Abstract
Organic acids have exhibited great potential as an antibiotic replacement and as an additive work tremendously for health maintenance of broiler chicken. To explore more about organic acids, a total of 900 day-old broiler chicks (Cobb-500) were procured from a local hatchery and distributed into 9 treatment groups having 5 replicates of 20 birds each; duration of the biological trial was of 35 days. Group T1 served as control group without any dietary supplementation. Other groups T2 and T3 were boosted with different levels (125 g/ton and 250 g/ton) of enramycin (antibiotic), T4, T5, and T6 were supplemented with different levels (2 kg/ton, 3 kg/ton, and 4 kg/ton) of ammonium formate and ammonium propionate, and T7, T8, and T9 were fed with different levels (2 kg/ton, 3 kg/ton, and 4 kg/ton) of calcium formate and calcium propionate. The findings declared significant improvement (P < 0.05) in body weight gain and FCR in groups T3, T5, and T9 while feed intake was not affected. Carcass evaluation depicted significantly better (P < 0.05) dressed and eviscerated weight along with carcass yield (T5, T7, T8, T9). Broilers fed organic acid supplemented diet had significantly lower (P < 0.05) total bacterial count (T3, T5, T8, T9) and positively improved (P < 0.05) villi length (T5, T6, T9) as compared with control group. However, total protein, globulin, HDL, and LDL levels were determined to be non-significant (P > 0.05) among different organic acids treatments. Hence, organic acids can be utilized as a better replacement for antibiotics. Supplementation of organic acids at a dose rate of 3 kg/ton and 4 kg/ton is recommended for efficient performance of broilers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aim of poultry production is to attain maximum growth and better feed conversion ratio (FCR), with optimum bird’s performance during the whole rearing period. A balanced diet with effective feed additives is necessary for efficient growth and production of the poultry industry. Utilization of specific feed additives results in better production and effective feed conversion to some extent (Khan and Iqbal 2016; Çetingül et al. 2020). Its been more than a century, antibiotic growth enhancers have been widely used for getting optimum growth in broilers. Non-prophylactic usage for the purpose of growth promotion in birds of antibiotics was discovered in the early 1940s. But antibiotic remnants embraced in poultry meat and eggs have aroused great concern about their supplementation. Transfer of antibiotic residues to humans via poultry products has been a matter of discussion. In many European countries, utilization of antibiotics has been strictly abolished and several actions have been taken to limit their use in many other countries as well for marketing and their use as growth promoters (Waldroup et al. 2003). The poultry industry is always inquisitive about non-antibiotic options to enhance growth performance. There are many antibiotic substitutions such as organic acids, probiotics, prebiotics, essential oils, and phytogenic products (Huyghebaert et al. 2011).
Organic acids have exhibited tremendous potential as a replacement to antibiotics and have been officially accepted by European Union (EU) to be utilized in the same manner as feed additives and preservatives since a long time in order to improve feed quality (Ricke 2003). Organic acids can be defined as carboxylic acids with acidic properties, having chemical structure of R-COOH, including fatty acids. However, it is not possible to make use of each one of them as additives in feed. Because of unique chemical and physical properties, short chain fatty acids such as acetic, propionic, butyric, lactic, and formic acid have been given preference as enhancers of growth in poultry diets. All these types are available commercially which are either directly added in the feed as feed additives or as supplements in drinking water (Khan and Iqbal 2016; Byrd et al. 2001; Russell and Diez Gonzales 1998). Organic acids have been used in various forms either individually, in a mixture, or with different salts. When organic acids are given in the form of salts, they are simple to handle due to their less volatile form and also because they are in solid state as salts, it’s easier to incorporate them in feed. They are considered less damaging, easily dissolves in water, and odorless (Huyghebaert et al. 2011).
