Abstract
West Nile fever (WNF) and Rift Valley fever (RVF) are emerging and re-emerging zoonotic diseases of veterinary and public health importance in Africa. Despite the existence of potential vectors and a wide range of hosts, the transmission of these diseases in domestic animals has not been well documented in the South Omo area of Ethiopia. This study aimed to estimate the sero-prevalence of IgG antibodies produced against West Nile virus (WNV) and Rift Valley fever virus (RVFV) infections among cattle in the South Omo area. Between May and June 2019, blood samples were collected from 397 cattle and screened for IgG antibodies against WNV and RVFV infections using enzyme-linked immunosorbent assay (ELISA). The overall sero-prevalence of IgG antibody to WNV infection was 4.8% (95% CI: 2.67–6.88%), while it was 5.0% to RVFV infection (95% CI: 2.87–7.18). Compared to 1–3 years old cattle, those in the age group ≥ 7 years had significantly higher odds of being positive for WNV (AOR = 6.82; 95% CI: 1.72–26.99) and RVFV (AOR = 4.38; 95% CI: 1.08–17.88) infections. The occurrence of WNV and RVFV infections in cattle population in the present study area indicates the risk of transmission to humans. Strengthening the surveillance system and conducting further studies to identify active cases in domestic and wild animals as well as in humans is crucial to reduce the risk of possible outbreaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emerging and re-emerging mosquito-borne zoonotic viral diseases including West Nile fever (WNF) and Rift Valley fever (RVF) pose a significant problem both in animals’ and humans’ health with an economic burden throughout the world, especially in the tropical and subtropical areas (Caminade et al. 2019). About 60% of infectious diseases and 70% of emerging/re-emerging infections of humans are zoonotic in origin. Among the emerging and re-emerging viral diseases of animals, WNF which is caused by West Nile virus (WNV) infection and RVF due to infection with Rift Valley fever virus (RVFV) are the most common ones. These viral diseases are transmitted through mosquitoes’ bite to their vertebrate hosts (Kortekaas 2014; Lustig et al. 2016). The documented outbreaks of these viruses in new geographical locations worldwide might be attributed to climate change, spread and abundance of competent vectors, increased global trade in animals and foodstuffs, persistence of the viruses in wild animals, wide host range, and a complex interface between the host populations (Caminade et al. 2019).
WNV belongs to the family Flaviviridae and genus Flavivirus. The virus was first isolated in Uganda in 1937 (Smithburn et al. 1940). The virus is transmitted to its vertebrate hosts including cattle through the biting of infected female Culex mosquitoes, while feeding blood meal. Birds are considered as the primary host for WNV in which the virus is maintained in a bird–mosquito–bird transmission cycle, while domestic animals and humans are incidental dead-end hosts that do not play a role in the transmission cycle of the virus (Davoust et al. 2016).WNV infection has been reported in many tropical, sub-tropical, and temperate countries in Africa, Europe, Asia, and America (Davoust et al. 2016; Ozkul et al. 2006; Thompson et al. 2012). There are inactivated and recombinant vaccines available for veterinary use only (Seino et al. 2007), but no proven effective treatments are available for both animal and human cases.
RVFV belongs to the family Phenuiviridae, genus Phlebovirus (Maes et al. 2019), and it was first identified in Kenya in 1931 (Daubney et al. 1931). The virus can cause a life-threatening disease both in animals and humans. In animals, RVFV is transmitted both by Aedes and Culex mosquitoes and causes abortions and stillbirths, with a high mortality in neonates and juveniles. Hence, RVFV outbreaks can lead to a significant economic loss at country level (Bett et al. 2018). In humans, RVFV infection is caused by direct contact of infected animal tissue and fluids or by mosquito bite (Bett et al. 2018). The occurrence of RVF has been reported from many African countries (Lagare et al. 2019; Ndengu et al. 2020; Nyakarahuka et al. 2018; Salekwa et al. 2019; Tshilenge et al. 2019; Umuhoza et al. 2017; Van Den Bergh et al. 2019). Countries having heavy rainfall, large mosquito populations, and neighboring to endemic countries are at increasing risk of the disease. Currently, there are vaccines for animals to limit the substantial economic impact of RVF, but there is no specific treatment for RVFV infection in animals or humans (Kortekaas 2014).
