Abstract
Successful preventive and control measures of zoonotic diseases require updated epidemiological data. Sylvatic rabies is endemic in Oman since 1990. Studying of the prevalence of animal rabies in Oman (2017–2019) was the goal of the current study besides the clinical–histopathological investigations of rabies in different animal species. A total of 117 whole brains of different animal species from different regions of Oman were examined by fluorescent antibody test (FAT) and histopathology for rabies during 2017–2019. Sixty-four samples (54.7%) were positive for rabies by FAT. The most affected species were goat (53.1%) followed by camel (18.8%), which pose a great risk to farmers and veterinarians. Positive fox cases were (10.9%). Most confirmed cases of animal rabies were submitted from Northern regions of Oman. Rabies was reported recently in Al Wusta among wild ruminants, Central Oman. The seasonal cycle of animal rabies in Oman was year-round with the peak from December to April. The clinical signs and neuropathological findings were nearly similar in different animal species. Histopathology-positive cases had Negri bodies in pyramidal and purkinje neurons, non-suppurative encephalitis features, and neuronal degeneration and necrosis. The sensitivity and specificity of histopathological diagnosis of rabies in different animals were 76.47% and 100.00%, respectively. Finally, sylvatic rabies remains a major challenge to the public and animal health in Oman. Although of the value of histopathological diagnosis of rabies if no other technique is available, other complementary tests must be employed to confirm negative results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rabies, OIE listed disease, is a fatal zoonotic viral disease that poses a significant risk to public health with 74,000 annually deaths, especially in the developing countries of Africa and Asia (Alaifan and Altamimi 2019; Kassiri et al. 2018; Obonyo et al. 2016; Sami and Ennaji 2020). It is usually transmitted through rabid animal bites resulting in encephalitis and death, if not treated (Nigg and Walker 2009; Wilde et al. 2012). It caused by a negative-stranded RNA virus (genus Lyssavirus, family Rhabdoviridae). Urban and sylvatic rabies are two distinct epidemiological cycles of the rabies virus: domestic dog is the main reservoir and transmitter of urban rabies; meanwhile, different wild species (foxes, wolves, bats, and others) are the main reservoir and transmitter of the sylvatic rabies (Cisterna et al. 2005; Ward 2012). Conversion of the sylvatic to the urban cycle of rabies can occur with the interaction between wildlife animals and unvaccinated and/or stray dogs (Bannazadeh Baghi et al. 2018). Rabies is endemic in the Arabian Peninsula (Bannazadeh Baghi et al. 2018). In Yemen, urban rabies was the most circulating form (Al-Shamahy et al. 2013). In Jordan, urban and sylvatic rabies were documented with a very low risk of human transmission (Yakobson et al. 2017). In Iraq, urban rabies constituted the major rabies cases and were confined to dog bites (Horton et al. 2013). Urban and sylvatic rabies cycles were reported in animals in Saudi Arabia, where dogs were the most common source of human bites (Kasem et al. 2019; Memish et al. 2015). In Oman, sylvatic rabies is endemic, where red fox (Vulpes vulpes) was the main reservoir and transmitter of rabies since first reported human case in 1990 (Al Ismaily et al. 2002; Hussain et al. 2013; Novelli and Malankar 1991). Rabies is not endemic in Kuwait, and scarce information about urban and sylvatic rabies could be found (Bannazadeh Baghi et al. 2018), while Memish et al. (2015) reported Kuwait as a rabies-free country. Bahrain, Qatar, and United Arab Emirates are rabies-free countries (Bannazadeh Baghi et al. 2018). First local human rabies in Saudi Arabia was reported in 2016 (Dhayhi et al. 2019). Eight local human rabies were reported in Oman with history of wild animal bites (Al Abaidani et al. 2015; Awaidy and Al Hashami 2020). The latest available data about the prevalence of rabies in Oman was published in 2015 (Al Abaidani et al. 2015; 2013). Updating of this information is crucial to the establishment of a rigid national rabies control plan in the Sultanate of Oman.
