf supply. The concentration and ingestion of protodioscin were adjusted to a quadratic regression model, in which the maximum concentration of protodioscin (g/kg) was 88.6 g/kg BW on offer and the maximum ingestion of protodioscin was 96.3 g/kg BW green supply (P> 0.05). The correlation between the DMI and the protodioscin concentration was negatively affected by 105 g/kg. The concentration of protodioscin influenced the CMS of lambs when evaluated in the four experimental periods and leaf stocks (Fig. 1).
In vitro digestibility and degradation kinetics
In vitro nutrient digestibility (Table 3) was not affected by different leaf supplies levels (60, 75, 90, or 105 g/kg) (P>0.05). Similar results were observed in in vitro degradation kinetics (Table 4) (P>0.05).
Ruminal, blood, and urinary parameters
Different leaf supplies levels did not affect (P > 0.05) ruminal parameters (Table 5), biochemical parameters (Table 6), or urinary compounds (Table 7). The results of the liver enzyme aspartate aminotransferase (AST) showed a mean higher than reference values. Those results can be a relationship to toxicity, mainly on levels 90 and 105g/kg BW.
Discussion
Dry matter intake and protodioscin concentration
Dry matter consumption was estimated by chromium oxide (Cr2O3) as an external marker and is considered a reliable marker (Costa et al. 2019). It was observed that consumption increased linearly as a function of leaf levels (Table 2). This result can be explained by the lower competition for forage as the supply of leaves increases, which allows greater selectivity on the part of the animals.
Our results demonstrated that there was a correlation between the forage protodioscin concentration and DMI up to the level of 90 g/kg BW of offering (Table 2). However, at the highest level of leaf supply, the correlation of protodioscin content and DMI was negative. Less intense grazing in the lower levels stimulated an increase in forage protodioscin concentration, which in turn, had a negative influence on the DMI in the subsequent period. This scenario could be explained by the higher concentration of protodioscin in the forage and, consequently, a possible intoxication by protodioscin in the animals, leading to a lower stimulus for ingestion of forage. This can be explained by the fact that grazing more lenient allows greater aging of forage; and, apparently, the plant has higher concentrations of protodioscin (Melo et al. 2019; Leal et al. 2020).
These results corroborate with other studies, as observed in Melo et al. (2019) and Leal et al. (2020) whose results demonstrated that at the time of lower rainfall concentration there is a higher concentration of protodioscin in the plant, indicating greater toxicity in sheep that consumed greater amounts of toxic compounds. With less water availability, the forage plant increases the life span of the leaves (Montagner et al. 2012), with older leaves showing a higher concentration of toxic compounds.
In vitro digestibility and degradation kinetics
There were no differences in in vitro digestibility of nutrients in relation to levels of dry matter of green leaves (Table 3). However, it is important to emphasize that DMivD average value found in our study was considered lower (698.4 g/kg) than other studies (Leal et al. 2020) that found Brachiaria possesses estimated DMivD values between 772 and 752 g/kg. This lower digestibility may be correlated with higher levels of NDF (677.8 g/kg), ADF (409.3 g/kg), and lignin (84.15 g/kg) (Table 1), while NDF is more correlated with reduced consumption, ADF and lignin are closely related to dry matter digestibility (Van Soest 1994), since lignin impregnates ADF and its low oxygen content and high in condensed structures hamper degradation and delay aerobic catabolism (Van Soest 1994), this fact may be related to the physiological stage of the plant evidenced by the value of lignin. Thus, this fact may be related to the physiological stage of the plant that could be evidenced by the lignin content found in this study.