The prevention and control of these zoonotic viral diseases mostly relies on an effective surveillance system, early detection, and vaccination of susceptible domestic animals (Caminade et al. 2019). Despite the proximity of the current study area to Kenya, a country reporting repeated outbreaks of zoonotic arboviruses including RVFV and WNV (Bett et al. 2018), the practice of unrestricted livestock and wildlife movements across the borders, and availability of a wide range of potential hosts and abundance of vectors, the occurrence of these diseases in the present study area has not been well studied. Therefore, this study aimed to investigate the sero-prevalence of IgG antibodies against WNV and RVFV infections among RVF unvaccinated cattle populations in the South Omo area, Southern Ethiopia.
Materials and methods
Study area, population, and period
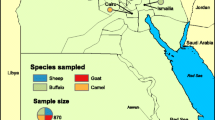
A cross-sectional study was conducted in the Bena Tsemay District of the South Omo Zone of Southern Ethiopia between May and June 2019. The area is located approximately 750 km from Addis Ababa (the capital city of Ethiopia) and borders Kenya to the South. The area resides within the Lower Omo Valley and contains Woito, Neri, and Kako rivers and Mago National Park where a large number of interactions of livestock (cattle, sheep, and goats), birds, wild animals, and human populations take place. The district covers an area of 2923 km2 with estimated human population of 69,099. The altitude of the district ranges between 496 and 2476 m above sea level. Geographical location is 5.010 N to 5.730 N latitude and 36.380 E to 37.070 E longitude. The mean annual rainfall ranges between 800 and 1300 mm, and the mean annual temperature ranges between 18 and 38 °C. For the current study, two kebeles (sub-districts), namely, Goldiya and Shalla-Luka kebeles (Fig. 1), were purposely selected. The presence of a large number of cattle farming and ecological suitability for WNV and RVFV were taken as selection criteria for the kebeles.
Goldiya kebele is located at an elevation of 1156 m above sea level (masl), latitude of 5.601 N and longitude of 36.517 E, and it is closer to Mago National Park. The residents of the Goldiya kebele practice both crop production and animal husbandry. Shalla-Luka kebele is located on the west of Woito river and southeast of Goldiya kebele at an elevation of 660 masl, latitude of 5.442 N and longitude of 36.825 E, and it is about 41 km far from Goldiya kebele. The residents of the Shalla-Luka Kebele are more of pastoralists. More detailed information about the sub-district and kebeles is described elsewhere (Legesse et al. 2018).
Sample size estimation and sampling techniques
To the best of our knowledge, there is no reliable information about the sero-prevalence of WNV or RVFV infection in cattle in the present study area or Ethiopia. Assuming 50% sero-prevalence of IgG antibodies to WNV and/or RVFV infections in cattle in the study area, with 95% confidence in the estimate and 5% margin of error, the minimum sample size was estimated to be 385. Prior to data collection, the total sample size was allocated proportionally to the cattle population in the study kebeles. The required number of cattle from each kebele was estimated after visiting livestock watering points. Six watering points from Goldiya kebele along Kako River closer to Mago National Park and seven community ponds from Shalla-Luka kebele were identified and recorded to Mobile Topographer Pro Application version 14.0.0 for Android using Keyhole markup language (KML). The KML layers’ file of watering points and community ponds were exported to Google Earth Pro7.3.2.5776 (64-bit) to measure distances in between watering points within kebeles aimed for reducing sample collection repetition. From Goldiya kebele, two watering points at distance of 7 km in between and in Shalla-Luka kebele three watering points at average distance of 8.7 km in between were selected for sample collection. Animal welfare concerns during restraint were taken into account; all samples were collected in improvised stock chute made with poles which were giving services for vaccinations.