In broiler’s diet, their supplementation results in better utilization of nutrients and improved performance. Dietary supplementation of organic acids in broilers has a positive impact on carcass yield and dressing percentage (Dehghani-Tafti and Jahanian 2016). Recently, the incorporation of organic acids is gaining popularity as these are supposed to enhance palatability, deplete gastric pH, have antimicrobial effects, cause depletion of bacterial counts in the gastrointestinal tract, and increase digestibility of nutrients. The antimicrobial mechanism of organic acids is still not completely known but they are able to exhibit bacteriostatic and bactericidal properties (Naseri et al. 2012; Wang et al. 2009). Most of the organic acids at low pH range are undissociated and lipophilic. Therefore, across the cell membrane of bacteria, their diffusion is remarkable. Once they are in the bacterial cell, dissociation of weak organic acid due to higher pH of bacterial cytoplasm happens and the cytoplasm pH falls down immediately and ultimately death of bacteria occurs (Dibner and Buttin 2002). In addition to the antimicrobial activity of organic acids, they possess some other biological activities as well such as better intestinal health for efficient utilization and absorption of nutrients, hence improving broiler’s overall health and performance. For intestinal villi, organic acids are considered readily accessible cause of energy and stimulate their differentiation and multiplication, and consequently escalates feed efficiency (Adil et al. 2011).
It was hypothesized, therefore, that supplementing organic acids in broiler’s diet would have a positive impact on overall broiler performance. The current study was carried out with the objectives to scout the effect of dietary supplementation of organic acids on growth performance, carcass characteristics, cecal microbiota, gut morphology, and serum biochemistry of the broiler chicken.
Materials and methods
A research trial was conducted at Research Center (Poultry Shed) of the University of Veterinary and Animal Sciences, Jhang Campus, Pakistan, with approval of project and ethics of research from the Directorate of the Advanced Studies and Research Board of the university (DAS/947/26-04-18). Birds were kept in controlled environment containing pan feeder for feed and nipple drinker for availability of water. Pellet feed and fresh drinking water were given ad libitum. The temperature of the shed was maintained at 32 °C for early 5 days then gradually declined according to standard management protocols up to a 25 °C. Humidity of the shed was kept (65 ± 5%). The lightening period was 23 h per day. Bedding material was rice husk (8 cm thickness) and floor space was 5.5 × 3.5 × 2 ft. (L × W × H) according to standard requirement of the chicks. Birds were vaccinated according to standard procedures. A total of 900 day-old broiler chicks (Cobb-500) were acquired from a local hatchery and distributed into 9 treatment groups having 5 replicates of 20 birds each according to completely randomized design. Group T1 served as control in the absence of any dietary supplementation. Other groups T2 and T3 were fed with different levels (125 g/ton and 250 g/ton) of enramycin (Enradin 8% MSD, US; Enrapak 4%, CN), T4, T5, and T6 were treated with different levels (2 kg/ton, 3 kg/ton, and 4 kg/ton) of ammonium formate and ammonium propionate (Sal-Zap by Alltech, Inc.), and T7, T8, and T9 were supplemented with different levels (2 kg/ton, 3 kg/ton, and 4 kg/ton) of calcium formate and calcium propionate (Addcon XF Superfine by ADDCON). The duration of biological trial was of 35 days. The experimental layout is depicted in Table 1 and basal ration composition is presented in Table 2. At weekly intervals, progressive feed consumption and body weight gain of birds were recorded on individual basis. Feed conversion ratio was also measured by taking into consideration weekly body weight gain and feed intake of a respective group. At the end of the research trial final feed intake, body weight gains and FCR were recorded. For carcass, total bacterial count, gut morphology, and serum biochemistry evaluation (35 days), 3 birds from each replicate (male) were chosen at random and slaughtered according to the Halal (Islamic) method. Dressed weight after slaughtering, defeathering, and evisceration of birds along with carcass yield was recorded by using digital weighing balance. Eviscerated weight was taken by removing all internal organs except the giblets and at the end giblets weight (heart, liver, gizzard) was taken. The ceca were removed post-slaughtering and placed in 0.9% NaCl solution along with digesta. A 10-fold dilution method was used and 1 ml of each dilution was inoculated on agar plates by spread plate method for the purpose of total bacterial count (TBC) and counting was done according to standard procedure (Hartemink and Rombouts 1999; Hassan et al. 2010). For gut morphology 2 cm tissue samples from Meckel’s diverticulum till ceca were collected and washed with distilled water. Samples were preserved in 10% neutral buffer formalin (SJQW03140 Sigma-Aldrich, Merck) till 48 h; tissues were then washed and treated with ethyl alcohol (L850107 BDH). After embedding in paraffin, with the help of microtome, 4 mm section was cut, on a slide carefully mounted and stained by HE (hematoxylin & eosin) stain (Medilines modified H 0706; E 920–921). Villus height was taken considering lamina propria up to crest of the villus. Between the crypt and villus, crypt depth was examined by using a light microscope (Panda et al. 2009). From the slaughtered birds, blood was collected for serum evaluation and placed into non-heparinized tubes, centrifuged for 15 min at 3000 rpm for serum separation and was stored at − 20 °C in the freezer until any further analysis. A standard protocol using commercial laboratory kits was conducted for biochemical metabolites determination present in serum including total proteins, globulin, HDL, and LDL (Kamal and Ragaa 2014).
