Abstract
The aim of this study was to evaluate the bioactive effect of Moringa oleifera leaves hydroalcoholic extract as a dietary feed additive on helminths load and growth performances of goats. Initially, the availability of bioactive compounds in M. oleifera hydroalcoholic extract was analysed using gas chromatography-mass spectrometry (GC-MS), which showed the presence of heneicosane (35.69%), 1,2-benzenedicarboxylic acid (22.89%), heptacosane (18.26%), pentatriacontane (4.77%), and hexadecanoic acid ethyl ester (3%) as predominant compounds in the leaves extract. The anthelmintic effect of M. oleifera extract (0 and 60 mL of extract animal-1) was evaluated against disparate nematodes using standard methodology. M. oleifera leaves extract exhibited significant (P = 0.002) anthelmintic activities against Trichuris sp. and Ostertagia sp. with reduced counts of eggs. A completely randomized experiment of 3 treatments comprised of 10 goats in each treatment was designed for the growth performance study. Treatments used in the present experiment were as follows: treatment 1 (T1), 0 mL of extract animal-1; treatment 2 (T2), 30 mL of extract animal-1; and treatment 3 (T3), 60 mL of extract animal-1. Growth performance parameters (body weight, daily weight gain, and feed intake values) of goats fed varied concentrations of M. oleifera extract were estimated as per standard protocols. The T2 and T3 groups’ goats offered significant (P < 0.05) increment in body weight. Daily weight gain of the T2 and T3 groups’ goats was also increased. Group T3 exhibited maximum feed intake value of 588, 678, 652, and 678 g d-1 at 0, 30, 45, and 60 days, respectively. Feed conversion efficiency was increased for T2 and T3 groups’ goats versus T1. Findings of this study concluded that M. oleifera hydroalcoholic extract can be used not only as an effective anthelmintic agent against disparate nematodes but also as a prominent feed additive to improve growth performances of goats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Livestock industries play a paramount socio-economic role in both the developing and developed countries in order to fulfil the growing global demands of meat. Among disparate ruminants and non-ruminants, goats are considered versatile animals due to their valuable contribution in economically deprived countries as pivotal sources of food and income (Moyo et al. 2013). Goats can adapt to adverse environments and can consume disparate kinds of grasses and leaves. Unfortunately, the productivity of goats in most of the sub-tropical regions is often limited because of the low quality or inadequacy of prominent feeds (Damor et al. 2017). On the other hand, currently, gastro-intestinal nematodes are colossal constraint in the productivity of goats of tropical regions. Nematodes of different genera such as Haemonchus, Trichostrongylus, Eimeria, Cooperia, Trichuris, and Ostertagia cause anaemia, weight loss, diarrhoea, reduced yield in milk and wool, alteration in the reproductive cycle, and high mortality of goats (Tayo et al. 2014). Levamisol, mebendazole, morantel, dormactin, thiabendazole, albendazole, and ivermectin are the commonly used conventional anthelminthic drugs. However, these drugs are the leading factor for the emergence of drug-resistant nematodes (Hafiz et al. 2009).
Generally, farmers feed goats with poor nutritious quality of forages containing not only low level of nitrogen, vitamins, and other minerals but also high concentration of lignin and cellulose (Sultana et al. 2014). Thus, it leads to the reduced digestibility and low feed intake (Gebregiorgis et al. 2012). In addition, feeding ruminants a supplement-free diet causes significant loss in weight and sometimes death of animal (Tona et al. 2014). Therefore, the availability of additive-free forages, increasing cost of nutritious feed, ban of antibiotic growth promoters, and development of drug resistant gastro-intestinal nematodes have emphasized veterinarians to identify suitable feedstuff for animals.