Data collection and laboratory investigation
Before commencement of the data collection, the herd/cattle owner was asked his/her willingness to include his/her cattle in the study. The cattle included in this study were those with age greater than or equal to 1 year, apparently in good health, born and grown in the study area. A 3 ml jugular vein blood sample was collected into serum separator vacutainer test tubes, serum separated and stored at − 20 °C until screening. Enzyme-linked immunosorbent assays (ELISA) for IgG antibodies against WNV and RVFV infections were performed according to the manufacture’s protocol (Abbexa Ltd., Cambridge UK/www.abbexa.com). The samples were analyzed in duplicate and the optical density reading was performed at 450 nm using a 96-well ELISA plate reader (Multiskan™ FC Microplate Photometer). The result was interpreted as positive or negative on the basis of the manufacturer’s recommended cut-off values (cut-off value = negative control + 0.15).
Additionally, interviews were carried out with the owners of the cattle regarding information related to age of the animal, number of parity and history of abortion in female, birth place of the study animal, introduction of new animal to the herd in the last 12 months, and migration to other areas for searching grass/water.
Data analysis
Data were entered into EpiData Software v.3.1 and analyzed using SPSS Version 25. Frequencies and percentages were used to summarize the general characteristics of the study cattle. The sero-prevalence of IgG antibody to WNV and RVFV infections were estimated by dividing the number of study cattle with positive test results by the total number of study cattle tested. Univariable logistic regression analysis was performed to assess the unadjusted association between the sero-positivity for IgG antibody and the hypothesized individual potential risk factors. Multivariable logistic regression analysis was performed to assess the effect of each of the independent variables on the outcome variable (sero-positivity) after adjusting each independent variable for all other variables. P value of below 0.05 was considered as indicator of statistically significant association.
Ethical considerations
The study protocol was approved by the Institutional Review Board (IRB) of the Aklilu Lemma Institute of Pathobiology, Addis Ababa University. The aim of the study was explained to each cattle owner and verbal consent was obtained. Blood sample collection was carried out under aseptic conditions by experienced veterinary laboratory technicians.
Results
General characteristics of the study cattle
A total of 397 cattle (50.1% males, age range from 1 to 12 years, mean age ± SD = 4.64 ± 2.74 years) were included in the current study. Among the study cattle, 17.4% live together with new animals introduced into the herd within the last 12 months and 4.5% of the female cattle had a history of abortion (Table 1).
Sero-prevalence of IgG antibody to WNV infection
Out of the 397 cattle tested for anti-WNV IgG antibody, 19 were sero-positive (4.8%; 95% CI: 2.67–6.88%) which was slightly higher in male cattle than female cattle (6.0% versus 3.5%; p = 0.24) though the difference was not statistically significant (Table 2). The sero-prevalence of IgG antibody to WNV infection increases with age of the cattle and the highest (15.4%) was observed in the age group of ≥ 10 years old (Fig. 2).
Sero-prevalence of IgG antibody to RVFV infection
Out of the 397 cattle tested for IgG antibody against RVFV infection, 20 were sero-positive giving an overall sero-prevalence of 5.0% (95% CI: 2.87–7.18) (Table 3). Sero-prevalence slightly increased with age and the highest sero-prevalence (7.7%) was observed in the age group of ≥ 10 years old (Fig. 3).
Independent predictors of IgG antibody sero-positivity for WNV infection
Results from multivariable logistic regression analysis are summarized in Table 4. Compared to cattle in the age group 1–3 years, those whose age is 7 years or above were about seven times more likely to be sero-positive for WNV infection (AOR = 6.82; 95% CI: 1.72–26.99).
Independent predictors of IgG antibody sero-positivity for RVFV infection
Multivariable logistic regression analysis also showed that cattle in the age group ≥ 7 years were about four times more likely to be sero-positive for RVFV infection compared to in the age group 1–3 years (AOR = 4.38; 95% CI: 1.08–17.88) (Table 4).