Statistical analysis
Data were analyzed by one-way ANOVA technique (Steel et al. 1997) using PROC GLM in SAS software (version, 9.1). Significant treatment means were compared by using Tukey’s (1953) HSD test at P ≤ 0.05 probability level.
Results
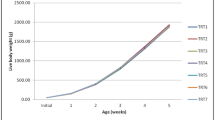
Dietary acidification influence on broiler growth performance is shown in Table 3. Broilers treated with organic acids showed no significant difference (P > 0.05) in feed intake among the treatments. Significantly higher (P < 0.05) final body weight gain and better final FCR were observed in the treatments supplemented with ammonium formate and ammonium propionate (T5 group), calcium formate and calcium propionate (T9 group), and enramycin (Chinese origin) T3 group, at the rate of 3 kg/ton, 4 kg/ton, and 250 g/ton, respectively.
Dressed and eviscerated weights were significantly improved (P < 0.05) in T5 (ammonium formate and ammonium propionate) at 3 kg/ton and T7, T8, and T9 (calcium formate and calcium propionate) at 2 kg/ton, 3 kg/ton, and 4 kg/ton supplemented group respectively. Significantly highest (P < 0.05) carcass yield was depicted among various treatment groups including T5 and T6 (ammonium formate and ammonium propionate) at 3 kg/ton and 4 kg/ton, and T7, T8, and T9 (calcium formate and calcium propionate) at 2 kg/ton, 3 kg/ton, and 4 kg/ton respectively as compared with the control group. There was a non-significant difference (P > 0.05) between the treatments for the giblets weight (heart, liver, and gizzard) (Tables 4 and 5).
Significantly lower (P < 0.05) total bacterial count was seen in treatments supplemented with enramycin (Chinese origin) T3 group at 250 g/ton, ammonium formate and ammonium propionate (T5 group) at 3 kg/ton, and calcium formate and calcium propionate (T8, T9 groups) at 3 kg/ton and 4 kg/ton in control group comparison (Table 6). Organic acids supplementation positively influenced the gut morphology. Significantly improved (P < 0.05) villi length was observed in treatment groups T5 and T6 (ammonium formate and ammonium propionate) at 3 kg/ton and 4 kg/ton each and T9 group (calcium formate and calcium propionate) at 4 kg/ton as compared with the control group whereas crypt depth was not significantly different (Table 7). However, total protein, globulin, HDL, and LDL levels showed non-significant (P > 0.05) findings among different organic acids treatments (Table 8).
Discussion
The experimental trial was escorted to scrutinize the influence of dietary acidification on overall health of broilers. The findings in the current research trial regarding body weight gain and feed conversion ratio agreed with Kamal and Ragaa (2014) who reported that butyric, formic, and lactic acid supplementation showed a significantly higher weight gain in contrast to control. Results of our study also seconds with Hassan et al. (2010) and Panda et al. (2009) who declared organic acid based diet showed an increase in body weight gain in broilers. The profusely higher gain may be attributed to the profound influence on the gut microbiota of organic acids in broilers diet. Similarly, Fathi et al. (2016) also observed that broilers treated with formic and propionic acid presented better BWG and FCR. These results were in harmony with Paul et al. (2007) who concluded that ammonium formate or calcium propionate supplementation at the rate of 3 g/kg depicted high live weight gain and better FCR. The outcomes of this trial were also in accordance with other researchers Mallo et al. (2012), Denli et al. (2003) and Adil et al. (2011) who described that addition of organic acids resulted in higher body weight gain and better FCR. Better body weight gain was observed because feed utilization by dietary acidification becomes more efficient (Mohamed et al. 2014). In line to these results of feed intake, a study performed by Akyurek and Yel (2011) depicted no significant effect on FI. Results of this experiment were in conflict with Isabel and Santos (2009), Kopecky et al. (2012) and El-Hakim et al. (2009) who concluded that feeding organic acids had minimal variation on weight gain.