Over the past few years, plethora of plants extracts and browse tree’s leaves has been exploited as promising alternative supplements to the existing antibiotics in order to promote growth performances with no toxicity, improve the intake of roughage, enhance the efficiency in animals, and determine anthelmintic properties (Shalaby 2013; Voemesse et al. 2018). Likewise, the incorporation of shrubs and fodder trees could be a potent approach to increase the availability and quality of forages during the dry season (Damor et al. 2017). Moringa oleifera (family: Moringaceae), also called as “miracle tree” or “drumstick tree”, is a fast-growing woody plant in tropical and sub-tropical zones of South Asia, Arabia, and Africa (Moyo et al. 2016). Seeds, flowers, and leaves of this plant have revealed broad spectrum therapeutic applications in the past (Mahfuz and Piao 2019). However, leaves of M. oleifera are highly nutritious containing lipids, proteins, vitamins (vitamin B complex, vitamin C, beta-carotene, and vitamin K), amino acids, and minerals (Onunkwo and George 2015). Leaf extracts constitute low level of polyphenols, thereby showing its potential impact on blood lipid metabolism (Leone et al. 2015). Additionally, varied phytocomponents present in the leaves of M. oleifera show anti-septic, anthelmintic, and antioxidant properties (Ogbunugafor et al. 2011; Moyo et al. 2013; Torondel et al. 2014).
Beside the colossal medicinal importance, M. oleifera has shown its potential as feed additives in animals too. Previous studies revealed the utilization of M. oleifera as alternative feed additives in broilers (Wapi et al. 2013; Voemesse et al. 2018), fish (Afuang et al. 2003), cattle (Mendieta-Araica et al. 2011), horse (Pedraza-Hernández et al. 2019), and goats (Qwele et al. 2013; Sultana et al. 2014; Damor et al. 2017). However, reports on its feeding effects on growth performances of goats and anthelmintic attributes are scarce. Considering this, the present context was focussed not only to determine the anthelmintic activity of M. oleifera leaves hydroalcoholic extract against certain nematodes but also evaluate its potential as a natural feed additive on improving growth performances of goats.
Materials and methods
Study location
The experiment was carried out at Rancho El Cajon located in the Libramiento Oriente Tejupilco-Amatepec. Animals were cared and managed in accordance with the official Mexican standards for animal care (AOAC 1997). This study was conducted in compliance with the guidelines of the animal care and performed in accordance with the ethical standard laid down in the 1996 declaration of Helsinki and its later amendments.
Plant material
Fresh leaves of five M. oleifera trees of different phenological stages were collected from South of the State of Mexico, Tejupilco, Rancho ICAMEX. Samples were brought to the laboratory inside a polythene bag and stored for further experimental purposes.
Hydroalcoholic extract preparation of M. oleifera leaves
Fresh leaves (1 kg) of M. oleifera were dried in shade for 7–10 days at room temperature. After drying, leaves were ground and mixed into 8 L of distilled water, ethanol (99.9%, analytical grade, Fermont®, Monterrey, Mexico), and methanol (99.8%, analytical grade, Fermont®, Monterrey, Mexico), prepared in 80:10:10 ratio. The mixture was incubated at 30 °C for 72 h in an orbital shaker. After required incubation period, the mixture was centrifuged to separate the solid fraction from solution. The collected solution was filtered further through filter paper (Whatman No. 1). The filtrate was concentrated by evaporating the solvent at 50 °C using a rotary evaporator. The hydroalcoholic extract obtained was stored at 4 °C until further analyses (Salem et al. 2015).
Gas chromatography-mass spectrometry (GC-MS) analysis of extract
M. oleifera hydroalcoholic extract was analysed for the presence of bioactive compounds using Focus GC-DSQ Mass Spectrometer (Thermo Scientific, Austin, TX, USA) apparatus with a direct capillary column TG–5MS (30 m × 0.25 μm × 0.25 μm film thickness). The column oven temperature was initially held at 45 °C, increased further by 5 °C min-1 to 250 °C, and then increased to 280 °C (10 °C min-1). The injector and detector (MS transfer line) temperatures were kept at 250 °C. Helium was used as a carrier gas at a constant flow rate of 1 mL min-1 (EL-Hefny et al. 2018). The solvent delay was 2 min, and diluted samples of 1 μL were injected automatically using Autosampler AS3000 coupled with GC in the split mode. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 40–550 in full scan mode. The ion source was set at 200. The components were identified by comparison of their retention times and mass spectra with those of WILEY 09 and NIST 11 mass spectral database (Adams 2009).