Discussion
We investigated sero-prevalence of WNV and RVFV infection among local cattle breeds in the South Omo area of Southern Ethiopia. None of the cattle enrolled in the current study was vaccinated for RVFV since the vaccine was not available in Ethiopia. The key findings are (1) the sero-prevalence of IgG antibody to WNV infection was 4.8%; (2) the sero-prevalence of IgG antibody to RVFV infection was 5.0%; and (3) the sero-positivity for WNV and RVFV among the study animals increased with age.
Although cattle are as susceptible to WNV infection as horses, there is limited published information on sero-prevalence IgG antibody to WNV infection in cattle. The sero-prevalence of IgG antibody to WNV infection revealed in this study (4.8%) was nearly similar to the sero-prevalence observed in Turkey (Ozkul et al. 2006), but higher than the sero-prevalence (2.2%) reported in a Palestine cattle (Darwish et al. 1983). The observed differences in the sero-prevalence of WNV infection might be attributed to difference in geographical factors like altitude, rainfall, and temperature of the countries or study areas. In the current study, the geography of the Omo river basin and of other small but permanent rivers like Woito river is favorable to mosquito vectors as well as to different species of birds including migratory birds (Debebe and Selemon 2019). These favorable climatic conditions may facilitate transmission of WNV infection in the area. Beyond animal health, the current finding has important implications for human health. Humans have a risk of acquiring the infection from the cattle they rear and live with which needs further investigation.
The current finding shows that the sero-prevalence of IgG antibody to RVFV infection is ~ 5.0% among the cattle population in the study area which is lower than reports elsewhere including 26% in Kenya (Bett et al. 2018), 7.7% in Tanzania (Salekwa et al. 2019), 27% in Uganda (Nyakarahuka et al. 2018; Budasha et al. 2018), 16.8% in Rwanda (Umuhoza et al. 2017), 12.37% in Democratic Republic of Congo (Tshilenge et al. 2019), 34% in South Africa (Van Den Bergh et al. 2019), and 53.3% in Niger (Lagare et al. 2019). However, the sero-prevalence in Southern Ethiopia was higher than the sero-prevalence (1.7%) recently reported from a study conducted in Zimbabwe (Ndengu et al. 2020). Since the cattle enrolled in this study were apparently healthy, the finding of the current study indicates that the observed infection of WNV and RVFV stems from past exposure or sub-clinical circulation of the viruses without demonstrating sign and symptoms of the clinical disease in the cattle population. The possibility of clinical or sub-clinical circulation of the virus not excluded as geographic knowledge–based modeling study (mapping) conducted in East African countries indicated Ethiopia as being a suitable area for RVFV amplification and spread (Tran et al. 2016), but this subject is of further investigation. In addition, Southern Ethiopia (the current study area) was prospectively identified as an area with a high risk for RVF outbreaks (Anyamba et al. 2009). This was suggested due to excessive rainfall and associated flooding in the areas as a result of El Niño phenomena that induce hatching of desiccated mosquito eggs from the soil which contains trans-ovarially transmitted RVFV with a potential to be transmitted to the local domestic animals (Rostal et al. 2010).
Higher sero-positivity of both WNV and RVFV infections were significantly associated with older age of cattle compared to younger cattle which is in line with the previous studies in Uganda (Nyakarahuka et al. 2018) and Rwanda (Umuhoza et al. 2017). This might be attributed to length of exposure, with a higher chance of exposure to the pathogen over a lifespan. The increase of sero-prevalence of both WNV and RVFV infections with age may also indicate the possibility of ongoing/stable transmission or inter-epidemic/endemic transmission of the viruses. However, the possible importation of the viruses from neighboring countries cannot be excluded since animals commonly move across the border between Ethiopia and Kenya.
Despite the current study reported the first sero-epidemiological evidence of WNV and RVF in cattle in the South Omo area of Ethiopia, the finding should be interpreted in light of the following limitation. Serologic cross-reaction of WNV with other related flaviviruses and that of RVFV with other Bunyaviruses were not excluded due to limited resources to perform confirmatory test using viral neutralization tests.
