Abstract
The aim of this study was to evaluate the compatibility among virus isolation (VI), ELISA, and PCR for diagnosis of the major viral agents (BPIV-3, BRSV, BVDV, and BoHV-1) responsible for BRD in the field samples. For that purpose, a total of 193 samples (133 nasal swabs and 60 lung tissue samples) from cattle with respiratory signs in northwestern Turkey were examined. For VI, all the samples were inoculated at least 3 blind passages onto MDBK cell culture. In addition, the samples were tested by hemadsorption assay and RT-PCR for BPIV-3; nested RT-PCR for BRSV; immunoperoxidase monolayer assay, antigen-ELISA, and RT-PCR for BVDV; and antigen-ELISA and PCR for BoHV-1. The detected 1 (0.52%) BPIV-3 isolate was found to be in the genotype BPIV-3c. No BRSV isolate could be obtained, while 5 (2.59%) samples were evaluated positive in nested-RT PCR. The presence of BVDV antigen in 10 (5.18%) samples and the BVDV genome in 5 (2.59%) samples were detected, while non-cytopathogenic BVDV isolates were obtained only in 2 (1.04%) samples. The detected BVDV strains fell into the genetic clusters of BVDV-1a, -1f, and -1l. For detection of BoHV-1, although viral isolation and Ag-ELISA results were negative, presence of BoHV-1.1 genome was detected in 2 (1.04%) samples. By the results of VI, ELISA, and PCRs, 10.88% (21/193) of samples were found positive for the evaluated viruses. Depending on the obtained data, combined uses of the diagnostic methods were evaluated to be more reliable for routine diagnosis of bovine respiratory viruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine respiratory disease (BRD) complex that causes significant economic losses is one of the major problems in cattle worldwide. BRD predisposing factors for cattle are insufficient colostrum feeding and/or stress factors such as transportation, weaning, poor maintenance conditions, and abnormal weather change. Economic losses are due to increase of mortality, slaughter, decrease in milk yield, bodyweight loss, reduced performance, and costs of therapy (Urban-Chmiel and Grooms 2012). In order to control and prevent losses, it is important to make timely and accurate diagnosis of diseased cattle (Fulton and Confer 2012).
Viral pathogens are usually acted as the primary cause of respiratory infections in cattle. The most important viral pathogens responsible for BRD include Bovine parainfluenza virus type 3 (BPIV-3; bovine respirovirus-3), Bovine respiratory syncytial virus (BRSV; bovine orthopneumovirus), Bovine viral diarrhea virus (BVDV), and Bovine herpesvirus type 1 (BoHV-1) (Autio et al. 2007; Urban-Chmiel and Grooms 2012). Because these viral pathogens cause immunosuppression through various mechanisms (Jones and Chowdhury 2010), animals become more susceptible to secondary infections, i.e., Mycoplasma bovis, Mannheimia haemolytica, Histophilus somni, and Pasteurella multocida (Autio et al. 2007). On the other side, BVDV infections can persist in breeding herds as immunotolerant persistent infection, and BoHV-1 can cause latency after primary infection in cattle.
Clinical findings related to BRD infections are mostly similar and includes fever, nasal and ocular discharge, depression, dyspnea, and cough. The lesions and clinical findings in cattle with BRD cannot provide precise information about specific viral etiology. Therefore, the definitive and reliable diagnosis of the etiology of BRD cases is important both for the determination of control measures and the monitoring of the infections. For definitive diagnosis of BRD cases, methods such as virus isolation (VI), enzyme-linked immunosorbent assay (ELISA), or polymerase chain reaction (PCR), which differ in terms of test duration, sensitivity, and specificity, are used (Caswell et al. 2012). Virus isolation for detection of viral pathogens has been widely used as a conventional diagnostic method until the development of molecular-based tests. Advantage and disadvantage of conventional and molecular methods have been discussed (Fulton and Confer 2012). Therefore, the combined application of these methods may provide more accurate results in the diagnosis of the agents.
This study aimed at the following: (1) detection of BPIV-3, BRSV, BVDV, and BoHV-1 in respiratory samples (nasal swab and lung) from BRD-affected cattle by various diagnostic methods; (2) sequencing and genotyping of detected agents; and (3) comparison of compatibility among the results of VI, ELISA, and PCR protocols.
Materials and methods
Animals and sampling
Total of 193 samples which include both nasal swabs (n = 133) from live animals and lung tissue samples (n = 60) from dead/slaughtered cattle at different life stages were collected. All the sampled animals were Holstein breed and the farms were closely managed. Sampled animals have exhibited clinical symptoms such as fever, nasal and ocular discharge, increased lung auscultation sound, dyspnea, and cough.