Anthelmintic activity
Animals used
Eight goats (age: 5 to 6 months) with an average weight of 15–17 kg were used for determining in vivo anthelmintic activity. Animals were fed balanced diet (Table 1) during two frequencies at 8:00 pm and 5:00 pm in order to maintain homogeneous ruminal fermentation. Animals were provided clean water throughout the day.
Treatments
Treatments used in the present experiment were as follows: treatment 1 or control (T1), 0 mL of extract animal-1; and treatment 2 (T2), 60 mL of extract animal-1.
Parasitology test
The oocyst and egg count technique were performed using the methodology as described by Hernandez et al. (2014). Briefly, faecal samples of each animal were collected individually directly from the rectum before morning feeding on day 0 (pre-extract administration) and thereafter on day 7 and day 15 after the first administration of the extract (on day 0). Faecal samples were evaluated for the presence of worm eggs or Eimeria oocysts by a salt flotation technique (MAFF 1979), and afterwards the parasite load was quantified using the McMaster method (Ojeda-Robertos et al. 2008). Faecal pellets were collected and weighed. One gram of faeces was mixed with 60 mL of NaCl solution. Pellets were broken by a mechanical stirrer and then strained into a 250-mL conical flask using sieve. The strained solution (10 mL) was used for the determination of faecal egg counts using a 2-McMaster chamber with a limit of detection of 200 eggs g-1 of faeces. Faecal cultures were prepared during each sampling time as two replicates of pooled samples from each animal in order to allow the development of third-stage larvae from strongylidae eggs. The genus of the parasite was identified after 12 days at 27 °C in a chamber with constant humidity and oxygenation. Larvae were then collected from a Baermann equipment, and generic identification of Strongylida nematodes was carried out using identification taxonomic keys (van Wyk and Mayhew 2013). Mean egg or oocyst counts (eggs g-1 of faeces) from each animal within each experimental period were used for statistical comparisons.
Growth performance
Animals used
A total of 30 males Boer breed, with an average live weight between 15 and 18 kg, were used in this study. Animals were kept in individual cages of 1 m × 1.5 m. Animals were fed balanced diet (Table 1) during two frequencies at 8:00 pm and 5:00 pm in order to maintain homogeneous ruminal fermentation. In addition, fresh water was provided throughout the day.
Treatments
A completely randomized experimental design of 3 treatments containing 10 goats in each treatment was used. Treatments used in the present experiment were as follows: treatment 1 or control (T1), 0 mL of extract animal-1; treatment 2 (T2), 30 mL of extract animal-1; and treatment 3 (T3), 60 mL of extract animal-1.
Each treatment was used as an experimental unit using the following statistical model:
where,
- Yij:
-
Response variable
- μ:
-
Overall mean
- Ti:
-
Treatment effect (3 treatments)
- εij:
-
Experimental error
Measurement and analysis parameters-
Body weight
Goats were fed non-supplemented and M. oleifera leaves hydroalcoholic extract supplemented diets as per the treatment groups. Body weight was measured at 30, 45, and 60 days (d) for control and treated goats.
Daily weight gain
Daily weight gain was calculated by weighing the goats every 15 days during 60 days of the experiment.
Feed intake
Each animal was offered 700 g of diet daily as per the treatment groups, and the rejection of the feed was measured the next day before offering the fresh diet to each animal in the group of animals for each treatment.
Statistical analyses
Data of the two experiments were analysed as a complete randomized design using SAS software (version 9.1, the ANOVA procedure), and the standard error (± SE) was considered for measuring differences between means. The polynomial contrasts of linear and quadratic effects were used to assess response values of each treatment. Value P < 0.05 was considered significant.