Forages of low quality may present decreased digestibility of fibrous fraction. Forages that are slow to digest require a longer period within the rumen to be hydrated, ruminated, and, therefore, to be broken into particles that are appropriately colonized and subsequently degraded by fermentation (Granzotto et al. 2011). Thus, a longer retention period of forage in the rumen provides a longer time that microorganisms degrade protodioscin, resulting in possible animal poisoning. This poisoning process may be explained due to protodioscin degradation by ruminal microbiota and its derivatives, such as sapogenins, which are transported along the digestive tract and eliminated in feces; however, some sapogenins are absorbed into the duodenum and transported to the liver, where these compounds are conjugated with bile and excreted by feces (Wina et al. 2005). This process of conjugation of sapogenins and glucuronic acid leads to calcium ion binding, which in turn, form insoluble salts that may precipitate into the bile ducts in the form of crystals (Cruz et al. 2001; Gracindo et al. 2014). Thus, these crystals may result in inflammation and biliary system obstruction in addition to periportal hepatocytes necrosis, resulting in icterus, photosensitization, and hepatitis (Santos et al. 2008). According to Leal et al. (2020), the protodioscin content of Brachiaria cultivars can negatively affect their digestibility and some parameters of degradation kinetics in the rumen.
In order to explain this process, we evaluated in vitro degradation kinetics from forages (Table 4), whose bacterial colonization time (Lag time = 5.2 h) was considered low. This data can be explained due to the lignification degree of the vegetal cell wall, which forms a barrier to adhere and degrade the food particles and, consequently, degrades the forage (McAllister 1994; Paciullo 2002). Furthermore, we observed decreased total gas production (14.22 mL) suggesting that the presence of lignin influenced the adhesion process and, consequently, reduced OM degradability. Sá et al. (2011) evaluated Brachiaria brizantha at three harvest stages (28, 35, and 54 days) and observed colonization time of 12.9, 13.2, and 14.6 h, respectively, and total gas production values of 95.75, 116.8, and 68 mL, respectively. Liu et al. (2002) showed that the gas production technique could be used as a measure of ruminal degradation of food, suggesting that in vitro degradability kinetic used in our study is well accepted in the literature.
On the other hand, Leal et al. (2016) evaluated the nutritional value of Brachiaria grasses (B. decumbens, B. humidicola, and B. ruziziensis) to quantify aspects of anti-quality and to identify their correlation with quality grasses and showed the protodioscin content influences negatively the digestibility and the gas production results. Similarly, Leal et al. (2020) studying the influence of protodioscin content on digestibility and degradation kinetics in Brachiaria brizantha cultivars observed that there is a negative correlation between protodioscin content and degradability, which reduces the quality and rumen fermentation capacity of the grasses.
Ruminal, blood, and urinary parameters
The ruminal parameters were not influenced by forage supplies. Rumen fluid pH averaged was 7.7 (Table 5), which is considered above the standard range. Church (1979) suggested that ruminants that consumed forage-based diets maintained rumen pH between 6.2 and 6.8. Van Soest (1994) mentioned that a pH range between 6.2 and 7.2 is usually accepted. In fact, rumen pH alterations provide reduced digestion rates and enhancement on the fiber colonization time (Grant and Mertens 1992), leading to forage intake reduction and, consequently, a decrease in the productive capacity of the animal. Nonetheless, it must be considered that our animals were 24-h food fasting before rumen fluid collection, suggesting that rumination and salivation may have promoted a buffering effect, besides the sampling procedure can stimulate the salivation.
The N-NH3 concentration (9.8 mg/dL) found in our study (Table 5) is above the limiting value for microbial growth (5 mg/dL) according to Satter and Slyter (1974) and near the optimum value of 10 mg/dL, (Van Soest 1994). One explanation of N-NH3 concentration found in this study is due to the consequence of the balance between production and the use by microorganisms, being the latter is dependent on the amount of energy available (Ítavo et al. 2002). Unused ammonia by rumen bacteria is transported to the blood through the rumen wall and reaches the liver where urea is formed. Therefore, the urea circulates in the blood and may be eliminated by urine or recycled. However, González and Silva (2017) reported that a prolonged fasting period may lead to increased endogenous proteolysis using amino acids as an energy source, leading to increased urea concentration since the urinary flux is reduced and inhibits renal urea excretion. These facts corroborate with higher levels of urea found in the blood (40.3 mg/dL) (Table 6) and urine (200.8 mg/dL) (Table 7) in our study, indicating a possibility of Brachiaria spp. poisoning, since increased urea levels on the plasma may be related to insufficient renal filtration (González and Silva 2017). Therefore, the protodioscin content average found in our study was 3.3 g/kg (Table 2).