The nasal swab and lung samples collected in Bursa and neighboring provinces (Balıkesir, Kutahya, Bilecik, and Kocaeli province) between years 2012 and 2017 were used in the study. Out of 193 samples, 126 (nasal swab = 113; lung tissue = 13) were collected between the years November 2016 and June 2017 through field sampling, while 67 samples (nasal swab = 20; lung tissue = 47) were archived materials submitted for routine diagnosis between dates 2012 and 2017 (Table 1). The sampling was conducted according to permission from local ethical committee for animal experiments (HADYEK 2016-05/03).
The collected nasal swab samples were placed into tubes containing 2 mL sterile phosphate-buffered saline (PBS) and brought to the laboratory under cold chain conditions. Swab samples were vortexed and centrifuged at + 4 °C, 3000 rpm for 20 min. The supernatant was passed through a 220-nm filter and collected into the stock tube. Because some of the respiratory viruses are affected by freeze-thawing, 10% (v/v) dimethylsulfoxide (DMSO) was added to the samples and stored at − 80 °C until testing.
Tissue samples collected from the lungs with lesions were also delivered to the laboratory under the cold chain conditions. Approximately 1 g of lung tissue material was homogenized in 9 mL sterile PBS. The prepared homogenate was centrifuged at + 4 °C, 3000 rpm for 20 min. The supernatant was filtered through a 220-nm filter and transferred to a stock tube. DMSO (10%, v/v) was added to the samples and stored at − 80 °C until testing.
Cell line and viruses
Madin Darby bovine kidney (MDBK) cell line was used for the production of control viruses as well as virus isolation and immunoperoxidase test steps. In the preparation of MDBK cell cultures, Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma, D7777, USA) which supplemented with 10% fetal calf serum (FCS) (Capricorn, FBS-11A, South America), 100 UI/mL penicillin/streptomycin (BI, 03-031-1B, Israel), and 250 μL/mL amphotericin B solution (BI, 03-028-1B, Israel) were used.
BPIV-3 strain SF-4, BRSV strain Atue, cytopathogenic reference strain BVDV-NADL, noncytopathogenic (ncp) strain BVDV-FLK, and BoHV-1 strain Cooper were used as control viruses during the tests. Viruses were obtained from the virus collection of Bursa Uludağ University, Faculty of Veterinary Medicine, Department of Virology.
Virus isolation in cell culture and typing of viruses
The collected samples were subjected to virus isolation (VI) onto MDBK cell line in order to obtain field isolates. Following 3 blind passages, immunoperoxidase monolayer assay (IPMA) was applied to detect the presence of non-cytopathogenic pestiviruses, and hemadsorption test was performed for possible quick detection of BPIV-3 in infected cell cultures.
For VI, 24-well plates were coated with MDBK cells at a concentration of 1 × 105cells/mL. After 24 h, the medium was removed and 200 μL of inoculum was added. Plates were incubated for 1 h at 37 °C for virus adsorption. Then, 1 mL of DMEM without FCS was added to the each well of the plates. The culture medium was changed 24 h after the inoculation. For the next 7 days, the cells were daily observed at inverted light microscope for cytopathogenic effects (CPE). At the end of 7th day, the cells were harvested by freezing at − 80 °C, and thawing at 37 °C. The 3 blind passages were applied to all the samples. In some suspicious samples, blind passages were continued after the third passage.
For demonstration of non-cytopathogenic Pestivirus propagation in inoculated cell cultures, the IPMA protocol was implemented as reported by Alpay and Yeşilbağ (2015). For hemadsorption test, MDBK cell suspension was settled into 24-well plate and after 24 h incubation, 100 μL of supernatant previously collected from blind passage of suspicious specimens was inoculated into wells. BPIV-3 (SF-4) was inoculated as the positive control and PBS as negative control. Subsequently, after 24 h, the cell monolayers were washed with sterile PBS. Two hundred microliters of 0.5% washed bovine erythrocyte suspension in Alsever’s solution was added in all of the wells. The plate was incubated at room temperature for 1 h, the erythrocyte suspension was removed, and cell monolayers were washed 3 times with sterile PBS. When the results were evaluated under inverted light microscope, the presence of erythrocytes attached to monolayer cells was recorded as the positive reaction for hemadsorption.
Detection of viral antigen with ELISA
All the samples were tested with commercially available antigen ELISA kits of BVDV (IDEXX, 99-43810) and BoHV-1 (Biox Diagnostics, BioK 335/1) for detecting viral antigens and to compare with the PCR results. The test protocol was applied as recommended by the manufacturers. The test plates were read by an automatic microplate reader (Thermo-Multiskan EX) at 450 nm wavelength, and the results were evaluated using suggested calculations.