In general, the current study revealed exposure of cattle population in South Omo area to WNV and RVFV as detected by IgG antibodies based ELISA. This is suggestive of either past exposure and/or subclinical circulation of the viruses. The risk of exposure to both WNV and RVFV is higher among older cattle as compared to younger cattle. The findings warrant the risk of transmission of WNV and RVFV to humans in the area. Therefore, strengthening surveillance system, risk management, and communication are essential. In addition, further studies of WNV and RVFV active cases in domestic and wild animals, as well as in humans in the area, is highly recommended to predict and reduce possible future outbreaks.
Data availability
All data generated or analyzed during this study are included in the manuscript and its supplementary information files.
References
Anyamba, A., Chretien, J. P., Small, J., et al., 2009. Prediction of a Rift Valley fever outbreak. Proceedings of the National Academy of Sciences of the United States of America,106: 955-959.
Bett, B., Lindahl, J., Sang, R., et al., 2018. Association between Rift Valley fever virus seroprevalences in livestock and humans and their respective intra-cluster correlation coefficients, Tana River County, Kenya. Epidemiology and Infection, 147: 1-9.
Budasha, N. H., Gonzalez, J. P., Sebhatu, T. T., et al., 2018. Rift Valley fever seroprevalence and abortion frequency among livestock of Kisoro district, South Western Uganda 2016: a prerequisite for zoonotic infection. BMC Veterinary Research,14: 271.
Caminade, C., Mcintyre, K. M. & Jones, A. E., 2019. Impact of recent and future climate change on vector-borne diseases. Annals of the New York Academy of Sciences,1436: 157-173.
Darwish, M. A., Hoogstraal, H., Roberts, T. J., et al., 1983. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene,77: 442-445.
Daubney, R., Hudson, J. & Garnham, P., 1931. Enzootic hepatitis of Rift Valley fever; an undescribed virus disease of sheep, cattle and man from East Africa. Journal of Pathology and Bacteriology, 34: 545–579.
Davoust, B., Maquart, M., Roqueplo, C., et al., 2016. Serological survey of West Nile virus in domestic animals from Northwest Senegal. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.),16: 359-361.
Debebe, D. & Selemon, T., 2019. Community composition, abundance and major conservation threats of bird fauna of South of Omo National Park, Ethiopia. International Journal of Conservation Science, 10: 565-574.
Kortekaas, J., 2014. One health approach to Rift Valley fever vaccine development. Antiviral Research,106: 24-32.
Lagare, A., Fall, G., Ibrahim, A., et al., 2019. First occurrence of Rift Valley fever outbreak in Niger, 2016. Veterinary Medicine and Science,5: 70-78.
Legesse, M., Endale, A., Erku, W., et al., 2018. Community knowledge, attitudes and practices on Yellow fever in South Omo area, Southern Ethiopia. PLoS Neglected Tropical Diseases,12: e0006409.
Lustig, Y., Hindiyeh, M., Orshan, L., et al., 2016. Mosquito surveillance for 15 years reveals high genetic diversity among West Nile viruses in Israel. The Journal of Infectious Diseases,213: 1107-1114.
Maes, P., Adkins, S., Alkhovsky, S. V., et al., 2019. Taxonomy of the order Bunyavirales: second update 2018. Archives of Virology,164: 927-941.
Ndengu, M., Matope, G., Tivapasi, M., et al., 2020. Seroprevalence and associated risk factors of Rift Valley fever in cattle and selected wildlife species at the livestock/wildlife interface areas of Gonarezhou National Park, Zimbabwe. Onderstepoort Journal of Veterinary Research, 87:e1-e7.
Nyakarahuka, L., De St Maurice, A., Purpura, L., et al., 2018. Prevalence and risk factors of Rift Valley fever in humans and animals from Kabale district in Southwestern Uganda, 2016. PLoS Neglected Tropical Diseases,12: e0006412.
Ozkul, A., Yildirim, Y., Pinar, D., et al., 2006. Serological evidence of West Nile Virus (WNV) in mammalian species in Turkey. Epidemiology and infection,134: 826-829.