An important result to be highlighted in our experiment was the AST concentration which is an important parameter evaluated for the detection of intoxications, since its high concentration in the blood circulation may be related to acute and chronic liver damage (Kaneko et al. 2008). Our result of AST concentration mean was 129.4 U/L (Table 6), indicating possible liver damage that may have resulted from poisoning by ingesting Brachiaria grass according to Kaneko et al. (2008) and Jain (1993) listed AST reference values of 0–90 U/L. It is important to note that two experimental animals died during the experimental period, showing all signs of intoxication by Brachiaria spp. The most of the studies so far that evaluated protodioscin are only case reports that demonstrated clinical toxicity signs in the animals. Few studies discuss the action of protodioscin within the rumen, although the protodioscin action is associated with forage quality. Protodioscin poisoning in animals happens when rumen microorganisms degrade the compound and the longer retention time of this particle in the rumen leads to the possibility that damage will be higher.
Conclusion
Our findings demonstrated that DMI by lambs was influenced by protodioscin content in the forage since grazing activity stimulates regrowth on different leaf offers, which in turn, stimulates higher protodioscin concentration in the forage. In vitro digestibility and in vitro degradation kinetics demonstrated decreased forage degradation and because of this a longer particle retention period within the rumen. Blood parameters confirmed natural Brachiaria spp. poisoning in the animals.
References
Camacho, L.F.; Silva, T.E.; Palma, M.N.N.; Assunção, A.; Rodrigues, J.P.; Costa e Silva, L.F.; Detmann, E. 2019. Evaluation of buffer solutions and urea addition for estimating the in vitro digestibility of feeds. Journal of Animal Science, 97(2), 922-–931. https://doi.org/10.1093/jas/sky464
Castro, M.B., Santos Junior, H.L., Mustafa, V.S., Gracindo, C.V., Moscardini, A.R.C., Louvandini, H., Paludo, G.R., Borges, J.R.J., Haragushi, M., Ferreira, M.B., and Riet-Correa, F., 2011. Brachiaria spp. poisoning in sheep in Brazil: experimental and epidemiological findings. In: Riet-Correa, F., Pfister, J., Schild, A.L., and Wierenga, T. (eds). Poisoning by plants, mycotoxins and related toxins. CAB Internacional, London.
Church, D.C., 1979. Digestive physiology and nutrition of ruminants. Oxford Press Inc., Oxford.
Costa, S.Z.R., Nespoli, P.B., Bezerra Júnior, P.S., Nogueira, V.A., Driemeier, D., Boabaid, F.M., Peixoto, P.V., Armién, A.M., França, T.N., 2013. Lectin histochemistry evaluation of liver and kidney of sheep with photosensitization caused by Brachiaria decumbens. Pesquisa Veterinaria Brasileira, 33(8), 955--962. https://doi.org/10.1590/S0100-736X2013000800001
Costa, M.C.M., Ítavo, L.C.V., Ítavo, C.C.B.F., Dias, A.M., Petit, H.V., Reis, F.A., Gomes, R.C., Leal, E.S., Niwa, M.V.G., Moraes, G.J., 2019. Evaluation of internal and external markers to estimate faecal output and feed intake in sheep fed fresh forage. Animal Production Science, 59(4), 741--748. https://doi.org/10.1071/AN16567
Cruz, C., Driemeier, D., Pires, V.S., Schenkel, E.P., 2001. Experimentally induced cholangiohepatopathy by dosing sheep with fractionated extracts from Brachiaria decumbens. Journal of Veterinary Diagnostic Investigation, 13, 170--172. https://doi.org/10.1177/104063870101300215
Detmann, E., Paulino, M.F., Zervoudakis, J.T., Valadares Filho, S.C., Lana, R.P., Queiroz, D.S., 2001. Suplementação de novilhos mestiços durante a época das águas: Parâmetros ingestivos e digestivos. Revista Brasileira de Zootecnia, 30, 340--1349. https://doi.org/10.1590/S1516-35982001000500030
Detmann, E., Souza, M.A., Valadares Filho, S.C., Queiroz, A.C., Berchielli, T.T., Saliba, E.O.S., Cabral, L.S., Pina, D.S., Ladeira, M.M., Azevedo, J.A.G., 2012. Métodos para análise de alimentos. Suprema, Visconde do Rio Branco, Minas Gerais, Brasil.