The results regarding carcass characteristics in our trial were in concurrency with Sultan et al. (2015) who said the same about organic acids and presented increased dressed weight and carcass yield as compared with other treatment groups. Another study executed by Dehghani-Tafti and Jahanian (2016) showed the demanding ability of citric and butyric acid of positively affecting the carcass yield; however, gizzard weight was relatively reduced. Similarly, Panda et al. (2009) also reported a positive effect on dressing percentage and abdominal fat content by dietary acidification in broilers. Likewise, organic acids (propionic and butyric acid) supplemented at 0.2% dose rate each produced higher breast meat yield (Vijaya Lakshmi and Shyam Sunder 2013). In contrast to these significant results showed by many researchers, Kopecky et al. (2012) and Garcia et al. (2007) were among those who concluded no positive impact on carcass yield and slaughter weight as compared with the control group. The outcome findings of giblets weight in this study were in line with Mohamed et al. (2014) who reported no significant variation in the heart, liver, and gizzard weights by the incorporation of organic acids.
Our current study findings regarding cecal microbiota were in line with Paul et al. (2007) who concluded that broilers fed ammonium formate showed lowest total bacterial count as compared with the control group; this was mainly because the antimicrobial activity is the forte of formic acid. These findings were also supported by Cengiz et al. (2012) and Emami et al. (2017) who showed drastic decrease in the bacterial count in broilers fed organic acids. Similarly, levels of Salmonella were significantly reduced in cecal samples of broilers fed calcium formate. Another study performed by Gunal et al. (2006) depicted that organic acid supplementation showed a drastic decrease in bacterial numbers in the broiler chicken and the maximum decrease was seen in gram-negative bacteria.
In conformity with current results of gut morphology, Garcia et al. (2007) reported great improvement in length of villi as compared with other groups by the supplementation of formic acid. Similarly, Smulikowska et al. (2009) and Liu et al. (2017) depicted considerable improvement in villi height by the supplementation of organic acids in broilers. Histological changes in the intestines result in improved surface area for better utilization and absorption of nutrients, thereby improving broiler’s health and productivity. Our findings were in concurrency with (Sultan et al. 2016) who concluded that significantly better villus height, villus width, and VH to CD ratio was observed which improved the absorption of nutrients in broilers when fed with organic acids. Similarly, morphometric results, in general, were in harmony with (Panda et al. 2009) who reported that organic acid in feed increased the villi length. In contrast to these findings, (Qaisrani et al. 2015) showed no as such difference in villi length by organic acid supplementation in broilers.
The results of serum biochemistry in the current research study were in compatibility with (Hakim et al. 2009) who concluded that albumin, globulin, and total protein proportion remain unchanged in broilers raised on organic acids supplementation. Similarly, serum concentration of total cholesterol and total protein did not differ significantly in broilers (Khosravi et al. 2008). In contrary to current results, Ghazalah et al. (2011) concluded a significantly higher serum levels of globulin and cholesterol due to organic acids feeding to broilers.
Conclusion
In conclusion, organic acids can be utilized as a better replacement for antibiotics. Supplementation of organic acids in broiler’s feed in current research trial showed significant positive differences among various parameters, hence proving the worth of organic acids as an excellent antibiotic replacer. Supplementation of organic acids at a dose rate of 3 kg/ton and 4 kg/ton is recommended for the efficient performance of broilers.
References
Adil, S., Banday, M., Bhat, G., Qureshi, S., Wani, S., 2011. Effect of supplemental organic acids on growth performance and gut microbial population of broiler chicken. Livestock Research Rural Development 23, 1-8.