Rostal, M. K., Evans, A. L., Sang, R., et al., 2010. Identification of potential vectors of and detection of antibodies against Rift Valley fever virus in livestock during interepizootic periods. American Journal of Veterinary Research,71: 522-526.
Salekwa, L. P., Wambura, P. N., Matiko, M. K., et al., 2019. Circulation of Rift Valley fever virus antibody in cattle during inter-epizootic/epidemic periods in selected regions of Tanzania. The American Journal of Tropical Medicine and Hygiene,101: 459-466.
Seino, K. K., Long, M. T., Gibbs, E. P., et al., 2007. Comparative efficacies of three commercially available vaccines against West Nile Virus (WNV) in a short-duration challenge trial involving an equine WNV encephalitis model. Clinical and Vaccine Immunology,14: 1465-1471.
Smithburn, K. H., Burke, A. & Paul, J., 1940. A neurotropic virus isolated from the blood of a native of Uganda. The American Journal of Tropical Medicine and Hygiene,20: 471–492.
Thompson, N. N., Auguste, A. J., Coombs, D., et al., 2012. Serological evidence of flaviviruses and alphaviruses in livestock and wildlife in Trinidad. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.),12: 969-978.
Tran, A., Trevennec, C., Lutwama, J., et al., 2016. Development and assessment of a geographic knowledge-based model for mapping suitable areas for Rift Valley fever transmission in Eastern Africa. PLoS Neglected Tropical Diseases,10: e0004999.
Tshilenge, G. M., Dundon, W. G., De Nardi, M., et al., 2019. Seroprevalence of rift Valley fever virus in cattle in the Democratic Republic of the Congo. Tropical Animal Health and Production,51: 537-543.
Umuhoza, T., Berkvens, D., Gafarasi, I., et al., 2017. Seroprevalence of Rift Valley fever in cattle along the Akagera-Nyabarongo rivers, Rwanda. Journal of the South African Veterinary Association,88: e1-e5.
Van Den Bergh, C., Venter, E. H., Swanepoel, R., et al., 2019. High seroconversion rate to Rift Valley fever virus in cattle and goats in far northern KwaZulu-Natal, South Africa, in the absence of reported outbreaks. PLoS Neglected Tropical Diseases,13: e0007296.
Acknowledgments
The authors would like to acknowledge data collectors, cattle owners, and South Omo Zone and Bena Tsemay District livestock and fishery resources offices and community leaders for facilitating the data collection.
Funding
The study was financially supported by the Office of Vice President for Research and Technology Transfer, Addis Ababa University (Ref No. RD/PY662/2016). ELISA kits used to analyze the collected samples were donated by Panorama Research Institute, CA, USA. The funders had no role in study design, data collection, analysis, decision to publish, or interpretation of the findings.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Adugna Endale, Mengistu Legesse, Woldaregay Erku Abegaz, Girmay Medhin, Bayilla Geda.
Acquisition of data: Adugna Endale, Mengistu Legesse, Woldaregay Erku Abegaz, Girmay Medhin, Bayilla Geda.
Formal analysis and interpretation: Adugna Endale, Mengistu Legesse, Woldaregay Erku Abegaz, Girmay Medhin, Bayilla Geda, Daniela Michlmayr, James W. Larrick, Getahun Asebe.
Writing—original draft: Adugna Endale.
Writing—review and editing: Adugna Endale, Mengistu Legesse, Woldaregay Erku Abegaz, Girmay Medhin, Bayilla Geda, Daniela Michlmayr, James W. Larrick, Getahun Asebe.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(SAV 12 kb)
Rights and permissions
About this article
Cite this article
Endale, A., Michlmayr, D., Abegaz, W.E. et al. Sero-prevalence of West Nile virus and Rift Valley fever virus infections among cattle under extensive production system in South Omo area, southern Ethiopia. Trop Anim Health Prod 53, 92 (2021). https://doi.org/10.1007/s11250-020-02506-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-020-02506-0