Materials and methods
Rabies surveillance among animals in Oman
Rabies is an obligatory notifiable disease in Oman by the Ministry of Agriculture and Fisheries (MAF) through a total of 69 governmental veterinary clinics and 252 private veterinary clinics throughout the country. Also, there is coordination between MAF and the Ministry of Environment and Climate Affair regarding the suspected cases among wild animals. Coordination between MAF and the Ministry of Health is already well established. Diagnosis of animal rabies in Oman was conducted at the national reference laboratory (Central Laboratory for Animal Health (CLAH)), MAF, which located in the capital city, Muscat. Rabies Specimen Submission Guidelines to CLAH were established by the MAF as following: All suspected animals showing nervous signs with fresh wounds and/or history of animal bite are strongly urged to be submitted to CLAH to rule out rabies through the examination of brain tissues.
From January 2017 till December 2019, a total of 117 whole brain samples either fresh or frozen with or without fixation in 10% neutral-buffered formalin were received at CLAH from different regions of Oman that belong to different domesticated and wild animal species. Impression smears of brain tissues (medulla, cerebellum, and hippocampus) were performed from all received samples for subsequent direct fluorescent antibody test (FAT). Fifty-seven formalin-fixed brain samples were subjected to histopathological examination, and the results were compared to FAT.
Diagnostic investigations
Whole brain samples were tested for rabies through FAT and histopathology. FAT was performed using brain impression smears fixed in cold 100% acetone at − 20 °C for at least 20 min (Garg 2014). Acetone-fixed brain impressions were stained for 30 min at 37 °C in a humid chamber using FITC- anti-Rabies monoclonal globulin conjugate (Fujirebio Diagnostics Inc.). Slides were then rinsed three times with PBS and air-dried. The slides were mounted with 90% glycerin buffer (pH 8.5) and covered with coverslip. Finally, the slides were examined using a fluorescent microscopy (Olympus BH3). Positive samples showed scattered bright green fluorescent foci of varying sizes. All tested samples were compared to positive and negative control slides.
Histopathological examination of formalin-fixed brain samples was performed for monitoring the presence of Negri bodies in neurons, the pathologic hallmark of the rabies (Lucas 2018). Fixed brain samples were processed using paraffin-embedded technique and stained with hematoxylin and eosin (H&E) (Bancroft and Gamble 2008).
Statistical analysis
Proportions of positivity between different animal species, different regions, and months were performed using MedCalc Statistical Software 18 (bvba 2018). P < 0.05 was considered statistically significant. Also, calculation of the sensitivity (the ability to determine the positive cases correctly) and the specificity (the ability to determine the negative cases correctly) of histopathological results compared to FAT results were performed using MedCalc Statistical Software 18 (bvba 2018). Geographical distribution of positive animal rabies in different provinces (Willayat), in Oman was created using open-source QGIS software 3.4.
Results
Clinical signs of rabid animals
The clinical signs of different rabid animals were summarized in Table 1.
Diagnostic laboratory investigations
FAT
Out of 117 suspected brain samples, 64 (54.7%) were found positive by FAT. Compared to control positive and control negative rabies slide, positive samples showed scattered bright green fluorescent foci of varying size like positive control slide (Fig. 1a). Meanwhile, no antigen expression was noticed in the negative control slide.
a Impression smear of a rabid fox’ brain tested by direct fluorescent antibody test (FAT) showing scattered bright green fluorescent foci of varying sizes, scale bar = 5 μ. b–e Neuropathological findings of confirmed cases of animal rabies (H&E): b diffuse massive perivascular hemorrhages in the cerebrum of a rabid fox (arrows; H&E); scale bar = 200 μ. c Perivascular lymphocytic and monocytic cuffs in in the cerebrum of a rabid fox (arrow); scale bar = 50 μ. Negri bodies (intracytoplasmic oval eosinophilic inclusion; arrows) in purkinje neuron in the cerebellum of a rabid goat; scale bar = 20 μ (d) and pyramidal neuron in the cerebral cortex of a rabid camel; scale bar = 20 μ (e). f She-camel has bitten by an Arabian red fox, provided by Dr. Abd-Elhady Abass (A veterinarian at Al Batinah North governorate in Oman; 2019)
Histopathology
Histopathological examination was performed on 57 formalin-fixed brain samples. Compared to FAT-positive (34 samples) and negative (23 samples) results, all FAT- negative brain samples were histologically normal. Out of thirty-four FAT-positive samples subjected to histopathological examination, all FAT-positive brain samples exhibited features of non-suppurative viral encephalitis including mild to moderate cerebral hemorrhage (Fig. 1b), perivascular lymphocytic and monocytic cuffs (Fig. 1c), and neuronal necrosis and gliosis. Meningitis and malacia were also noticed in few cases. Meanwhile, twenty-six FAT-positive brain samples (76.5%) showed pathognomonic eosinophilic intracytoplasmic Negri bodies in Purkinje neurons (Fig. 1d) in goats, sheep, and cattle or pyramidal neurons (Fig. 1e) in foxes and camels.