Nucleic acid isolation, PCR, RT-PCR, and sequencing
A commercial nucleic acid isolation kit (Macherey-Nagel Nucleospin Virus) was used for extraction of total viral nucleic acid from 133 nasal swab and 60 lung tissue samples. The kit protocol was applied as recommended by the manufacturer. For RT-PCR, the cDNA was synthesized using iScript cDNA synthesis kit (Biorad, 170-8891). All the samples were tested either by polymerase chain reaction (PCR) method for BoHV-1, or by RT-PCR for BRSV, BPIV-3, and panpestivirus. Gene-specific primers were used for identification of BPIV-3 (hemagglutinin-neuraminidase gene), BRSV (fusion gene), panpestivirus (5′UTR), and BoHV-1 (gC gene). Some optimization studies have been applied to PCR and RT-PCR protocols based on previously published studies (Table 2).
The PCR and RT-PCR products were visualized by electrophoresis on the 1.5% agarose gel including 0.05 μL/mL Safe View Classic (Applied Biological Materials, Canada). The samples that were positive in electrophoresis were selected for Sanger sequencing (Macrogen, South Korea). Sequence data were aligned by the ClustalW multiple alignment tool using the BioEdit software. Maximum likelihood analysis was performed with 1000 bootstrap replication using MEGA7. For designing the phylogenetic trees, the reference sequences were obtained from GenBank.
Statistical analyses
Chi-square test was applied to evaluate the data obtained from this study in terms of both the viral infections and diagnostic materials (nasal swab and lung tissue sample). For this purpose, IBM SPSS Statistics 20 (Chicago, IL, USA) package program was used. p < 0.05 was accepted as the statistical significance limit for all evaluations.
Results
VI, IPMA, hemadsorption test, and antigen ELISA
All the 193 samples were entirely subjected to blind passages in MDBK cell culture at least for three times. During the virus isolation, total of 5 nasal swab samples were detected to produce CPE in the MDBK cells. Among these samples, the sample ET-81 created CPE at the 4th passage level, while it was shown CPE in the first passage of the other 4 samples but not continued in the following passage levels. By hemadsorption test applied at 4th passage level, only 1 nasal swab sample (ET-81) was found positive.
IPMA test was performed to investigate the presence of ncp pestiviruses in 193 samples which were subjected to 3 blind passages. By this method, 2 samples which are 1 nasal swab (ET-94) and 1 lung tissue sample (DO-48) were found positive for ncp pestivirus.
All of the 193 samples (133 nasal swabs and 60 lung tissue samples) were tested by commercial ELISAs both for BVDV and BoHV-1. Despite BVDV antigens being detected in 2 nasal swab samples (ET-94 and ET-109) and 8 lung tissue samples (DO-1, DO-2, DO-14, DO-15, DO-16, DO-48, DO-49, and DO-60), there was no positive result obtained for BoHV-1 antigens.
PCR/RT-PCR and phylogenetic analyses
For nucleic acid detection of mentioned viruses (BPIV-3, BRSV, Pestivirus, and BoHV-1) in the samples, PCR and RT-PCR were employed. Only one sample which is nasal swab sample ET-81 was found positive for BPIV-3. Two nasal swabs (ET-63 and ET-124) and 3 lung tissue samples (DO-7, DO-18, and DO-19) were positive for BRSV by nested RT-PCR. Four nasal swabs (ET-64, ET-84, ET-101, and ET-102) and 1 lung tissue sample (DO-48) were positive for Pestivirus viral RNA by RT-PCR. BoHV-1 viral DNA was not detected in 60 lung samples tested by PCR, while 2 out of 133 nasal swab samples (ET-120 and ET-121) were positive.
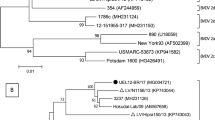
The phylogenetic tree generated by comparison of the HN gene sequences of BPIV-3 genome and the isolate ET-81 demonstrated that Turkish isolate is located in the cluster of BPIV-3 genotype C (Fig. 1). Three out of 5 samples (DO-7, ET-63, and ET-124) positive for BRSV partial F gene region were selected for sequencing. The phylogenetic analysis showed that the BRSV sequences obtained in this study are closely related to each other, and these sequences dropped in the same cluster with previously detected BRSV/TR/Erz/2014 sequence in Turkey (Fig. 2). Another 3 samples (DO-48, ET-64, and ET-94) were selected for sequence analysis for Pestivirus in 5′UTR. By phylogenetic analysis, the sample DO-48 was characterized as BVDV-1f, while ET-64 was in subgenotype BVDV-1a and ET-94 was in subgenotype BVDV-1l (Fig. 3). The phylogenetic analysis of the gC gene region of BoHV-1 showed that identified ET-121 sequence was in the same cluster with reference strain Cooper (DQ173733) within BoHV-1.1 (Fig. 4).