Results
GC-MS analysis of M. oleifera extract
The GC-MS analysis showed the presence of 14 bioactive compounds in M. oleifera leaves hydroalcoholic extract. Among them, Heneicosane (35.69%), 1,2-benzenedicarboxylic acid (22.89%), Heptacosane (18.26%), Pentatriacontane (4.77%), and Hexadecanoic acid ethyl ester (3%) were predominantly present (Table 2).
Anthelmintic activity
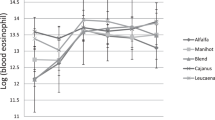
The anthelmintic characteristics of M. oleifera leaves hydroalcoholic extract against the eggs of Eimeria sp., Cooperia sp., Chabertia sp., Moniezia sp., Trichuris sp., Trichostrongylus sp., and Ostertagia sp. were determined which revealed a significant (P = 0.002) effect against Trichuris sp. and Ostertagia sp. with reduced numbers of eggs/gram of faeces (Table 3). However, sampling days and treatment × sampling day exhibited no significant (P > 0.05) effect on the egg output (Fig. 1).
Effect of sampling day (0, 7, and 15 days) after the start of the M. oleifera hydroalcoholic extract administration on the oocyst or egg output of parasites (eggs/gram of faeces) of Eimeria sp. (EIM), Chabertia sp. (CHA), Trichuris sp. (TRH), Trichostrongylus sp. (TRS), and Ostertagia sp. (OST) in growing goats (n = 4)
Body weight
Goats fed with M. oleifera leaves hydroalcoholic extract at varied doses showed significant (P < 0.05) increment in the body weight at 30, 45, and 60 days (Table 4). Consequently, the non-supplemented goats showed significant (P < 0.05) reduction in body weight from 16.58 kg (day 0) to 15.1 (day 30), 15.73 (day 45), and 16.49 kg (day 60). The supplementation of 30 mL of extract into the diet (group T2) exhibited significant (P < 0.05) improvement in body weight of goats from 14.74 kg (day 0) to 16.52 (day 30), 17.72 (day 45), and 19.64 kg (day 60). Likewise, the addition of 60 mL of extract into the diet (group T3) significantly (P < 0.05) increased the body weight of goats from 15.41 kg (day 0) to 19.19 (day 30), 20.87 (day 45), and 22.73 kg (day 60).
Daily weight gain
Goats fed with 30 mL of extract (group T2) and 60 mL of extract (group T3) had significant (P < 0.05) influence on daily weight gain up to 60 days (Fig. 2). The body weight gain increased from 14.66 g (day 0) to 14.74 (day 15), 16.27 (day 30), 17.44 (day 45), and 19.34 g (day 60) in group T2. On the other hand, in group T3, the daily weight gain increased from 15.41 g (day 0) to 17.16 (day 15), 18.9 (day 30), 20.55 (day 45), and 22.38 g (day 60).
Feed intake
The influence of M. oleifera leaves hydroalcoholic extract supplementation on feed intake is illustrated in Table 5. The dry matter intake in group T1 at 0, 30, 45, and 60 days were estimated 490, 533, 473, and 501 g d-1, respectively. In group T2, results showed significant (P < 0.05) increment in the feed intake value of 533, 604, 571, and 604 g d-1 at 0, 30, 45, and 60 days, respectively. In contrary, group T3 exhibited maximum feed intake value of 588, 678, 652, and 678 g d-1 at 0, 30, 45, and 60 days, respectively. In a like manner, the feed conversion efficiency was estimated to be increased for groups T2 and T3 as compared to group T1 (Table 6).