The calculated sensitivity and specificity of the histopathological diagnosis of rabies in different animals were 76.47% and 100.00%; respectively. The characteristic histopathological lesions in different rabid animals were summarized in Table 1.
Rabies in animals and regions
As shown in Table 2, the overall positivity of rabies among species during 2017–2019 was 64/117 (54.7%), of which seven foxes (10.9%), twelve camels (18.8%), four cattle (6.2%), three sheep (4.7%), thirty-four goats (53.1%), two Goitered gazelle (3.1%), one Mountain gazelle (1.6%), and one Arabian Oryx (1.6%). The difference between the proportions of positivity between different animal species was statistically significant (P < 0.005).
All positive cases were reported with nervous signs and a history of bite. Twenty-two (excluding fox) rabid cases (38.6%) were reported with a history of fox bites in extremities and muzzle (Fig. 1d); five rabid cases (8.8%) were reported with a history of stray dog bite in Ibri province (AL Dhahirah) in eastern Oman and four cases with history of contact with other rabid animals (7%).
During 2017–2019, the positivity percentage of animal rabies was 54.7% (64/117), 17 cases were reported in 2017 (26.6%), 23 cases were reported in 2018 (35.9%), and 24 cases were reported in 2019 (37.5%) as shown in Fig. 2.
As shown in Table 3, the majority of positive cases received from Ash- Sharqiyah South (18/64; 28.1%), Al Dakhiliyah (14/64; 21.9%), Al Batinah North and Al Wusta (8/64; 12.5% for each), and Al Dhahirah (7/64; 10.9%). Fewer rabid animals were recorded in Dhofar (3/64; 4.7%) and Al Batinah South and Ash -Sharqiyah North and Muscat (2/64; 3.1% for each). No positive cases were recorded from Al Buraymi and Musandam. The difference between the proportions of positivity between different governorates was statistically significant (P < 0.005).
The geographical distribution of positive animal rabies in different provinces in Oman is shown in Fig. 3.
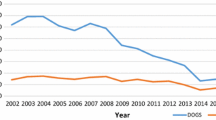
During 2017–2019, the annual cycle of animal rabies in Oman occurs each year with the highest peak in April (18.7%) and the lowest positivity in October (1.6%), as shown in Fig. 4. The difference between the proportions of positivity between months during 2017–2019 was statistically significant (P < 0.05).
Discussion
Rabies is still a neglected zoonotic disease especially in the Middle Eastern countries (Bannazadeh Baghi et al. 2018; Barecha et al. 2017; Bourhy et al. 2010). It remains underreported in animals due to the lack of surveillance and diagnostic laboratory facilities beside the death of most of the victims without laboratory confirmation (Banyard et al. 2013; Naji et al. 2019). Although sylvatic rabies is endemic in Oman with history of eight human rabies since 1990 (Al Ismaily et al. 2002; Hussain et al. 2013), there is a scarce and outdated information about rabies situation among animals from 1990 to 2000 (Al Ismaily et al. 2002) and 2006–2010 (Hussain et al. 2013). So, the current study aimed to update the prevalence of animal rabies in Oman in the last 3 years (2017–2019).
All suspected samples were submitted from different regions in Oman to CLAH. The diagnosis of rabies in CLAH depends mainly on FAT, the gold standard test approved by both OIE and WHO for the definitive diagnosis of rabies (Hayman et al. 2011; WHO 2013).
Recently, brain postmortem and histopathology were useful for the differential diagnosis of some nervous diseases of the livestock that reported for first time in Oman (El-Neweshy et al. 2019a, b). The presence of Negri bodies (Eosinophilic intracytoplasmic inclusions) in the brain is a unique and pathognomonic histopathological finding in rabies (Jamadagni et al. 2007; Singh 2001). Meanwhile, 50–80% of rabies positive animals actually have Negri bodies (Beck et al. 2017; Mrak and Young 1993; Singh and Grewal 1998). In the current study, insufficient sensitivity (76.47%) and the required fresh or formalin-fixed brain samples for histopathological diagnosis limit its application as primary diagnostic tool of animal rabies.