Phylogenetic tree for partial F gene sequences of BRSV. DO-7 (lung), ET-63 (swab), and ET-124 (swab) samples were originated from locations Bursa-Yenişehir (at December 2017), Kocaeli-Karamürsel (at November 2016), and Bursa-Yenişehir (at January 2013), respectively. Reference sequences were downloaded from GenBank. Accession numbers are shown on the phylogeny tree
Phylogenetic tree for 5′UTR gene sequences of BVDV. DO-48 (lung), ET-94 (swab), and ET-64 (swab) samples were originated from the locations Bursa-Mustafakemalpaşa (at January 2011), Bilecik-Pazaryeri (at May 2017), and Kocaeli-Karamürsel (at November 2016), respectively. Reference sequences were downloaded from GenBank. Accession numbers are shown on the phylogeny tree
Comparison of diagnostic methods
During this study, all the samples were inoculated onto MDBK cell culture for at least 3 blind passages for virus isolation. Also all samples were analyzed by hemadsorption test and RT-PCR for BPIV-3, by nested RT-PCR for BRSV, by immunoperoxidase monolayer assay, antigen-ELISA, and RT-PCR for Pestivirus and by antigen-ELISA and PCR methods for BoHV-1. According to results of applied methods, 10.88% (n = 21; 10/133 nasal swab and 11/60 lung tissue) of total 193 samples were evaluated to be positive for BPIV-3, BRSV, BVDV, or BoHV-1. During the analyses, no multiple infections were detected in the samples. Ten samples were found positive by BVDV-antigen ELISA (Pestivirus), and 13 samples were found positive by PCRs, while isolates were obtained only from 3 samples.
BPIV-3 virus isolate was obtained from 1 nasal swab sample in the 4th passage level by virus isolation. In the same sample, the presence of BPIV-3 viral genome was detected by RT-PCR and it was also found positive by hemadsorption test (0.52%; 1/193). Although no BRSV isolate could be determined by virus isolation method, 5 samples (2.59%; 5/193) were positive for BRSV nucleic acid. Cytopathogenic BVDV biotype was not detected in the samples by virus isolation. In 2 samples, the results of IPMA, ELISA, and RT-PCR methods were compatible with each other, while 3 samples were positive by only RT-PCR and 8 samples were positive only by ELISA. For BoHV-1, virus isolation and ELISA results were found to be negative for all the samples, while only 2 (1.04%; 2/193) samples were positive for the presence of BoHV-1 viral DNA (Table 3).
Statistical analyses
In terms of positive detection rates for tested samples, there were a statistically significant differences between BVDV and BPIV-3 data and between BVDV and BoHV-1 data (p < 0.05) by chi-square test. In addition, considering the detection of at least one of the viral agents (BPIV-3, BRSV, BVDV, and BoHV-1), the positivity rate detected in lung samples (11/60) was found to be statistically significantly higher than the nasal swab samples (10/133) (p < 0.05).
Discussion
Respiratory system infections of cattle with high fever, wheezing, cough, nasal and ocular discharge, and loss of condition are among the most common problems in cattle herds (Autio et al. 2007). BRD-causing viruses may lead persistent and/or latent infections in the herd, creating increased economic losses. Therefore, laboratory tests are used to identify and eliminate the causative agent of infection, which is an important step in disease prevention and control programs (Fulton and Confer 2012).
Bovine respiratory infections are common all around the world, and almost 25% of the cattle examined clinically are suffering from respiratory diseases (Kennerman et al. 2003). BRD cases are demonstrated by serological and virological studies in Turkey (Alkan et al. 2000; Okur Gumusova et al. 2007; Yeşilbaǧ et al. 2008; Öner and Yeşilbağ 2018). In the mentioned studies, seroprevalence for BPIV-3, BRSV, BVDV, and BoHV-1 agents was determined in between the rates of 18–99%, 40–100%, 41–86%, and 17–98%, respectively.
In the present study, 133 nasal swabs and 60 lung tissue samples collected from 193 cattle showing BRD clinical findings were evaluated for the presence of BPIV-3, BRSV, BVDV, and BoHV-1. The obtained samples were analyzed by PCR for each of the viral agents, and also were examined by conventional virological methods including virus isolation, immunoperoxidase monolayer assay, and antigen ELISA. In a previous study, 95 nasal swab samples investigated for BRD agents (BPIV-3, BRSV, BVDV, and BoHV-1) in Turkey, 20.0% (n = 19) were found positive by direct immunofluorescence technique (Alkan et al. 2000). As a result of the real-time PCR method applied to 1364 nasal swab samples collected during a respiratory epidemics of cattle in Ireland, 29.7% of the samples were reported positive for the presence of BRSV, BPIV-3, BVDV, and BoHV-1 (O’Neill et al. 2014). In a study conducted in Australia, 6.5% (n = 97) of 1484 nasal swab specimens were positive for the same agents by quantitative RT-PCR (Moore et al. 2015). As well as the sensitivity of the diagnostic methods, the differences in the prevalence values determined in the mentioned studies may vary depending on the age ranges in the sampled animals and sampling periods. Changes in breeding systems, animal movements, and health practices in different countries should also be considered an important factor.
