Abstract
In this study, the prevalence of ESBL/pAmpC-producing Escherichia coli and their molecular characterization from cloacal swab samples were investigated. All samples were obtained from broiler flocks that are located in Hatay, Adana, and Mersin provinces of Turkey. Antimicrobial susceptibilities of the isolates were determined by disk diffusion method following the CLSI criteria. Genetic mechanisms mediating resistance in ESBL/pAmpC-producing E. coli isolates were identified by polymerase chain reaction (PCR) and followed by DNA sequencing. Phylogenetic groups and plasmid replicon types of the isolates were also investigated by PCR. The clonal relationship of selected isolates was investigated by enterobacterial repetitive intergenic consensus (ERIC)-PCR and multilocus sequence typing (MLST) method. Of 430 cloacal swab samples, 154 (35.8%) were positive for ESBL/pAmpC-producing E. coli. The ESBL/pAmpC type beta-lactamases were as follows: CMY-2 (n = 46), CMY-2 + TEM-1b (n = 63), SHV-12 (n = 5), SHV-12 + TEM-1b (n = 12), CTX-M-3 (n = 14), CTX-M-3 + TEM-1b (n = 1), CTX-M-15 (n = 4), CTX-M-15 + TEM-1b (n = 4), and CTX-M-1 (n = 3). Moreover, various rates of resistance to different antimicrobials were determined such as nalidixic acid (92.9%), ciprofloxacin (76%), sulfamethoxazole-trimethoprim (78.6%), tetracycline (73.4%), streptomycin (52.6%), chloramphenicol (44.2%), kanamycin (27.9%), tobramycin (24.7%), gentamicin (19.5%), and amikacin (0.6%). Furthermore, 148 (96.1%) isolates were found to be MDR. The ESBL/pAmpC-producing isolates were distributed into the following phylogroups: E (n = 61), B1 (n = 30), F (n = 20), A (n = 19), B2 (n = 11), D (n = 10), and C (n = 3). ERIC-PCR analysis showed 51 unrelated patterns. Out of the 28 selected isolates, the following sequence types (STs) were detected: ST354 (n = 3), ST114 (n = 3), ST5696 (n = 2), ST156 (n = 2), ST174 (n = 2), ST362 (n = 2), ST157 (n = 2), ST5114 (n = 2), ST6635, ST539, ST457, ST1640, ST95, ST5843, ST1158, ST10, ST648, and ST4248. The results of the current study revealed that broilers in Turkey are important reservoir of ESBL/pAmpC-producing E. coli, which suggest that these agents have a great potential of transmission to humans by food chain or direct contact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emergence and dissemination of extended-spectrum beta-lactamase (ESBL) and plasmid-mediated AmpC-type (pAmpC) beta-lactamase-producing Escherichia coli in food-producing animals are major concerns for human and veterinary health worldwide (Ewers et al. 2012). Due to multidrug resistance (MDR) character of these bacteria, therapeutic options of infections caused by these agents are very limited (Pitout and Laupland 2008; Pitout 2010; Ewers et al. 2012). Especially, broiler flocks are considered as a potential reservoir for ESBL/pAmpC-producing E. coli, and contaminated chicken meat are linked with colonization or infection of humans (Ewers et al. 2012; Huijbers et al. 2014; Dierikx et al. 2013).
ESBL’s can confer resistance to a variety of antimicrobials such as 3rd and 4th generation cephalosporins, which are listed “critically important antimicrobials for human health” (WHO 2015). ESBLs were divided into three major families: TEM, SHV, and CTX-M. To date, CTX-M replaced TEM- and SHV-type beta-lactamases and became dominant ESBL type with an increasing prevalence throughout the world (Olsen et al. 2014; Ewers et al. 2009). In addition to the 3rd and 4th generation cephalosporins, AmpC-type beta-lactamases can confer resistance to the beta-lactams that are combined with beta-lactamase inhibitors and cephamycin (i.e., cefoxitin and cefotetan). AmpC-type beta-lactamases are either chromosomal- or plasmid-mediated. Although there are a number of plasmid-mediated AmpC beta-lactamase types, CMY-2 is the most commonly encountered among Enterobacteriaceae family (Philippon et al. 2002).
A few reports on the prevalence of ESBL/pAmpC-producing E. coli in broiler flocks are available in Turkey. Başaran Kahraman et al. (2016) reported prevalence of ESBL/AmpC-producing E. coli as 11.5% from 43 flocks belonged to 14 farms located in the Marmara Region. Ünal et al. (2017) surveyed three integrated broiler firms (two in the Aegean Region and one in the Black Sea region) and found the prevalence of ESBL-producing E. coli as 8.3%. On the other hand, prevalence of ESBL/AmpC-producing E. coli on the broiler farm level has been reported to be low between EU member states, except Lithuania (prevalence of ESBL and AmpC-producing E. coli were 17% and 36%, respectively) (EFSA and ECDC 2018).