The current data clearly demonstrate that the overall positivity (54.7%) of animal rabies in the last 3 years (2017–2019) was decreased compared to previous reports 64% (Al Ismaily et al. 2002) and 62.8% (Hussain et al. 2013). Many reports (Ahmed et al. 2020; Body et al. 2018; Body et al. 2014; Hussain et al. 2013) documented that rabies transmission in Oman has been confined to sylvatic cycle since 1990 where red fox has been reported as the main reservoir. These reports confirmed that one genetic rabies virus group circulating among animals with 99% homology. Only 7 confirmed fox cases were recorded in the current study compared to 37 cases during the period 2006–2010. Despite the history of dog bites in 5 confirmed cases in the current study, no confirmed dog rabies was reported. Previously, 2 confirmed dog cases in Oman were reported in 1990 (WHO 1992), 9 cases in 1991–1992 (MOH 1998), one case 1993–2000 (unpublished data), and one case in 2011(Hussain et al. 2013). Till April 2020, no further confirmed rabies in dogs in Oman since 2011, which augments (Al Abaidani et al. 2015) hypothesis who suggested that dogs are not a maintenance host for rabies virus in Oman.
In Oman, rabies surveillance program was integrated into the national communicable diseases program since August 1990 and efforts given to prevent the establishment of dog-mediated cycle of rabies (Awaidy and Al Hashami 2020). The control program of the animal rabies in Oman is based on the obligatory notification of all suspected animal cases, the accurate laboratory diagnosis, ring vaccination of all animals in contact, and yearly mass vaccination of endemic areas of all animal at risk using an inactivated adjuvant cell culture vaccine (Rabivac vaccine).
In the current study, sampling was more frequent from North and North-Central governorates of Ash-Sharqiyah South, Al Dakhiliyah, and Al Batinah North compared to other governorates. About 65% of animal livestock concentrated in these governorates with efficient veterinary services that encouraged reporting of suspected cases with biting incident to local authorities. Also, the provinces of these governorates share the mountainous terrain without natural boundaries, so rabid foxes moved freely between neighboring provinces. However, these results may have been affected by sampling bias due to the nearness of these governorates to the diagnostic laboratory.
The current information revealed significantly changes in the percentage and distribution of positive cases compared to the latest available information in 2015 (Al Abaidani et al. 2015) where percentage of positive cases was highest in Al Dakhiliyah (47.3%), followed by Al Batinah North (20.9%), Ash-Sharqiyah South (12.2%), Dhofar (6%), Ash-Sharqiyah North (5.2%), Al Dhahirah (4.0%), Al Batinah South (2.4%), Muscat (1.6%), and Al Buraymi (0.2%). Also, animal rabies was established recently in Al Wusta governorate (12.5%) with 4 positive cases in fox and 4 wild ruminants. This variation is likely related to the population dynamics of the fox host species. Blanford’s Fox (Vulpes cana), known to be distributed in the Hajar Mountains in northern and southern Oman, was recently recorded for the first time in Al Wusta, Central Oman (Alsaid et al. 2019). Musandam governorate (The Musandam Peninsula) is still without suspected/confirmed cases. Similar to the previous study in 2015 (Al Abaidani et al. 2015), goats and camels were the highest animal species with positive cases.
In the current study, the high prevalence of rabies in camels and goats might be due to their free roaming and grazing nature that increase their exposure to the rabid fox’s bite. In contrast, supervised grazing of sheep flocks and cattle within farms limits their exposure to rabid foxes (Hussain et al. 2013). Approximately 90% of the confirmed cases were > 6 months old, which more vulnerable to get rabies during free roaming or grazing, while young animals usually graze under supervision or in farms.
The seasonal variation of positive samples in the current study is nearly similar to the previous information in Oman (Hussain et al. 2013) where the peak of positive cases was recorded in March and the lowest number of positive cases were reported in August, which may related to grazing season. The peak of red fox-related rabies in Canada was reported in December and was least in June (Johnston and Beauregard 1969). Johnston and Beauregard (1969) suggested the increase of red fox-rabies during late summer, and early fall was related to the time of the reproductive maturation of juvenile male. Meanwhile, the peak of red fox-related rabies in March was related to the time of parturition of yearling females that showed a higher rate of aggressive contact. In the current study, winter-spring animal rabies peak was probably related to the mating season during late winter.