There are various studies in different countries to report detection and isolation of BPIV-3 from clinical cases of BRD (Autio et al. 2007; O’Neill et al. 2014; Thanthrige-Don et al. 2018). Similar to the present study, Timurkan et al. (2019) reported 3 (1.93%) out of 155 samples in Eastern Turkey found positive for BPIV-3 by RT-PCR (Timurkan et al. 2019). The data obtained both by this study and by Timurkan et al. (2019) show that the detection rate of BPIV-3 is low in clinical BRD cases in Turkey.
The isolate BPIV-3 ET-81, found to be genetically closely related to Japanese and Chinese isolates, was located in the genotype BPIV-3c. In Turkish cattle, the presence of BPIV-3 nucleic acid was previously reported by two different research groups (Albayrak et al. 2019; Timurkan et al. 2019). For phylogenetic analyses, Albayrak et al. (2019) targeted the BPIV-3 F gene region, while Timurkan et al. (2019) targeted the BPIV-3 M gene region. In our study, molecular analyses were performed according to the HN gene region, because the HN gene of HPIV-3 and BPIV-3 has more epitopic differences as compared to F and N gene regions (Klippmark et al. 1990) and between HPIV-3 and BPIV-3 has no cross-reaction for HN gene region in the RT-PCR (de Almeida Vaucher et al. 2008). Although phylogenetic analyses of Turkish BPIV-3 isolates were performed according to three different gene region (F, M, and HN), the phylogenetic characterization data have been met in the same results. Thus, the current data confirms the common circulation of genotype BPIV-3c among Turkish cattle populations all around the country. Moreover, present study reports genetic typing of BPIV-3 from western Turkey for the first time.
It has been widely demonstrated that BRSV plays a significant role in bovine respiratory disease complex (Alkan et al. 2000; Roshtkhari et al. 2012; Fulton et al. 2016; Öner and Yeşilbağ 2018). Presence of BRSV by RT-PCR from 121 nasal swabs and lung tissues from BRD cases was demonstrated, but no virus isolation occurred (Fulton et al. 2016). For an example of successful virus isolation, one of 2 CPE positive samples was determined on the 5th day of the first passage, whereas, in the other sample, it was found to form CPE on the 12th day of the 5th passage (Smith et al. 1975). Due to the slow virus replication, virus isolation studies for BRSV are laborious and require long passaging to detect CPE in cell culture. In addition, isolation studies are mostly unsuccessful due to the excessive lability of the virus (Smith et al. 1975; Kimman et al. 1986). This situation explains the failure of BRSV isolation despite that 5 samples were positive by RT-PCR in this study.
Since the F gene region can detect most strains of BRSV, does not cross-react between BRSV and HRSV (Oberst et al. 1993), and has few variable regions (Eleraky et al. 2003), the nested RT-PCR method was applied in this study using primers specific to the F gene region. In a molecular characterization study in Erzurum province of Turkey, 2 out of 155 samples (1.29%) were determined positive for BRSV (Timurkan et al. 2019). By phylogenetic analysis, sequences detected in our study and in Erzurum province (Access. no: KY499619) dropped in the same cluster. It is shown that the BRSV strains circulating in Turkey are close to each other, because of 98–99% identities between our sequences (DO-7, ET-63, and ET-124) and the KY499619 sequence according to the nucleotide BLAST database.
There are many studies both on the role of BVDV in respiratory system infections in Turkey (Alkan et al. 2000; Yeşilbaǧ et al. 2014) and evaluating the sensitivity of diagnostic methods for BVDV (Tuncer Göktuna and Yeşilbağ 2017). Also, there is a study of different diagnostic materials used for the diagnosis of BVDV (VanderLey et al. 2011). In this study, differences were detected between the results of the tests applied for BVDV detection. As pointed out previously (Tuncer Göktuna and Yeşilbağ 2017), the discrepancy between ELISA and RT-PCR results may be due to the high RNase activity in tissue samples and consequently the degradation of viral RNA. The higher rate of positivity detected by ELISA and RT-PCR methods in the present study suggests that BVD virus particles may lose infectivity for cell culture during the process.
BVDV-1 strains are more common than BVDV-2 in nasal swab and lung samples collected from cattle with respiratory system problems (Fulton et al. 2000). Moreover, subgenotype BVDV-1b is dominant in the USA, India, Argentina, and South America; and BVDV-1d is dominant in Africa (Obando et al. 1999; Fulton et al. 2002; Pogranichniy et al. 2011; Pecora et al. 2014). In a survey for an outbreak of severe pneumonia and hemorrhagic enteritis in Turkey, one out of 4 isolates was reported to be BVDV-1l subgenotype and other 3 isolate were in BVDV-1r, which created a new subgroup. In this study, 3 different BVDV subgenotypes (BVDV-1f, BVDV-1a, and BVDV-1l) were detected in the samples and these results are similar to those previously identified subgenotypes in Turkey (Yeşilbaǧ et al. 2008, 2014; Oǧuzoǧlu et al. 2012).