Although many molecular typing methods such as multilocus variable-number tandem repeat analysis (MLVA), pulsed field gel electrophoresis (PFGE), random amplified polymorphic DNA (RAPD), multiple-locus variable number tandem repeat (VNTR) and multilocus sequence typing (MLST) have been used to determine the clonal relationship between isolates, enterobacterial repetitive intergenic consensus (ERIC-PCR) has widely been used for the typing of E. coli strains isolated from different sources because it is cheaper and labor effective in compare with the molecular typing methods (Ranjbar et al. 2017; Lim et al. 2009; Madoshi et al. 2016; Ibrahim et al. 2016). However, in recent years, whole genome sequence (WGS) analysis has become a very affordable and fast tool for typing of bacterial isolates, due to the continuous decline in costs, and provides higher resolution than the molecular typing methods (Quainoo et al. 2017). In addition, information on the genetic basis of antimicrobial resistance (AMR) and plasmids harboring AMR genes can be readily obtained from WGS assemblies (Zankari et al. 2013; Carattoli et al. 2014).
To date, there are no comprehensive study regarding to the occurrence and molecular characterization of ESBL/pAmpC-producing E. coli in Turkey. In this study, therefore, it was aimed to (i) determine the prevalence and molecular characterization of ESBL/pAmpC-producing E. coli, (ii) investigate antibiotic susceptibilities of the isolates by disk diffusion method, (iii) determine ESBL/pAmpC types by PCR and DNA sequencing analysis, (iv) investigate plasmid replicon types and phylogenetic groups of the isolates, and (v) determine clonal relationship of the isolates by enterobacterial repetitive intergenic consensus (ERIC)-PCR and multilocus sequence (MLST) in selected isolates.
Materials and methods
Study design and sampling
Three provinces (Mersin, Adana, and Hatay) located in Southern Turkey were selected for the collection of the samples since these provinces, especially Mersin and Adana, provide almost all of the broiler to be consumed in these provinces and their surrounding provinces. Sampling was performed in a total of 21 broiler flocks, including 7 flocks from each province with a median capacity of 50,000 broilers. From January 2017 to June 2017, a total of 21 flocks (seven flocks from each province) were sampled, and 430 cloacal swab samples using Stuart Transport Medium were collected.
In Turkey, commercial broiler production involves the total depopulation of the broiler premises and removal of the litter after every flock, cleaning and disinfection of the broiler houses before the arrival of the next batch. All sampled flocks were commercially reared and slaughtered at the age of 6 to 7 weeks. All sampled flocks belonged to different companies, and from each flock, 20–22 cloacal swab samples were collected. The collected cloacal swab samples were transported on ice to the laboratory within 5 h of collection and were inoculated onto Eosin Methylene Blue (EMB) agar supplemented with 2 μl/ml cefotaxime (Sigma, Germany) for selective isolation (Costa et al. 2009). The plates were incubated overnight at 37 °C for 24 h. One typical colony was selected and plated onto blood agar with 5% of defibrinated sheep blood in order to obtain pure culture, and then, the isolates were stored at − 20 °C in TSB containing 20% (v/v) glycerol until studied. If one broiler in a flock was found to be genotypically positive, the entire flock was considered as positive.
Phenotypic confirmation of ESBL- and pAmpC-producing isolates
Phenotypic confirmation of ESBL-producing E. coli isolates was evaluated by combination disc method (CLSI 2012) and double disc synergy (Jarlier et al. 1988). Cefoxitin and amoxicillin-clavulanic acid resistance was considered as an indication of AmpC production.
Following biochemical tests, all isolates were confirmed using E. coli species-specific primers E16S-F 5’-CCC CCT GGA CGA AGA CTG AC-3 ‘and E16S-R 5’-ACC GCT GGC AAC AAA GGA TA-3′ (Wang et al. 2002).