Faccin, T.C., Riet-Correa, F., Rodrigues, F.S., Santos, A.C., Melo, G.K.A., Silva, J.A., Ferreira, R., Ítavo, C.C.B.F., Lemos, R.A.A., 2014. Poisoning by Brachiaria Brizantha in flocks of Naive and experienced sheep. Toxicon, 82, 1--8. https://doi.org/10.1016/j.toxicon.2014.02.008
Ganzera, M., Bedir, E., Khan, I.A., 2001. Determination of steroidal saponins in Tribulus terrestris by reversed-phase high-performance liquid chromatography and evaporative light scattering detection. Journal of Pharmaceutical Sciences 90(11), 1752–1758. https://doi.org/10.1002/jps.1124
González, F.H.D., Silva, S.C., 2017. Introdução à bioquímica clínica veterinária. (3rd ed.). Porto Alegre-RS, Brazil. UFRGS ed. 538 p
Gracindo, C.V., Louvandini, H., Riet-Correa, F., Ferreira, M.B., Castro, M.B., 2014. Performance of sheep grazing in pastures of Brachiaria decumbens, Brachiaria brizantha, Panicum maximum, and Andropogon gayanus with different protodioscin concentrations. Tropical Animal Health and Production, 49(5), 733--737. https://doi.org/10.1007/s11250-014-0556-y
Grant, R.J., Mertens, D.R., 1992. Development of buffer systems for ph control and evaluation of ph effects on fiber digestion in vitro. Journal of Dairy Science, 75(6), 1581–1587. https://doi.org/10.3168/jds.S0022-0302(92)77915-6
Granzotto, F., Branco, A.F., Santos, A.L., Barreto, J.C., Teixeira, S., Serrano, R.C., Barbosa, O.R., Mano, D.S., Ferelli, F., Coneglian, S.M., 2011) Proteic supplements with and without sulfur sources on intake behavior of steers fed with low quality hay. Semina: Ciências Agrárias, 32, 1151--1162. https://doi.org/10.5433/1679-0359.2011v32n3p1151
Hall, M.B., 2000. Neutral detergent-soluble carbohydrates. Nutritional relevance and analysis. Gainesville: University of Florida, 2000. 76p. https://animal.ifas.ufl.edu/media/animalifasufledu/dairy/pdf/Manual_-_Neutral_Detergent-Soluble_Carbohydrates_Nutritional_Relevance_and_Analysis.pdf
IBGE - Pesquisa Pecuária Municipal. Tabela 3939 – Efetivo dos rebanhos, por tipo de rebanho, 2016. https://sidra.ibge.gov.br/tabela/3939.
Ítavo, L.C.V., Valadares Filho, S.C., Silva, F.F., Valadares, R.F.D., Leão, M.I., Cecon, P.R., Ítavo, C.C.B.F., Moraes, E.H.B.K., Rennó, L.N., Paulino, P.V.R., 2002. Produção Microbiana e Parâmetros Ruminais de Novilhos Alimentados com Dietas Contendo Vários Níveis de Concentrado. Revista Brasileira de Zootecnia, 31(3), 1553—1561. https://doi.org/10.1590/S1516-35982002000600027
Jain, N.C., 1993. Essentials of veterinary hematology. Lea e Febiger, Philadelphia. 417p.