Discussion
Plants-based anthelmintics are considered a sustainable and unique strategy for controlling gastro-intestinal nematodes in ruminants. In this study, M. oleifera leaves hydroalcoholic extract showed a significant (P = 0.002) effect against Trichuris sp. and Ostertagia sp. with reduced numbers of eggs/gram of faeces. This attribute was further supported by few investigations reporting anthelmintic activities of leaves and seeds extracts of M. oleifera against different nematodes of ruminants (Asaolu et al. 2012; Salles et al. 2014). In addition, previous studies depicted potent anthelmintic activity of M. oleifera extract against Haemonchus contortus eggs (Tayo et al. 2014; Cabardo Jr and Portugaliza 2017). To the best of our knowledge, the role of M. oleifera extract as an anthelmintic agent against other nematodes is scanty and probably lacks data suggesting nematocidal activity of M. oleifera leaves hydroalcoholic extract against Trichuris sp. and Ostertagia sp.
M. oleifera has been used as a potent feed additive for improving diversified factors of ruminants and non-ruminants. In this study, we had undertaken a significant attempt to improve growth performances of goats using M. oleifera as dietary feed additive. Goats fed with M. oleifera leaves hydroalcoholic extract showed higher growth performances as compared to the control. This might be mainly due to the presence of high protein content. In the line of our findings, previous studies demonstrated improved performances of goats fed with fodders constituting M. oleifera leaves (Dougnon et al. 2012; Moyo et al. 2016; Mataveia et al. 2019).
In the present investigation, goats fed with M. oleifera leaves hydroalcoholic extract at varied doses showed a significant (P < 0.05) increment in the body weight. Similar observations were reported by Sultana et al. (2014) and Damor et al. (2017) who demonstrated a significant enhancement in the body weight of goats due to the supplementation of M. oleifera leaves. This characteristic might be because of the high palatability and significant amount of protein contents of the feeding diet. In contrary, low fibre content in the control diet might have caused decreased growth rate and, thus, reduced body weight of goats as compared to the treated ones. In this context, goats fed with M. oleifera hydroalcoholic extract exhibited significant (P < 0.05) influence on daily weight gain too. Our findings agreed with the reports of Babeker and Bdalbagi (2015) and Sultana et al. (2015) who depicted significant increment in the daily weight gain by adding M. oleifera leaves in the diet of goats. The variation in the daily weight gain of our study from prior reports might be due to the difference in the intake of dry matter, composition of diet, and physiological nature of the animals used (Sultana et al. 2015).
In the present study, the supplementation of M. oleifera leaves in the feeding diet increased dry matter intake and feed conversion efficiency. Similar findings were reported by Asaolu et al. (2009), Sultana et al. (2015), and Kholif et al. (2016) too who demonstrated a significant (P < 0.05) enhancement in dry matter intake and feed conversion ratio in Moringa foliage treated groups. In contrary, Fadiyimu et al. (2010) determined decrement in dry matter intake with increase in the concentrations of Moringa foliage.
GC-MS analysis revealed the predominance of Heneicosane (35.69%), 1,2-benzenedicarboxylic acid (22.89%), Heptacosane (18.26%), Pentatriacontane (4.77%), and Hexadecanoic acid ethyl ester (3%) in M. oleifera leaves hydroalcoholic extract. These bioactive compounds might be responsible towards the anthelmintic activity and improvement of growth performances of goats. In a different study, Khusro et al. (2020) demonstrated a prominent role of varied compounds (3,5-bis(1,1-dimethylethyl)-phenol, Kaempferol, Moringyne, Niazimicin, and Tetradecanoic acid) of M. oleifera as anti-methanogenic agents in non-ruminants. These compounds might have inhibited methyl-coenzyme M reductase receptor, thereby mitigating methanogenesis in non-ruminants. Tetradecanoic acid revealed potential binding interaction with methyl-coenzyme M reductase, thereby decreasing the methanogenesis process, which had direct impact on increasing animal’s performance via distributing metabolic hydrogen to fermentation pathways (Khusro et al. 2020).