Determination of antimicrobial susceptibilities of E. coli isolates
The antimicrobial susceptibilities of ESBL/AmpC-producing E. coli strains were determined by disk diffusion method according to CLSI (2012) criteria. Antimicrobial disks used were ampicillin (AM, 10 μg), amoxicillin-clavulanic acid (AMC, 10/20 μg), cefpodoxime (CPD, 10 μg), cefepime (FEB, 30 μg), cefoxitin (FOX, 30 μg), cefotaxime (CTX, 10 μg), ceftazidime (CAZ, 30 μg), cefuroxime (CXM, 30 μg), cefotetan (CTT, 30 μg), ceftriaxon (CRO, 30 μg), cephalothin (KF, 30 μg), aztreonam (ATM, 30 μg), nalidixic acid (NA, 30 μg), ciprofloxacin (CIP, 5 μg), imipenem (IPM, 10 μg), chloramphenicol (C, 30 μg), gentamicin (CN, 10 μg), tobramycin (TOB, 10 μg), amikacin (AK, 30 μg), streptomycin (S, 10 μg), kanamycin (K, 30 μg), tetracycline (TE, 30 μg), and sulfamethoxazole-trimethoprim (SXT, 1.25 / 23.75 μg). E. coli ATCC 25922 was used as control strain. The isolates that show resistance to three or more than three antimicrobials of different classes were accepted as MDR.
Detection of ESBL/AmpC genes
ESBL genes (blaSHV, blaTEM, and blaCTX-M) were screened in phenotypically positive isolates as previously reported by Ahmed et al. (2007). pAmpC genes were investigated according to multiplex PCR methodology described by Pérez-Pérez and Hanson (2002). Amplicons from ESBL- and AmpC-positive strains were subjected to sequencing in both directions, and the obtained sequences were analyzed using the nucleotide-nucleotide BLAST program (http://www.ncbi.nlm.nih.gov) on the National Center for Biotechnology Information (NCBI).
Phylogenetic grouping
Phylogrouping of the isolates was performed according to quadruplex PCR methodology as previously described by Clermont et al. (2013).
Plasmid replicon typing
Plasmid replicon types of ESBL- and AmpC-producing isolates were determined using extracted plasmids of each strain, as previously described by Carattoli et al. (2005).
Eric-PCR
Clonal relationship of ESBL- and pAmpC-producing E. coli isolates was determined using ERIC-PCR, as previously described by Versalovic et al. (1991). ERIC-PCR profiles were analyzed using NTSYS-pc software (Version 2.02 K, Applied Biostatistics, Inc., NY, USA). The similarity between the strains was determined on the basis of the Jaccard coefficients. The dendrogram was constructed based on the average relatedness of the matrix using the algorithm of the unweighted pair-group method (UPGMA).
Multilocus sequence typing (MLST)
MLST typing was performed as previously reported by Wirth et al. (2006). Seven E. coli housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) were amplified, and sequence analysis of both the forward and reverse strands was performed using the services of Medsantek (Medsantek Inc., İstanbul, Turkey). Forward and reverse sequences were aligned for each strain, and alignments were edited to equally sized fragments. Allele numbers for seven gene fragments of each isolate were obtained by comparing them with corresponding alleles available in MLST E. coli database submission page (http://mlst.warwick.ac.uk/mlst/ /Ecoli/), and sequence type (ST) of each isolate was determined by combining seven allelic profiles.
Results
Prevalence of ESBL/pAmpC-positive isolates and determination of genes encoding beta-lactamases
Of 21 flocks sampled, 16 flocks were positive for ESBL and/or pAmpC, of which three flocks were only positive for pAmpC and two were only positive for ESBL. The rest of the flocks (n = 11) was positive for both ESBL and pAmpC (Table 1). In total, 154 isolates were phenotypically found as cefotaxime resistant, and all were positive for ESBL and/or pAmpC genes. The most prevalent beta-lactamase gene was blaCMY-2 gene (70.8%, 109/154), followed by blaSHV-12 (11.04%, 17/154), blaCTX-M-3 (11.04%, 17/154), blaCTX-M-15 (5.2%, 8/154), and blaCTX-M-1 (1.9%, 3/154). blaTEM-1b was found together with CMY-2 in 63 isolates, in 12 isolates with SHV-12, in 4 isolates with CTX-M-15, and in 1 isolate with CTX-M-3.
Resistance phenotypes of the ESBL/pAmpC-producing E. coli isolates
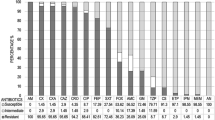
The resistance phenotypes of the ESBL/pAmpC-producing E. coli isolates are given in Fig. 1. Resistance rates to nalidixic acid, ciprofloxacin, sulfamethoxazole-trimethoprim, tetracycline, streptomycin, chloramphenicol, kanamycin, tobramycin, gentamicin, and amikacin were determined as 92.9%, 76%, 78.6%, 73.4%, 52.6%, 44.2%, 27.9%, 24.7%, 19.5%, and 0.6%, respectively (Fig. 2). No resistance to imipenem was determined. One hundred and forty-eight isolates (96.1%) were found to be MDR.