The only source of samples in the current and previous studies (Al Abaidani et al. 2015; Al Ismaily et al. 2002; Hussain et al. 2013) was the suspected animal cases with a history of bite and/or nervous signs those submitted to the diagnostic laboratories.
This passive nature of surveillance is likely to lessen the actual number of positive cases. The limitation of veterinary laboratories that equipped to undertake rabies diagnosis in Oman lessen the true burden of animal rabies. Moreover, transportation of suspected samples over long distances in hot weather adversely affects the sample quality and the laboratory result (McElhinney et al. 2014), so decentralized laboratory facilities is critical in rapid and accurate laboratory diagnosis.
Sylvatic rabies is still a major challenge to public and animal health in Oman. Also, updating the complete genomic sequencing and phylogenetic tree of the circulating rabies virus would be of great importance in future preventive and control measures.
References
Ahmed, M.S. et al., 2020. Molecular characterization and diagnostic investigations of rabies encephalitis in camels (Camelus dromedaries) in Oman: a retrospective study, Tropical Animal Health and Production, 1-6
Al-Shamahy, H.A., Sunhope, A., and Al-Moyed, K.A., 2013. Prevalence of rabies in various species in Yemen and risk factors contributing to the spread of the disease, Sultan Qaboos University Medical Journal, 13, 404
Al Abaidani, I. et al., 2015. Epidemiology of rabies in Oman: a retrospective study [1991-2013], EMHJ-Eastern Mediterranean Health Journal, 21, 591-597
Al Ismaily, S. et al., 2002. Retrospective studies of rabies in the Sultanate of Oman 1990–2000, Agric. Fish. Res. Bull, 2, 25-28
Alaifan, T., and Altamimi, A., 2019. A systematic review of epidemiology of rabies in Arab countries, Journal of Health Informatics in Developing Countries, 13, 1-15
Alsaid, T. et al., 2019. New record of Blanford’s Fox Vulpes cana (Mammalia: Carnivora: Canidae) in central Oman: a connection between the northern and southern populations, Journal of Threatened Taxa, 11, 14244-14246
Awaidy, S.A., and Al Hashami, H., 2020. Zoonotic diseases in Oman: successes, challenges, and future directions, vector-borne and zoonotic diseases, 20, 1-9
Bancroft, J.D. and Gamble, M. eds., 2008. Theory and practice of histological techniques. Elsevier health sciences.
Bannazadeh Baghi, H. et al., 2018. A perspective on rabies in the Middle East—beyond neglect, Veterinary sciences, 5, 67
Banyard, A.C. et al., 2013. Control and prevention of canine rabies: the need for building laboratory-based surveillance capacity, Antiviral Research, 98, 357-364
Barecha, C.B. et al., 2017. Epidemiology and public health significance of rabies, Perspectives in Medical Research, 5, 55-67
Beck, S. et al., 2017. Pathobiological investigation of naturally infected canine rabies cases from Sri Lanka, BMC veterinary research, 13, 99
Body, M.H. et al., 2018. Molecular characterization of rabies virus from foxes in the sultanate of Oman, Animal and Veterinary Sciences, 6, 17
Body, M.H.H. et al., 2014. Study on molecular characterization of rabies virus N gene segment from different animal species in the Sultanate of Oman, J. Vet. Med. Anim. Heal, 6, 295-301
Bourhy, H. et al., 2010. Rabies, still neglected after 125 years of vaccination, PLoS neglected tropical diseases, 4, e839.