Conclusions
The GC-MS analysis of M. oleifera leaves hydroalcoholic extracts showed the presence of distinct bioactive components, including Heneicosane (35.69%), 1,2-benzenedicarboxylic acid (22.89%), Heptacosane (18.26%), Pentatriacontane (4.77%), and Hexadecanoic acid ethyl ester (3%) as predominant compounds. M. oleifera leaves hydroalcoholic extract (60 mL of extract animal-1) revealed potential anthelmintic activities against Trichuris sp. and Ostertagia sp. Furthermore, the supplementation of M. oleifera leaves hydroalcoholic extract at different concentrations (30 and 60 mL of extract animal-1) into the feeding diet of goats enhanced body weight, daily weight gain, dry matter intake, and feed conversion efficiency. Further in vivo studies and toxicological assays of M. oleifera leaves hydroalcoholic extract are essential for combating the gastro-intestinal nematodes in future.
Data availability
Not available.
References
Adams, R.P. (2009). Geographic variation in the leaf essential oils of Hesperocyparis (cupressus) abramsiana, H. goveniana and H. macrocarpa: systematic implications. Phytologia 91, 226–243.
Afuang, W., Siddhuraju, P., Becker, K. (2003). Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of Moringa (Moringa oleifera Lam) leaves on growth performance and feed utilization in Nile tilapia (Oreochromis niloticus L). Aquaculture Research 34, 1147–1159.
AOAC (Association of Official Analytical Chemists). (1997). Official methods of analysis. 16th ed. Arlington, VA: AOAC.
Asaolu, V., Odeyinka, S., Akinbamijo, O. (2012). Evaluation of anthelmintic attributes of Moringa and bamboo leaves in gastrointestinal nematode-infested West African dwarf goats. Journal of Natural Sciences Research 2, 45–53.
Asaolu, V.O., Odeyinka, S.M., Akinbamijo, O.O., Sodeinde, F.G. (2009). Feed intake, nutrient digestibility and nitrogen utilization of graded levels of Moringa and Bamboo leaves by West African dwarf goats. Bulletin of Animal Health and Production in Africa 57, 361-368.
Babeker, E.A., Bdalbagi, Y.M. (2015). Effect of feeding different levels of Moringa oleifera leaves on performance, haematological, biochemical and some physiological parameters of Sudan Nubian goats. Journal of Animal and Feed Research 5, 50-61.
Cabardo Jr, J.E., Portugaliza, H.P. (2017). Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. International Journal of Veterinary Science and Medicine 5, 30–34.
Damor, S.V., Pawar, M.M., Ankuya, K.J., Gami, Y.M., Srivastava, A.K., Chauhan, H.D., et al. (2017). Effect of feeding different levels of Moringa (Moringa oleifera) leaves on growth performance of Mehsana goat kids. Trends in Biosciences 10, 3190-3193.
Dougnon, T.J., Aboh, B.A., Kpodékon, T.M., Honvou, S., Youssao, I. (2012). Effects of substituition of a pellet of Moringa oleifera to commercial feed on rabbit’s digestion, growth performance and carcass trait. Journal of Applied Pharmaceutical Sciences 2, 15-19.
EL-Hefny, M., Mohamed, A.A., Salem, M.Z.M, Abd El-Kareem, M.S.M., Ali, H.M. (2018). Chemical composition, antioxidant capacity and antibacterial activity against some potato bacterial pathogens of fruit extracts from Phytolacca dioica and Ziziphus spinachristi grown in Egypt. Scientia Horticulturae 233, 225–232.
Fadiyimu, A.A., Alokan, J.A., Fajemisin, A.N. (2010). Digestibility, nitrogen balance and haematological profile of West African dwarf sheep fed dietary levels of Moringa oleifera as supplement to Panicum maximum. Journal of American Science 6, 634-643.
Gebregiorgis, F., Negesse, T., Nurfeta, A. (2012). Feed intake and utilization in sheep fed graded levels of dried moringa (Moringa stenopetala) leaf as a supplement to Rhodes grass hay. Tropical Animal Health and Production 44, 511-517.