Kaneko, J.J., Harvey, J.W., Bruss, M.L., 2008. Clinical biochemistry of domestic animals, sixth ed. Academic Press, San Diego. https://www.sciencedirect.com/book/9780123704917/clinical-biochemistry-of-domestic-animals
Leal, E.S., Ítavo, L.C.V., Valle, C.B., Ítavo, C.C.B.F., Dias, A.M., Barbosa-Ferreira, M., Soares, C.M., de Melo, G.K.A., Ferreira, V.B.N., 2016. Anti-nutritional potential of protodioscin and kinetics of degradation in Urochloa grasses. Semina: Ciências Agrárias, 37(4), 2247--2258. https://doi.org/10.5433/1679-0359.2016v37n4p2247
Leal, E.S., Ítavo, L.C.V., Valle, C.B., Ítavo, C.C.B.F., Dias, A.M., Difante, G.S., Barbosa-Ferreira, M., Nonato, L.M., de Melo, G.K.A., Gurgel, A.L.C., 2020. Influence of protodioscin content on digestibility and in vitro degradation kinetics in Urochloa brizantha cultivars. Crop & Pasture Science, 72, 278-–28. https://doi.org/10.1071/CP18357
Lippke, H., 2002. Estimation of forage intake by ruminants on pasture. Crop Science, 42, 869--872. https://doi.org/10.2135/cropsci2002.8690
Liu, J.X., Susenbeth, A., Südekum, K.H., 2002. In vitro gas production measurements to evaluate interactions between untreated and chemically treated rice straws, grass hay, and mulberry leaves. Journal of Animal Science, 80(2), 517-524. https://doi.org/10.2527/2002.802517x
McAllister, T.A., 1994. Microbial attachment and feed digestion in the rumen. Journal of Animal Science, 72(11), 3004--3018. https://doi.org/10.2527/1994.72113004x
McDougall, E.I., (1948). Studies on ruminant saliva. The composition and output of sheep’s saliva. Biochemistry Journal, 43(1), 99--109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1274641/pdf/biochemj00946-0114.pdf
McMeniman, N.P., 1997. Methods of estimating intake of grazing animals. In: Reunião Anual da Sociedade Brasileira de Zootecnia, Simpósio Sobre Tópicos Especiais em Zootecnia, Juiz de Fora, 1997. Campos, O.F., Lizieire, R.S., Figueiredo, E.A.P. (eds). Proceedings...Sociedade Brasileira de Zootecnia, pp 131--168.
Melo, G.K.A., Silva, W.J., Pupin, R.C., Heckler, H.F., Nonato, L.M., Ítavo, C.C.B.F., Ferreira, V.B.N., Lemos, R.A.A., 2019. Comparison of two collection methods to determine protodioscin concentrations in Brachiaria spp. and to monitor Brachiaria spp. Poisoning. Pesquisa Veterinaria Brasileira, 39, 710-714. https://doi.org/10.1590/1678-5150-PVB-6226
Montagner, D.B., Nascimento Júnior, D., Sousa, B.M.L., Vilela, H.H., Silveira, M.C.T., Euclides, V.P.B., Silva, S.C., Carloto, M.N., 2012. Morphogenesis in guinea grass pastures under rotational grazing strategies. Revista Brasileira de Zootecnia, 41, 883-888. https://doi.org/10.1590/S1516-35982012000400008
Mustafa, V.S., Moscardini, A.R.C., Borges, J.R.J., Reckziegel, G.C., Riet-Correa, F., Castro, M.B., 2012. Intoxicação natural por Brachiaria spp. em ovinos no Brasil Central. Pesquisa Veterinaria Brasileira, 32(12), 1272--1280. https://doi.org/10.1590/S0100-736X2012001200010
Paciullo, D.S.C., 2002. Anatomical traits related with nutritive value of forage grasses. Ciência Rural, 32(2), 357--364. https://doi.org/10.1590/S0103-84782002000200029
Riet-Correa, F., Medeiros, R.M., 2001. Intoxicação por plantas no Brasil e no Uruguai: importância econômica, controle e riscos para a Saúde Pública. Pesquisa Veterinaria Brasileira, 21(1), 38--42. https://doi.org/10.1590/S0100-736X2001000100008
Riet-Correa, B., Castro, M.B., Lemos, R.A.A., Riet-Correa, G., Mustafa, V., Riet-Correa, F., 2011. Brachiaria spp. Poisoning of ruminants in Brazil. Pesquisa Veterinaria Brasileira, 31(3), 183--192. https://doi.org/10.1590/S0100-736X2011000300001