Various studies showed that virus isolation, ELISA, and PCR methods should be used in a combined approach for detection of BoHV-1 (El-Kholy 2005; Peshev and Christova 2010). Considering the results of our present work, for the detection of BoHV-1, PCR method is a sensitive and practical diagnostic method compared to the virus isolation and ELISA.
Significant differences were reported between the gC amino acid sequences of BoHV-1 and BoHV-5 (Claus et al. 2005). Therefore, the gC gene is considered to be an important target for molecular typing. In the present study, the gene region of ET-121 which was detected as BoHV-1 positive by PCR was sequenced, by phylogenetic analysis revealing that the obtained sequence was included in the subtype BoHV-1.1. Previously, both BoHV-1.1 and BoHV-1.2 were characterized by sequence analysis of gC gene region of BoHV-1 isolates in Turkey (Bilge-Dağalp et al. 2017; Yeşilbaǧ et al. 2018). The sequence ET-121 of this study was characterized in the same cluster with Cooper (USA), UY1999 (Uruguay), and Turkish sequences (Bilge-Dağalp et al. 2017), indexing in BoHV-1.1 subtype.
Consequently, the presence of BPIV-3, BRSV, BVDV, and BoHV-1 agents was determined through the phylogenetic analyses and the different diagnostic methods in respiratory system infections in Northwestern Turkey (Bursa, Balıkesir, Kütahya, Bilecik, and Kocaeli provinces). In addition, the compatibility of results collected by virus isolation, antigen ELISA, and PCR was compared. In general, it was shown that more reliable results can be obtained by combined application of diagnostic methods in the routine diagnostic studies for viral respiratory infections in cattle.
References
Albayrak, H., Yazici, Z., Ozan, E., Tamer, C., Abd, A., Wahed, E., Wehner, S., Ulrich, K. and Weidmann, M., 2019. Characterisation of the First Bovine Parainfluenza Virus 3 Isolate Detected in Cattle in Turkey. Veterinary Sciences, 6, 1–6
Alkan, F., Özkul, A., Bilge-Dagalp, S., Yeşilbağ, K., Oguzoglu, T.C., Akça, Y. and Burgu, I., 2000. Virological and serological studies on the role of PI-3 virus, BRSV, BVDV and BHV-1 on respiratory infections of cattle I.The detection of etiological agents by direct immunofluorescence technique. Deutsche Tierarztliche Wochenschrift, 107, 193–195
Alpay, G. and Yeşilbağ, K., 2015. Serological relationships among subgroups in bovine viral diarrhea virus genotype 1 (BVDV-1). Veterinary Microbiology, 175, 1–6
Autio, T., Pohjanvirta, T., Holopainen, R., Rikula, U., Pentikäinen, J., Huovilainen, A., Rusanen, H., Soveri, T., Sihvonen, L. and Pelkonen, S., 2007. Etiology of respiratory disease in non-vaccinated, non-medicated calves in rearing herds. Veterinary Microbiology, 119, 256–265
Bilge-Dağalp, S., Farzani, T. and Doğan, F., 2017. The molecular and antigenic characterization of Turkish Bovine Herpevirus Type 1 (BoHV-1) isolates In:, 5th Veterinary Herpesvirus Symposium of the European Society For Veterinary Virology,
Caswell, J.L., Hewson, J., Slavić, D., DeLay, J. and Bateman, K., 2012. Laboratory and Postmortem Diagnosis of Bovine Respiratory Disease Veterinary Clinics of North America - Food Animal Practice, 28, 419–441
Claus, M.P., Alfieri, A.F., Folgueras-Flatschart, A. V, Wosiacki, S.R., Médici, K.C. and Alfieri, A.A., 2005. Rapid detection and differentiation of bovine herpesvirus 1 and 5 glycoprotein C gene in clinical specimens by multiplex-PCR. Journal of Virological Methods, 128, 183–188
de Almeida Vaucher, R., Simonetti, A.B. and Roehe, P.M., 2008. RT-PCR for detection of bovine parainfluenza virus type 3 (bPIV-3) Acta Scientiae Veterinariae, 36, 215–220
Eleraky, N.Z., Kania, S.A., Evermann, J.F. and Potgieter, L.N.D., 2003. Comparison of targeting F and G protein genes to detect bovine and ovine respiratory syncytial viruses. Journal of Veterinary Diagnostic Investigation, 15, 277–280
El-Kholy, A.A., 2005. Molecular and immunological detection of bovine herpesvirus-1 in clinical specimens. The Egyptian journal of immunology / Egyptian Association of Immunologists, 12, 125–136