Hafiz, A.B., Zafar, I., Muhammad, N.K., Zia-ud-Din, S., Abdul, J. (2009). Anthelmintic activity of Ziziphus nummularia (Back) and Acacia nilotica (fruit) against Trichostrongylid nematodes of sheep. Journal of Ethnopharmacology 123, 325-329.
Hernandez, P.M., Salem, A.Z.M., Elghandour, M.M.M.Y., Cipriano-Salazar, M., Cruz-Lagunas, B., Camacho, L.M. (2014). Anthelmintic effects of Salix babylonica L. and Leucaena leucocephala Lam. extracts in growing lambs. Tropical Animal Health and Production 46, 173–178.
Kholif, A.E., Morsy, T.A., Gouda, G.A., Anele, U.Y., Galyean, M.L. (2016). Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Animal Feed Science and Technology 217, 45-55.
Khusro, A., Aarti, C., Salem, A.Z.M., Barbabosa-Pliego, A., Rivas-Caceres, R.R. (2020). Methyl-coenzyme M reductase (MCR) receptor as potential drug target for inhibiting methanogenesis in horses using Moringa oleifera L.: An in silico docking study. Journal of Equine Veterinary Science 88, 102949, https://doi.org/10.1016/j.jevs.2020.102949
Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., Bertoli, S. (2015). Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. International Journal of Molecular Science 16, 12791–12835.
MAFF. (1979). Parasitological laboratory techniques, Tech. Bull., No. 18. Ministry of agriculture fisheries and food manual of veterinary. Her Majestey’s Stationary Office, London.
Mahfuz, S., Piao, X.S. (2019). Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 9, 431; doi:https://doi.org/10.3390/ani9070431.
Mataveia, G.A., Garrine, C.M.L.P., Pondja, A., Hassen, A., Visser, C. (2019). Impact of supplementation of Moringa oleifera and Leucaena leucacephala tree fodder on the production performance of indigenous goats in Mozambique. Black Sea Journal of Agriculture 2, 93-102.
Mendieta-Araica, B., Sporndly, R., Sanchez, N.R., Sporndly, E. (2011). Moringa (Moringa oleifera) leaf meal as a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livestock Science 137, 10–17.
Moyo, B., Masika, P.J., and Muchenje, V. (2013). Effects of supplementing cross-bred Xhosa lop eared goats with Moringa oleifera Lam. on helminth load and corresponding body condition score, packed cell volume. African Journal of. Agricultural Research 8, 5327-5335.
Moyo, B., Masika, P.J., Muchenje, V. (2016). Potential use of Moringa oleifera leaf in animal feeding: a review. International Journal of Current Agricultural Research 4, 187-194.
Ogbunugafor, H.A., Eeneh, F.U., Ozumba, A.N., Igwo-ezikpe, M.N., Okpuzor, J., Igwilo, I.O., et al. (2011). Physico-chemical and anti-oxidant properties of Moringa oleifera seed oil. Pakistan Journal of Nutrition 10: 409–414.
Ojeda-Robertos, N.F., Torres-Acosta, J.F.J., Ayala-Burgos, A., Aguilar Caballero, A.J., Cob-Galera, L.A., et al. (2008). A technique for the quantification of Duddingtonia flagrans chlamydospores in sheep faeces. Veterinary Parasitology 152, 339–343.
Onunkwo, D.N., George, O.S. (2015). Effects of Moringa oleifera leaf meal on the growth performance and carcass characteristics of broiler birds. Journal of Agriculture and Veterinary Science 8, 63–66.
Pedraza-Hernández, J., Elghandour, M.M.M.Y., Khusro, A., Camacho-Diaz, L.M., Vallejo, L.H., Barbabosa-Pliego, A., et al. (2019). Mitigation of ruminal biogases production from goats using Moringa oleifera extract and live yeast culture for a cleaner agriculture environment. Journal of Cleaner Production 234, 779-786.
Qwele, K., Hugo, A., Oyedemi, S.O., Moyo, B., Masika, P.J., Muchenje, V. (2013). Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sun flower cake and grass hay. Meat Science 2013, 93, 455–462.