Phylogenetic groups
The phylogroup distribution of ESBL/pAmpC-producing E. coli isolates is given in Table 2. There were statistically significant association between phylogroups and ESBL/pAmpC types (0.001 < P). As seen in Table 2, SHV-12- and CMY-2-positive isolates, to great extent, belonged to phylogroup E.
Characterization of the isolates by ERIC-PCR
ERIC-PCR analysis revealed 51 different clonal types, based on a similarity cutoff value of ≥ 92 (supplementary material). ERIC-PCR analysis showed that the isolates had similar profiles on a poultry houses and grouped ESBL- and pAmpC-producing isolates separately.
Plasmid replicon typing
A total of 35 plasmid replicon combinations were detected among the isolates (Table 3). The most common replicon types were FIB, I1, and Frep (Fig. 2).
MLST analysis
Nineteen sequence types (STs) were detected among the 28 selected isolates. The following STs were found in more than one isolate: ST354 (n = 3), ST114 (n = 3), ST5696 (n = 2), ST156 (n = 2), ST174 (n = 2), ST157 (n = 2), ST362 (n = 2), and ST5114 (n = 2). ST6635, ST539, ST457, ST1640, ST95, ST5843, ST1158, ST10, ST648, and ST4248 were detected in only one isolate.
Discussion
The aim of this study was to investigate the prevalence of ESBL- and/or pAmpC-producing E. coli in the southeast region of Turkey, which is one of the regions that broiler farming most intensively carried out. This kind of data obtained from the region makes this study a valuable report demonstrating high flock prevalence (76.2%) with ESBL- and/or pAmpC-producing E. coli. Nevertheless, all sampled broiler farms were found to be positive for ESBL- and/or pAmpC-producing E. coli in the Netherlands (Dierikx et al. 2013), Belgium (Smet et al. 2008), and Spain (Mesa et al. 2006).
This study is also the first to reveal presence of blaCMY-2 among cefotaxime-resistant E. coli isolates in Turkey. In previous studies conducted in Turkey, despite low rates, presence of CMY-2 has previously been reported in chicken meat (Pehlivanlar Önen et al. 2015), dogs (Aslantaş and Yilmaz 2017), and cattle (Aslantaş et al. 2017). Similarly, high prevalence of blaCMY-2 was reported in Colombia (Castellanos et al. 2017), Sweden (Nilsson et al. 2014), the Netherlands (Dierikx et al. 2013), and Japan (Kameyama et al. 2013). On the contrary, while Bortolaia et al. (2010) identified all isolates as being ESBL producer, Smet et al. (2008) identified, out of 295 ceftiofur-resistant E. coli isolates, 45% (133) as being ESBL producer, 43% (127) as being AmpC producer, and 12% (35) as coproducers. Differences observed in epidemiological patterns of beta-lactamase-producing E. coli among different countries could be explained by implication of ecological factors (Vinueza-Burgos et al. 2019).
Out of the 19 ST types identified in the study, only one has previously been identified in ESBL/AmpC-producing E. coli from both broilers and humans, namely, ST10 (Day et al. 2016). ST648, ST156, ST1158, ST354, and ST1640 in ESBL/AmpC-producing E. coli from broilers (Day et al. 2016; Pires-dos-Santos et al. 2013; Castellanos et al. 2017; Börjesson et al. 2013; Mo et al. 2016), ST15 in wild birds (Liakopoulos et al. 2016), and ST5114, ST457, and ST95 in E. coli strains from human clinical cases (Giray et al. 2016; Hertz et al. 2016; Stephens et al. 2015) have been previously reported. Among them, ST5114 have recently been reported from human urosepsis cases in Turkey by Giray et al. (2016). To the best knowledge of the author, this study is the first to report that ESBL/pAmpC producers are associated with ST114, ST174, ST6635, ST539, ST362, ST5843, ST4248, and ST5696.
The determination of antimicrobial susceptibility of the microorganisms has been performed in either a quantitative or qualitative methods. The former method provides determination of the minimum inhibitory concentration (MIC) of the tested bacteria. In contrast, the disc diffusion method does not provide quantitative data, but this method is widely preferred due to relatively easy to setup, simplicity, low cost, the ability to test a large numbers of microorganisms and antimicrobial agents, and the ease of interpretation (Balouiri et al. 2016). Therefore, in this study, disc diffusion method was used to determine the antimicrobial susceptibility of the isolates. In several studies, ESBL/pAmpC-producing E. coli isolates have also been shown to be resistant to different classes of antimicrobials (Maciuca et al. 2015; Maamar et al. 2016;Pehlivanlar Önen et al. 2015). Similarly, in this study, 96.1% of the isolates showed MDR phenotype. In addition to high resistance rates of beta-lactam antibiotics, ESBL/pAmpC-producing isolates also showed resistance to non-beta-lactams. Although the use of antimicrobials as growth promoters in food producing animals has been banned in Turkey since 2006 (TMAF 2006), high resistance rates among commensal E. coli isolates indicate that these antimicrobials are probably still widely used in broiler flocks (Lambrecht et al. 2018). On the other hand, it has to be kept in mind the fact that the ban of antibiotic use in poultry production systems may not always result in diminishing ESBL/AmpC-producing E. coli. Ecological factors may also play a role in the epidemiology of these MDR bacteria (Huijber et al. 2016; Smith et al. 2007).