bvba, M.S., 2018. MedCalc Statistical Software version 18. 2018, (Belgium Ostend,
Cisterna, D. et al., 2005. Antigenic and molecular characterization of rabies virus in Argentina, Virus research, 109, 139-147
Dhayhi, N.S. et al., 2019. First confirmed case of local human rabies in Saudi Arabia, International journal of infectious diseases, 87, 117-118
El-Neweshy, M.S. et al., 2019a. Natural Ehrlichia ruminantium infection in two captive Arabian tahrs (Arabitragus jayakari) in Oman, Tropical Animal Health and Production, 51, 2539-2545
El-Neweshy, M.S. et al., 2019b. First report of an outbreak of cerebral coenurosis in Dhofari goats in Oman, Revista Brasileira de Parasitologia Veterinária, 28, 479-488
Garg, S.R., 2014. Rabies in man and animals, (Springer)
Hayman, D.T. et al., 2011. A universal real-time assay for the detection of Lyssaviruses, Journal of Virological Methods, 177, 87-93
Horton, D.L. et al., 2013. Rabies in Iraq: trends in human cases 2001–2010 and characterisation of animal rabies strains from Baghdad, PLoS neglected tropical diseases, 7, e2075
Hussain, M.H. et al., 2013. Spatio-temporal pattern of sylvatic rabies in the Sultanate of Oman, 2006–2010, Preventive veterinary medicine, 110, 281-289
Jamadagni, S., Singh, C., and Sandhu, B., 2007. Histopathological alterations in brains of rabies infected buffaloes and cattle, Italian Journal of Animal Science, 6, 872-874
Johnston, D., and Beauregard, M., 1969. Rabies epidemiology in Ontario, Bulletin of the Wildlife Disease Association, 5, 357-370
Kasem, S. et al., 2019. Rabies among animals in Saudi Arabia, Journal of infection and public health, 12, 445-447
Kassiri, H., Ebrahimi, A., and Lotfi, M., 2018. Animal bites: epidemiological considerations in the east of Ahvaz County, Southwestern Iran (2011-2013), Archives of Clinical Infectious Diseases, 13, e62384
Lucas, S., 2018. Investigating infectious diseases at autopsy, Diagnostic Histopathology, 24, 357-364
McElhinney, L.M. et al., 2014. Effects of carcase decomposition on rabies virus infectivity and detection, Journal of Virological Methods, 207, 110-113
Memish, Z.A., Assiri, A.M., and Gautret, P., 2015. Rabies in Saudi Arabia: a need for epidemiological data, International journal of infectious diseases, 34, 99-101
MOH, 1998. Ministry of Health (MOH), Community Health and Disease Surveillance Newsletter., VII, 1-9
Mrak, R.E., and Young, L., 1993. Rabies encephalitis in a patient with no history of exposure, Human pathology, 24, 109-110
Naji, E. et al., 2019. Comparison of reverse transcription loop-mediated isothermal amplification method with SYBR green real-time RT-PCR and direct fluorescent antibody test for diagnosis of rabies, Japanese journal of infectious diseases, JJID. 2019.2009
Nigg, A.J., and Walker, P.L., 2009. Overview, prevention, and treatment of rabies, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 29, 1182-1195
Novelli, V., and Malankar, P., 1991. Epizootic of fox rabies in the Sultanate of Oman, Transactions of the Royal Society of Tropical Medicine and Hygiene, 85, 543-543
Obonyo, M. et al., 2016. Suspected rabies in humans and animals, Laikipia County, Kenya, Emerging infectious diseases, 22, 551–553.
Sami, D., and Ennaji, M.M., 2020. Global epidemiology and genetic variability of rabies viruses. emerging and reemerging viral pathogens, 2020, (Elsevier, 259-275
Singh, C., 2001. Laboratory diagnosis of rabies by elisa in buffalo calves experimentally infected with rabies virus, (Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana)
Singh, C., and Grewal, G., 1998. Early pathogenic study in experimental rabies in buffalo calves with street rabies virus, Buff. J, 3, 361-373
Ward, M., 2012. Review of rabies epidemiology and control in South, South East and East Asia: past, present and prospects for elimination, Zoonoses and public health, 59, 451-467
WHO, 1992. Wildlife rabies in Oman and the United Arab Emirates, Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire, 67, 65-68
WHO, 2013. WHO expert consultation on rabies: second report, (World Health Organization)
Wilde, H. et al., 2012. Rabies in Asia: the classical zoonosis. One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases, 2012, (Springer, 185-203
Yakobson, B. et al., 2017. Impact of rabies vaccination history on attainment of an adequate antibody titre among dogs tested for international travel certification, Israel–2010–2014, Zoonoses and public health, 64, 281-289
Acknowledgements
All field veterinarians in MAF, Oman, are sincerely thanked by the authors for their valuable role in sample collection. The authors wish to thank Mr. Taha Al Sabhi, Central Laboratory for Animal Health, for his valuable help and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Neweshy, M.S., Al Mayahi, N., Al Mamari, W. et al. Animal rabies situation in Sultanate of Oman (2017–2019). Trop Anim Health Prod 52, 3069–3076 (2020). https://doi.org/10.1007/s11250-020-02328-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02328-0