Akyurek, H., Yel, A., 2011. Influence of dietary thymol and carvacrol preparation and/or an organic acid blend on growth performance, digestive organs and intestinal microbiota of broiler chickens. Africian Journal of Microbiology Research 5, 979-984.
Byrd, J.A., Hargis, B.M., Caldwell, D.J., Bailey, R.H., Herron, K.L., McReynolds, J.L., Brewer, R.L., Anderson, R.C., Bischoff, K.M., Callaway, T.R., Kubena, L.F., 2001. Effect of lactic acid administration in the drinking water during pre-slaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poultry Science 80, 278–283.
Cengiz, O., Koksal, B., Tatli, O., Sevim, O., Avci, H., Epikmen, T., Beyaz, D., Buyukyoruk, S., Boyacioglu, M., Uner, A., 2012. Influence of dietary organic acid blend supplementation and interaction with delayed feed access after hatch on broiler growth performance and intestinal health. Veterinary Medicine 57, 515-528.
Çetingül, I.S., Gültepe, E.E., Rahman, A., Iqbal, A., Uyarlar, C., Hacısalihoğlu, S., Özçınar U., Bayram, I., 2020. Pistacia terebinthus as a dietary supplement for laying hens. South African Journal of Animal Sciences. https://doi.org/10.4314/sajas.v50i1.5
Dehghani-Tafti, N., Jahanian, R., 2016. Effect of supplemental organic acids on performance, carcass characteristics, and serum biochemical metabolites in broilers fed diets containing different crude protein levels. Animal Feed Science and Technology 211, 109-116.
Denli, M., Okan, F., Celik, K., 2003. Effect of dietary probiotic, organic acid and antibiotic supplementation to diets on broiler performance and carcass yield. Pakistan Journal of Nutrition 2, 89-91.
Dibner, J., Buttin, P., 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. Journal of Applied Poultry Research 11, 453-463.
El-Hakim, A.A., Cherian, G., Ali, M., 2009. Use of organic acid, herbs and their combination to improve the utilization of commercial low protein broiler diets. International Journal of Poultry Science 8, 14-20.
Emami, N.K., Daneshmand, A., Naeini, S.Z., Graystone, E., Broom, L., 2017. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: Performance, microbiology, intestinal morphology, and immune response. Poultry Science 106, 3254-3263.
Fathi, R., Samadi, MS., Qotbi, A.A., Seidavi, A.,.Marín, A.L.M., 2016. Effects of feed supplementation with increasing levels of organic acids on growth performance, carcass traits, gut microbiota and pH, plasma metabolites, and immune response of broilers. Animal Science Papers and Reports 34(2), 195-206.
Garcia, V., Catala-Gregori, P., Hernandez, F., Megias, M., Madrid, J., 2007. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. Journal of Applied Poultry Research 16, 555-562.
Ghazalah, A., Atta, A., Elkloub, K., .Moustafa, M.E., Shata, R.F., 2011. Effect of dietary supplementation of organic acids on performance, nutrients digestibility and health of broiler chicks. International Journal of Poultry Science 10, 176-184.
Gunal, M., Yayli, G., Kaya,O., Karahan, N., Sulak, O., 2006. The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. International Journal of Poultry Science 5, 149-155.
Hartemink, R., Rombouts, F.M., 1999. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples. Journal of Microbiology Methods 36, 181-192.
Hassan, H., Mohamed, M., Youssef, A.W., Hassan, E.R., 2010. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian-Australian Journal of Animal Science 23, 1348-1353.
Huyghebaert, G., Ducatelle, R., Van Immerseel, F., 2011. An update on alternatives to antimicrobial growth promoters for broilers. Veterinary Journal 187, 182-188.
Isabel, B., Santos, Y., 2009. Effects of dietary organic acids and essential oils on growth performance and carcass characteristics of broiler chickens. Journal of Poultry Research. 18, 472-476.
Kamal, A.M., Ragaa, N.M., 2014. Effect of dietary supplementation of organic acids on performance and serum biochemistry of broiler chicken. National Science 12, 38-45.
Khan, S.H., Iqbal, J., 2016. Recent advances in the role of organic acids in poultry nutrition. Journal of Applied Animal Research 44, 359-369.