Based on the presence of large genetic variations among E. coli strains isolated from different sources, E. coli strains were assigned to one of eight phylogenetic groups (A, B1, B2, C, D, E, F, and clade I) by a new method developed by Clermont et al. (2013). According to this method, the isolates that cause extraintestinal infections mainly belonged to B2 or D phylogroups, whereas the commensal isolates predominantly belonged to A or B1 phylogroups (Clermont et al. 2013; Picard et al. 1999; Bailey et al. 2010). In previous studies, A, B1, and to lesser extent D have been reported as the most common phylogroups among ESBL/pAmpC-producing E. coli isolates from broilers (Costa et al. 2009; Maamar et al. 2016; Mo et al. 2016), using triplex PCR method (Clermont et al. 2000). In contrast to previous studies, by using quadruplex method, phylogenetic group E was found as the most common phylogroup among the isolates. In this study, nearly half of SHV-12- and CMY-2-producing E. coli isolates were found to be belonged to this phylogroup. By using quadruplex PCR method, Touzain et al. (2018) reported phylogenetic distribution of 33 ESBL/pAmpC-producing E. coli isolates from broilers as A(7), B1(3), D(1), B2(2), C(6), F(9), and E(5) in France.
Plasmids based on their replication origin were classified into incompatibility (Inc) groups (Carattoli et al. 2005). According to this typing scheme, plasmids with same replication origin are classified as “incompatible,” whereas plasmids with different replication origin are classified as “compatible.” In this study, IncFIB, IncI1, and IncFrep were the most prevalent incompatibility groups among studied isolates. In previous studies, ESBL and pAmpC genes have been detected on plasmids, belonging to Inc. groups I1, FIA, K/B, P (Maciuca et al. 2015), K (Mo et al. 2016), I1, A/C, B/O, and HI2 (Castellanos et al. 2017). IncF plasmids have a narrow host spectrum, are well adapted to E. coli, and associated not only with ESBLs (such as CTX-M-15) but also pAmpC (blaCMY and blaDHA), quinolone (aac(6′)-Ib-cr, qnr, qepA), and aminoglycoside (armA and rmtB) resistance genes (Villa et al. 2010). Morever, IncF plasmids, like I1 plasmids, have multi-replicon status, allowing both acquisition of plasmids carrying incompatible replicons and broad host range replication (Toukdarian 2004). Moreover, Fischer et al. (2014) reported that IncI1-carrying plasmids lead to no or negligible fitness costs on its E. coli host and have the ability to persist even in the absence of antimicrobial selection pressure.
In conclusion, the current study is the first comprehensive study to determine the prevalence and diversity of ESBL/pAmpC-producing E. coli in broiler flocks in Turkey, indicating that broiler flocks were mostly colonized with blaCMY-2-carrying E. coli strains and could be considered as one of the hotspots for antimicrobial resistance. The findings of the study necessitate further molecular characterization of ESBL/pAmpC-producing E. coli isolates from humans and broilers to elucidate transmission dynamics of these isolates.
References
Ahmed, A.M., Motoi, Y., Sato, M., Maruyama, A., Watanabe, H., Fukumoto, Y., Shimamoto, T., 2007. Zoo animals as a reservoir of gram negative bacteria harboring integrons and antimicrobial resistance genes. Appl Environ Microbiol. 73, 6686–6690.
Aslantaş, Ö., Yilmaz, E.Ş., 2017. Prevalence and molecular characterization of extended-spectrum β-lactamase (ESBL) and plasmidic AmpC β-lactamase (pAmpC) producing Escherichia coli in dogs. J Vet Med Sci. 79, 1024–1030.
Aslantaş, Ö., Elmacıoğlu, S., Yılmaz, E.Ş., 2017. Prevalence and Characterization of ESBL- and AmpC-producing Escherichia coli from Cattle. Kafkas Univ Vet Fak Derg. 23, 63–67.