Esteves, P., Dellagostin, O., Pinto, L., Silva, A., Spilki, F., Ciacci-Zanella, J., Hübner, S., Puentes, R., Maisonnave, J., Franco, A., Rijsewijk, F., Batista, H., Teixeira, T., Dezen, D., Oliveira, A., David, C., Arns, C. and Roehe, P., 2008. Phylogenetic comparison of the carboxy-terminal region of glycoprotein C (gC) of bovine herpesviruses (BoHV) 1.1, 1.2 and 5 from South America (SA) Virus Research, 131, 16–22
Fulton, R.W. and Confer, A.W., 2012. Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Canadian Veterinary Journal, 53, 754–761
Fulton, R.W., Purdy, C.W., Confer, A.W., Saliki, J.T., Loan, R.W., Briggs, R.E. and Burge, L.J., 2000. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Canadian Journal of Veterinary Research, 64, 151–159
Fulton, R.W., Ridpath, J.F., Saliki, J.T., Briggs, R.E., Confer, A.W., Burge, L.J., Purdy, C.W., Loan, R.W., Duff, G.C. and Payton, M.E., 2002. Bovine viral diarrhea virus (BVDV) 1b: Predominant BVDV subtype in calves with respiratory disease. Canadian Journal of Veterinary Research, 66, 181–190
Fulton, R.W., d’Offay, J.M., Landis, C., Miles, D.G., Smith, R.A., Saliki, J.T., Ridpath, J.F., Confer, A.W., Neill, J.D., Eberle, R., Clement, T.J., Chase, C.C.L., Burge, L.J. and Payton, M.E., 2016. Detection and characterization of viruses as field and vaccine strains in feedlot cattle with bovine respiratory disease Vaccine, https://doi.org/10.1016/j.vaccine.2016.04.020
Jones, C. and Chowdhury, S., 2010. Bovine herpesvirus type 1 (BHV-1) is an important cofactor in the bovine respiratory disease complex Veterinary Clinics of North America - Food Animal Practice, 26, 303–321
Kennerman, E., Yilmaz, Z. and Şentürk, S., 2003. Evaluation of Cattle and Sheep Admited to Large Animal Clinics of Internal Medicine Department of Uludağ University Veterinary Faculty (1990 - 2000) (in Turkish) Uludag Univ. J. Fac. Vet. Med., 22, 19–25
Kimman, T., Zimmer, G., Straver, P. and de Leeuw, P., 1986. Diagnosis of bovine respiratory syncytial virus infections improved by virus detection in lung lavage samples. American Journal of Veterinary Research, 47, 143–147
Klippmark, E., Rydbeck, R., Shibuta, H. and Norrby, E., 1990. Antigenic variation of human and bovine parainfluenza virus type 3 strains. Journal of General Virology, 71, 1577–1580
Moore, S.J., O’Dea, M.A., Perkins, N. and O’Hara, A.J., 2015. Estimation of nasal shedding and seroprevalence of organisms known to be associated with bovine respiratory disease in Australian live export cattle. Journal of Veterinary Diagnostic Investigation, 27, 6–17
O’Neill, R., Mooney, J., Connaghan, E., Furphy, C. and Graham, D.A., 2014. Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: A retrospective study Veterinary Record, https://doi.org/10.1136/vr.102574
Obando, R.C., Hidalgo, M., Merza, M., Montoya, A., Klingeborn, B. and Moreno-López, J., 1999. Seroprevalence to bovine virus diarrhoea virus and other viruses of the bovine respiratory complex in Venezuela (Apure State) Preventive Veterinary Medicine, 41, 271–278
Oberst, R.D., Hays, M.P., Hennessy, K.J., Stine, L.C., Evermann, J.F. and Kelling, C.L., 1993. Identifying bovine respiratory syncytial virus by reverse transcription- polymerase chain reaction and oligonucleotide hybridizations. Journal of Clinical Microbiology, 31, 1237–1240
Oǧuzoǧlu, T.C., Muz, D., Yilmaz, V., Timurkan, M.Ö., Alkan, F., Akça, Y. and Burgu, I., 2012. Molecular Characteristics of Bovine Virus Diarrhoea Virus 1 Isolates from Turkey: Approaches for an Eradication Programme Transboundary and Emerging Diseases, 59, 303–310
Okur Gumusova, S., Yazici, Z., Albayrak, H. and Cakiroglu, D., 2007. Seroprevalence of bovine viral respiratory diseases Acta Veterinaria, 57, 11–16
Öner, E.B. and Yeşilbağ, K., 2018. Seroprevalance of respiratory viruses and detection of persistent BVD virus infection in beef cattle (in Turkish) Ankara Üniv Vet Fak Derg, 65, 1–7
Pecora, A., Malacari, D.A., Ridpath, J.F., Perez Aguirreburualde, M.S., Combessies, G., Odeón, A.C., Romera, S.A., Golemba, M.D. and Wigdorovitz, A., 2014. First finding of genetic and antigenic diversity in 1b-BVDV isolates from Argentina Research in Veterinary Science, 96, 204–212