Sá, J.F., Pedreira, M.S., Silva, F.F., Figueiredo, M.P., Rebouças, G.M.N., Souza, D.R., 2011. In vitro ruminal fermentation kinetics of Marandu grass at different harvest ages. Acta Scientiarum. Animal Science, 33(3), 225--231. https://doi.org/10.4025/actascianimsci.v33i3.9462
Santos, J.C.A., Riet-Correa, F., Simões, S.V.D., Barros, C.S.L., 2008. Pathogenesis, clinical signs and pathology of diseases caused by hepatotoxic plants in ruminants and horses in Brazil. Pesquisa Veterinaria Brasileira, 28(1), 1--14. https://doi.org/10.1590/S0100-736X2008000100001
Satter, L.D., Slyter, L.L., 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. British Journal of Nutrition, 32(2), 199--208. https://doi.org/10.1079/BJN19740073
Schofield, P., Pitt, R.E., Pell, A.N., 1994. Kinetics of fiber digestion from in vitro gas production. Journal of Animal Science, 72(11), 2980--2991. https://doi.org/10.2527/1994.72112980x
Statistical Analysis System Institute – SAS, 2002. User’s guide: statistics. Cary.
Tilley, J.M.A., Terry, R.A., 1963. A two stage technique for the in vitro digestion of forage crops. Journal British Grassland Society, 18(2), 104--111. https://doi.org/10.1111/j.1365-2494.1963.tb00335.x
Valadares, R.F.D., Broderick, G.A., Valadares Filho, S.C., Clayton, M.K., 1999. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives. Journal of Dairy Science, 8, 1590--1603. https://doi.org/10.3168/jds.S0022-0302(99)75525-6
Van Soest, P.J., 1994. Nutritional ecology of the ruminant. Cornell University Press, Ithaca, New York, United States of America.
Wina, E., Muetzel, S., Becker, K., 2005. The impact of saponins or saponin-containing plant materials on ruminant production – a review. Journal of Agricultural and Food Chemistry, 53, 8093--8105. https://doi.org/10.1021/jf048053d
Implications
This study demonstrated adverse effects of protodioscin content on different leaf offerings. Due to the fact that more intense grazing can stimulate the sprout and the appearance of new leaves, the dry matter intake of lambs can be influenced by the content of protodioscin in the forages. In vitro digestibility and degradation kinetics revealed less forage degradation, suggesting that protodioscin could be retained for longer in the rumen and, consequently, could lead to natural intoxication by Brachiaria spp. in lambs.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul—FUNDECT, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Financing Code 001).
Author information
Authors and Affiliations
Contributions
MCM Costa, LCV Ítavo, CCBF Ítavo, and AM Dias conceived and designed research, and wrote the manuscript. LCV Ítavo, GS Difante, and RHTB Goes reviewed the manuscript. ES Leal, LM Nonato, ND Kozerski, GJ Moraes, and MVG Niwa conducted experiment, laboratorial analysis, and collection of samples. ALC Gurgel and TFFS Arco conducted data analysis and discussion of results, and reviewed the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics statement
The project was approved by the Committee on Ethics in Animal Use of Federal University of Mato Grosso do Sul (protocol 481/2012).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Costa, M.C.M., Ítavo, L.C.V., Ítavo, C.C.B.F. et al. Natural intoxication caused by protodioscin in lambs kept in Brachiaria pastures. Trop Anim Health Prod 53, 336 (2021). https://doi.org/10.1007/s11250-021-02775-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-02775-3