Bailey, J.K., Pinyon, J.L., Anantham, S., Hall R.M., 2010. Distribution of human commensal Escherichia coli phylogenetic groups. J Clin Microbiol. 48, 3455–3456.
Balouiri, M., Sadiki, M., Ibnsouda, S.K., 2016. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 6, 71–79.
Başaran Kahraman, B., Diren Sığırcı, B., Çelik, B., Gümüş, B., Metiner, K., Adıgüzel M.C, Bağcıgil, A.F., İkiz, S., Özgür, N.Y., Ak, S., 2016. Detection of extended-spectrum β-lactamase and AmpC β-lactamase producing Escherichia coli isolates from chickens. Kafkas Univ Vet Fak Derg. 22, 591–596.
Börjesson, S., Bengtsson, B., Jernberg, C., Englund, S., 2013. Spread of extended-spectrum beta-lactamase producing Escherichia coli isolates in Swedish broilers mediated by an incl plasmid carrying bla(CTX-M-1). Acta Vet. Scand. 55, 3.
Bortolaia, V., Guardabassi, L., Trevisani, M., Bisgaard, M., Venturi, L., Bojesen, A.M., 2010. High diversity of extended-spectrum beta-lactamases in Escherichia coli isolates from Italian broiler flocks. Antimicrob Agents Chemother. 54, 1623–1626.
Carattoli, A., Bertini, A., Villa, L., Falbo, V., Hopkins, K.L., Threlfall, E.J., 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods. 63, 219–228.
Carattoli, A., Zankari, E., García-Fernández, A., Voldby Larsen, M., Lund, O., Villa, L., Møller Aarestrup, F., Hasman, H., 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58, 3895–3903.
Castellanos, L.R., Donado-Godoy, P., León, M., Clavijo, V., Arevalo, A., Bernal, J.F., Timmerman, A.J., Mevius, D.J., Wagenaar, J.A., Hordijk, J., 2017. High heterogeneity of Escherichia coli sequence types harbouring ESBL/AmpC genes on IncI1 Plasmids in the Colombian poultry chain. PLoS One. 12, e0170777.
Clermont, O., Bonacorsi, S., Bingen, E., 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 66, 4555–4558.
Clermont, O., Christenson, J.K., Denamur, E., Gordon, D.M. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 5, 58–65.
Clinical and Laboratory Standard Institute (CLSI): Performance Standards for Antimicrobial Susceptibility Testing; Nineteenth Informational Supplements. CLSI Document M100-S19. Wayne, PA, USA, 2012.
Costa, D., Vinué, L., Poeta, P., Coelho, A.C., Matos, M., Sáenz, Y., Somalo, S., Zarazaga, M., Rodrigues, J., Torres, C., 2009. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolates in faecal samples of broilers. Vet Microbiol. 138, 339–344.
Day, M.J., Rodríguez, I., van Essen-Zandbergen, A., Dierikx, C., Kadlec, K., Schink, A.K., Wu, G., Chattaway, M.A., DoNascimento, V., Wain, J., Helmuth, R., Guerra, B., Schwarz, S., Threlfall, J., Woodward, M.J., Coldham, N., Mevius, D., Woodford, N., 2016. Diversity of STs, plasmids and ESBL genes among Escherichia coli from humans, animals and food in Germany, the Netherlands and the UK. J Antimicrob Chemother. 71, 1178–1182.
Dierikx, C.M., van der Goot, J.A., Smith, H.E., Kant, A., Mevius, D.J. 2013. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One. 8, e79005.
EFSA, ECDC, 2018. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 16, 5182.
Ewers, C., Antao, E.M., Diehl, I., Philipp, H.C., Wieler, L.H., 2009. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl Environ Microbiol. 75, 184–192.
Ewers, C., Bethe, A., Semmler, T., Guenther, S., Wieler, L.H., 2012. Extended-spectrum beta-lactamaseproducing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect. 18, 646–655.
Fischer, E.A., Dierikx, C.M., van Essen-Zandbergen, A., van Roermund, H.J., Mevius, D.J., Stegeman, A., Klinkenberg, D., 2014. The IncI1 plasmid carrying the blaCTX-M-1 gene persists in in vitro culture of a Escherichia coli strain from broilers. BMC Microbiol. 14, 77.
Giray, B., Uçar, F.B., Aydemir, S.Ş., 2016. Genotypic analysis of Escherichia coli strains that cause urosepsis in the Aegean region. Turk J Med Sci. 46, 1518–1527.