Peshev, R. and Christova, L., 2010. Diagnostic and molecular epidemiological investigation of Bulgarian bovine herpes virus 1 strains by PCR and restriction enzyme 6–11
Pogranichniy, R.M., Schnur, M.E., Raizman, E.A., Murphy, D.A., Negron, M. and Thacker, H.L., 2011. Isolation and genetic analysis of bovine viral Diarrhea virus from infected cattle in Indiana Veterinary Medicine International, 2011
Roshtkhari, F., Mohammadi, G. and Mayameei, A., 2012. Serological evaluation of relationship between viral pathogens (BHV-1, BVDV, BRSV, PI-3V, and Adeno3) and dairy calf pneumonia by indirect ELISA Tropical Animal Health and Production, 44, 1105–1110
Smith, M.H., Frey, M.L. and Dierks, R.E., 1975. Isolation, characterization, and pathogenicity studies of a bovine respiratory syncytial virus. Archives of Virology, 47, 237–247
Thanthrige-Don, N., Lung, O., Furukawa-Stoffer, T., Buchanan, C., Joseph, T., Godson, D.L., Gilleard, J., Alexander, T. and Ambagala, A., 2018. A novel multiplex PCR-electronic microarray assay for rapid and simultaneous detection of bovine respiratory and enteric pathogens. Journal of Virological Methods, 261, 51–62
Timurkan, M.O., Aydin, H. and Sait, A., 2019. Identification and molecular characterisation of bovine parainfluenza virus-3 and bovine respiratory syncytial virus: First report from Turkey. Journal of Veterinary Research (Poland), 167–173
Tuncer Göktuna, P. and Yeşilbağ, K., 2017. Evaluation of diagnostic methods for the detection of pestiviruses in clinical samples. Turkish Journal of Veterinary and Animal Sciences, 41, 175–179
Urban-Chmiel, R. and Grooms, D.L., 2012. Prevention and Control of Bovine Respiratory Disease. Journal of Livestock Science, 3, 27–36
VanderLey, B., Ridpath, J. and Sweiger, S., 2011. Comparison of detection of bovine virus diarrhea virus antigen in various types of tissue and fluid samples collected from persistently infected cattle. Journal of Veterinary Diagnostic Investigation, 23, 84–86
Vilcek, S., Elvander, M., Ballagi-Pordany, A. and Belak, S., 1994. Development of Nested PCR Assays for Detection of Bovine Respiratory Syncytial Virus in Clinical Samples. Journal Of Clinical Microbiology, 32, 2225–2231
Vilcek, S., Nettleton, P., Paton, D. and Belak, S., 1997. Molecular characterization of ovine pestiviruses. Journal of General Virology, 78, 725–735
Yeşilbaǧ, K., Förster, C., Bank Wolf, B., Yilmaz, Z., Alkan, F., Ozkul, A., Burgu, I., Rosales, S.C., Thiel, H. and Matthias, K., 2008. Genetic heterogeneity of bovine viral diarrhoea virus (BVDV) isolates from Turkey: Identification of a new subgroup in BVDV-1 Veterinary Microbiology, 130, 258–267
Yeşilbaǧ, K., Förster, C., Ozyiǧit, M.O., Alpay, G., Tuncer, P., Thiel, H.J. and König, M., 2014. Characterisation of bovine viral diarrhoea virus (BVDV) isolates from an outbreak with haemorrhagic enteritis and severe pneumonia. Veterinary Microbiology, 169, 42–49
Yeşilbaǧ, K., Alpay, G. and Öner, E.B., 2018. Isolation and characterization of BoHV-1.2 in Turkish cattle. In:, 11th International Congress for Veterinary Virology – 12th Annual Meeting of EPIZONE, (Vienna)
Zhu, Y.-M., Shi, H.-F., Gao, Y.-R., Xin, J.-Q., Liu, N.-H., Xiang, W.-H., Ren, X.-G., Feng, J.-K., Zhao, L.-P. and Xue, F., 2011. Isolation and genetic characterization of bovine parainfluenza virus type 3 from cattle in China. Veterinary Microbiology, 149, 446–451
Funding
This study was supported by Bursa Uludag University Research Fund (Project No: DDP(V)-2016/09) as a PhD thesis on “Molecular Diagnosis of Bovine Respiratory Viruses in Clinical and Necropsy Samples”. Dr. E.B. Toker is currently supported by Scientific and Technological Research Council of Turkey (TUBİTAK) as postdoc position, Project No: 119 O 571.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toker, E.B., Yeşilbağ, K. Molecular characterization and comparison of diagnostic methods for bovine respiratory viruses (BPIV-3, BRSV, BVDV, and BoHV-1) in field samples in northwestern Turkey. Trop Anim Health Prod 53, 79 (2021). https://doi.org/10.1007/s11250-020-02489-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-020-02489-y