Salem, M.Z.M., Zeidler, A., Böhm, M., Mohamed, M.E.A., Ali, H.M. (2015). GC/MS analysis of oil extractives from wood and bark extractives from Pinus sylvestris, Abies alba, Peciaabies, and Larix decidua. BioResources 10, 7725–7737.
Salles, H.O., Braga, A.C.L., do Nascimento, M.T.D.S, Sousa, A.M.P, Lima, A.R., Vieira, L.D.S, et al. (2014). Lectin, hemolysin and protease inhibitors in seed fractions with ovicidal activity against Haemonchus contortus. Brazilian Journal of Veterinary Parasitology 23, 136–143.
Shalaby, H.A. (2013). Anthelmintics resistance; how to overcome it? Iranian Journal of Parasitology 8, 18–32.
Sultana, N., Alimon, A.R., Haque, K.S., Sazili, A.Q., Yaakub, H., Hossain, S.M. (2014). The effect of cutting interval on yield and nutrient composition of different plant fraction of Moringa oleifera tree. Journal of Food, Agriculture and Environment 12, 599-604.
Sultana, N., Alimon, A.R., Huque, K.S., Baba, M., Hossain, J. (2015). Evaluation of Moringa foliage (Moringa oleifera) as goat feed. Iranian Journal of Applied Animal Science 5, 865-871.
Tayo, G.M., Poné, J.W., Komtangi, M.C., Yondo, J., Ngangout, A.M., Mbida, M. (2014). Anthelminthic activity of Moringa oleifera leaf extracts evaluated in vitro on four developmental stages of Haemonchus contortus from goats. American Journal of Plant Sciences 5, 1702-1710.
Tona, G.O., Ogunbosoye, D.O., Bakare, B.A. (2014). The growth performance and nutrient digestibility of West African Dwarf goats fed graded levels of concentrate diet containing Moringa oleifera leaf meal. International Journal of Current Microbiology and Applied Sciences 3, 99-106.
Torondel, B., Opare, D., Brandberg, B., Cobb, E., Cairncross, S. (2014). Efficacy of Moringa oleifera leaf powder as a hand- washing product: A crossover controlled study among healthy volunteers. BMC Complementary and Alternative Medicine 14, 57.
van Wyk, J.A., Mayhew, E. (2013). Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort Journal of Veterinary Research 80, 539
Voemesse, K., Teteh, A., Nideou, D., N'nanlé, O., Gbeassor, M., Decuypere, E., Tona, K. (2018). Effect of Moringa oleifera leaf meal on growth performance and blood parameters of egg type chicken during juvenile growth. International Journal of Poultry Science 17, 154-159.
Wapi, C., Nkukwana, T.T., Hoffman, L.C., Dzama, K., Pieterse E, Mabusela, T., et al. (2013). Physico-chemical shelf-life indicators of meat from broilers given Moringa oleifera leaf meal. South African Journal of Animal Science 43, 43–47.
Funding
This work was financed by the project #UAEM 4304/2017/CI of the Universidad Autónoma del Estado de México, México.
Author information
Authors and Affiliations
Contributions
JPH, AZMS, and MMMYE conceived and designed research. AZMS, MMMYE Supervisores. JPH, ABP, MMMYE and AZMS conducted experiments. MZMS Analysis the bioactive compounds of the M. oleifera leaves. JPH, LMCD, and ABP Helped in parasitology analysis and contributed new reagents or analytical tools. JPH, LMCD, and AZMS analysed data. JPH, MMMYE, AK, and AZMS wrote the manuscript. AK, AZMS, and MMMYE edited and revised the manuscript as well as prepared it for journal submission. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pedraza-Hernández, J., Elghandour, M.M.M.Y., Khusro, A. et al. Assessment on bioactive role of Moringa oleifera leaves as anthelmintic agent and improved growth performance in goats. Trop Anim Health Prod 53, 318 (2021). https://doi.org/10.1007/s11250-021-02745-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-02745-9