Hertz, F.B., Nielsen, J.B., Schønning, K., Littauer, P., Knudsen, J.D., Løbner-Olesen, A., Frimodt-Møller, N., 2016. Population structure of drug-susceptible,-resistant and ESBL-producing Escherichia coli from community-acquired urinary tract. BMC Microbiol. 16, 63.
Huijber, P.M.C., Graat, E.A.M., van Hoek, A.H.A.M., Veenman, C., de Jong, M.C.M., van Duijkeren, E., 2016. Transmission dynamics of extended-spectrum β-lactamase and AmpC β-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev Vet Med. 131, 12–19.
Huijbers, P.M.C., Graat, E.A.M., Haenen, A.P.J., van Santen, M.G., van Essen-Zandbergen, A., Mevius, D.J., van Duijkeren, E., van Hoek A.H.A.M., 2014. Extended-spectrum β-lactamase- and AmpC β-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: prevalence, risk factors, and molecular characteristics. J Antimicrob Chemother. 69, 2669–2675.
Ibrahim, D.R., Dodd, C.E., Stekel, D.J., Ramsden, S.J., Hobman, J.L., 2016. Multidrug resistant, extended spectrum β-lactamase (ESBL)-producing Escherichia coli isolated from a dairy farm. FEMS Microbiol Ecol. 92, fiw013
Jarlier, V., Nicolas, M.H., Fournier, G., Philippon, A., 1988. Extended broad-spectrum β- lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 10, 867–878.
Kameyama, M., Chuma, T., Yabata, J., Tominaga, K., Iwata, H., Okamoto, K., 2013. Prevalence and epidemiological relationship of CMY-2 AmpC β-lactamase and CTX-M extended-spectrum β-lactamase-producing Escherichia coli isolates from broiler farms in Japan. J Vet Med Sci. 75, 1009–1015.
Lambrecht, E., Van Meervenne, E., Boon, N., Van de Wiele, T., Wattiau, P., Herman, L., Heyndrickx, M., Van Coillie, E., 2018. Characterization of cefotaxime- and ciprofloxacin-resistant commensal Escherichia coli originating from Belgian farm animals indicates high antibiotic resistance transfer rates. Microb Drug Resist. 24, 707–717.
Liakopoulos, A., Olsen, B., Geurts, Y., Artursson, K., Berg, C., Mevius, D.J., Bonnedahl, J., 2016. Molecular Characterization of extended-spectrum-cephalosporin-resistant Enterobacteriaceae from wild kelp gulls in South America. Antimicrob Agents Chemother. 60, 6924–6927.
Lim, K.T., Yasin, R., Yeo, C.C., Puthucheary, S., Thong, K.L., 2009. Characterization of multidrug resistant ESBL-producing Escherichia coli isolates from hospitals in Malaysia. J Biomed Biotechnol. 2009, 165637.
Maamar, E., Hammami, S., Alonso, C.A., Dakhli, N., Abbassi, M.S., Ferjani, S., Hamzaoui, Z., Saidani, M., Torres, C., Boutiba-Ben Boubaker, I., 2016. High prevalence of extended-spectrum and plasmidic AmpC beta-lactamase-producing Escherichia coli from poultry in Tunisia. Int J Food Microbiol. 231, 69–75.
Maciuca, I.E., Williams, N.J., Tuchilus, C., Dorneanu, O., Guguianu, E., Carp-Carare, C., Rimbu, C., Timofte, D., 2015. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb Drug Resist. 21, 651–662.
Madoshi, B.P., Kudirkiene, E., Mtambo, M.M., Muhairwa, A.P., Lupindu, A.M., Olsen, J.E., 2016. Characterisation of commensal Escherichia coli isolated from apparently healthy cattle and their attendants in Tanzania. PLoS One, 11, e0168160.
Mesa, R.J., Blanc, V., Blanch, A.R., Cortés, P., González, J.J., Lavilla, S., Miró, E., Muniesa, M., Saco, M., Tórtola, M.T., Mirelis, B., Coll, P., Llagostera, M., Prats, G., Navarro, F., 2006. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J Antimicrob Chemother. 58, 211–215.
Mo, S.S., Slettemeås, J.S., Berg, E.S., Norström, M., Sunde, M., 2016. Plasmid and host strain characteristics of Escherichia coli resistant to extended-spectrum cephalosporins in the Norwegian broiler production. PLoS One. 11, e0154019.
Nilsson, O., Börjesson, S., Landén, A., Bengtsson, B., 2014. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother. 69, 1497–500.
Olsen, R.H., Bisgaard, M., Löhren, U., Robineau, B., Christensen, H., 2014. Extended-spectrum β-lactamase-producing Escherichia coli isolated from poultry: a review of current problems, illustrated with some laboratory findings. Avian Pathol. 43, 199–208.