Khosravi, A., Boldaji, F., Dastar, B., Hasani, S., 2008. The use of some feed additives as growth promoters in broilers nutrition. International Journal of Poultry Science 7, 1095-1099.
Kopecky, J., Hrnčár, C., Weis, J., 2012. Effect of organic acids supplement on performance of broiler chickens. Animal Science Biotechnology 45, 51-54.
Liu, Y., Yang, X., Xin, H., Chen, S., Yang, C., Duan, Y., Yang, X., 2017. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Animal Science Journal 88, 1414-1424.
Mallo, J.J., Puyalto, M., Rao, S.R., 2012. Evaluation of the effect of sodium butyrate addition to broiler diets on energy and protein digestibility, productive parameters and size of intestinal villi of animals. Feed Compound 32, 30-33.
Mohamed, M., El-Daly, E.F., El-Azeem, N.A.A., Youssef, A.W., Hassan, H., 2014. Growth performance and histological changes in ileum and immune related organs of broilers fed organic acids or antibiotic growth promoter International Journal of Poultry Science 13, 602-610.
Naseri, K.G., Rahimi, S., Khaki, P., 2012. Comparison of the effects of probiotic, organic acid and medicinal plant on Campylobacter jejuni challenged broiler chickens. Journal of Agricultural Science and Technology 14, 1485–1496.
Panda, A., Rao, S.R., Raju, M., Sunder, G.S., 2009. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-Austrlian Journal of Animal Science 22, 1026-1031.
Paul, S.K., Halder, G., Mondal, M.K., Samanta, G., 2007. Effect of organic acid salt on the performance and gut health of broiler chicken. Journal of Poultry Science 44, 389-395.
Qaisrani, S.N., van Krimpen, M.M., Kwakkel, R.P., . Verstegen, M.W.A., Hendriks, W.H., 2015. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poultry Science 94, 2152-2164.
Ricke, S., 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science 82, 632-639.
Russell, J.B., Diez Gonzales F., 1998. The effects of fermentation acids on bacterial growth. Advances in Microbial Physiology 39, 223–234.
Smulikowska, S., Czerwinski, J., Mieczkowska, A., Jankowiak, J., 2009. The effect of fat-coated organic acid salts and a feed enzyme on growth performance, nutrient utilization, microflora activity, and morphology of the small intestine in broiler chickens. Journal of Animal Feed Science 18, 478-489.
Steel, R.G.D., Torrie, G.H.,.Dickey, D.A., 1997. Principles and procedures of statisitics, a biometrical approach. 3rd Ed. MacGraw Hill Co., New York.
Sultan, A., Ullah, T., Khan, S., Khan, R.U., 2015. Effect of organic acid supplementation on the performance and ileal microflora of broiler during finishing period. Pakistan Journal of Zoology. 47, 635-639.
Tukey, J.W., 1953. The problem of multiple comparisons. In: The Collected Works of John W. Tukey VIII. Multiple Comparisons. Chapman and Hall, New York.
Vijaya Lakshmi, K., Shyam Sunder, G., 2013. Supplementation of propionic acid (PA), butyric acid (BA) or antibiotic (AB) in diets and their influence on broiler performance, carcass parameters and immune response. International Journal of Science Research 2319-7064.
Waldroup, P., Oviedo-Rondon, E.O., Fritts, C., 2003. Comparison of Bio-Mos and antibiotic feeding programs in broiler diets containing copper sulfate. International Journal of Poultry Science 2, 28-31.
Wang, J.P., Yoo, J.S., Lee, J.H., Zhou, T.X., Jang, H.D., Kim, H.J., Kim, I.H., 2009. Effects of phenyllactic acid on production performance, egg quality parameters and blood characteristics in laying hens. Journal of Applied Poultry Research 18, 203–209.
Author information
Authors and Affiliations
Contributions
KS executed the trial; S, TNP, AM conceived the idea and supervised; AR, ZH worked in trial and manuscript write up.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleem, K., Saima, Rahman, A. et al. Effects of dietary organic acids on performance, cecal microbiota, and gut morphology in broilers. Trop Anim Health Prod 52, 3589–3596 (2020). https://doi.org/10.1007/s11250-020-02396-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02396-2