Pehlivanlar Önen, S., Aslantaş, Ö., Yılmaz, E.Ş., Kürekci, C., 2015. Prevalence of β-Lactamase Producing Escherichia coli from Retail Meat in Turkey. J Food Sci. 80, M2023–2029.
Pérez-Pérez, F.J., Hanson, N.D., 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 40, 2153–2162.
Philippon, A., Arlet, G., Jacoby, G.A., 2002. Plasmid-determined AmpC-type lactamases. Antimicrob Agents Chemother. 46, 1–11.
Picard, B., Garcia, J.S., Gouriou, S., Duriez, P., Brahimi, N., Bingen, E., Elion, J., Denamur, E. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun. 67, 546–553.
Pires-dos-Santos, T., Bisgaard, M., Christensen, H. 2013. Genetic diversity and virulence profiles of Escherichia coli causing salpingitis and peritonitis in broiler breeders. Vet Microbiol. 162, 873–880.
Pitout, J.D. (2010): Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: Changing epidemiology and drug treatment choices. Drugs. 70, 313–33.
Pitout, J.D.D., Laupland, K.B. (2008): Extended-spectrum b-lactamase-producing Enterobacteriaceae. An emerging public-health concern. Lancet Infect Dis. 8, 159–166.
Quainoo, S., Coolen, J.P.M, van Hijum, S.A.F.T, Huynen, M.A., Melchers, W.J.G., van Schaik, W., Wertheim, H.F.L., 2017). Whole-genome sequencing of bacterial pathogens: the future of nosocomial outbreak analysis. Review. Clin Microbiol Rev. 30, 1015–1063.
Ranjbar, R., Pezeshknejad, P., Khamesipour, F., Amini, K., Kheiri, R., 2017. Genomic fingerprints of Escherichia coli strains isolated from surface water in Alborz province, Iran. BMC Res Notes. 10, 295.
Smet, A., Martel, A., Persoons, D., Dewulf, J., Heyndrickx, M., Catry, B., Herman, L., Haesebrouck, F., Butaye, P., 2008. Diversity of extended-spectrum beta-lactamases and class C beta-lactamases among cloacal Escherichia coli Isolates in Belgian broiler farms. Antimicrob Agents Chemother. 52, 1238–1243.
Smith, J.L., Drum, D.J., Dai, Y., Kim, J.M., Sanchez, S., Maurer, J.J., Hofacre, C.L., Lee, M.D., 2007. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl Environ Microbiol. 73, 1404–1414.
Stephens, C.M., Skerker, J.M., Sekhon, M.S., Arkin, A.P., Riley, L.W., 2015. Complete genome sequences of four Escherichia coli ST95 isolates from bloodstream infections. Genome Announc. 3, e01241–15.
TMAF (Turkish Ministry of Agriculture and Forestry): Amendment pertaining Directive on production, importation, exportation, sale and use of feed additives and premixes Directive no:21.01.2006/26056. Available online: http://www.resmigazete.gov.tr/eskiler/2006/01/20060121.htm/ 28.08.2018
Toukdarian A (2004): Plasmid strategies for broad-host-range replication in Gram negative bacteria. In: Funnel B, Philips G, Eds. Plasmid Biology. Washington, DC: ASM Press,259–270.
Touzain, F., Le Devendec, L., de Boisséson, C., Baron, S., Jouy, E., Perrin-Guyomard, A., Blanchard, Y., Kempf, I., 2018. Characterization of plasmids harboring blaCTX-M and blaCMY genes in E. coli from French broilers. PLoS One. 13, e0188768.
Ünal N, Karagöz A, Aşkar Ş, Dilik Z, Yurteri Z (2017): Extended-spectrum β-lactamases among cloacal Escherichia coli isolates in healthy broilers in Turkey. Turk J Vet Anim Sci. 41: 72–76
Versalovic, J., Koeuth, T., Lupski, J.R., 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19, 6823–31.
Villa, L., García-Fernández, A., Fortini, D., Carattoli, A., 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 65, 2518–2529.
Vinueza-Burgos, C., Ortega-Paredes, D., Narváez, C., De Zutter, L., Zurita, J., 2019. Characterization of cefotaxime resistant Escherichia coli isolated from broiler farms in Ecuador. PLoS One. 14, e0207567.
Wang, G., Clark, C.G., Rodgers, F.G., 2002. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J Clin Microbiol. 40, 3613–3619.
WHO. 2015. World Health Organization: Worldwide country situation analysis: response to antimicrobial resistance. http://www.who.int/antimicrobial-resistance/publications/situationanalysis/en/. Accessed 05 September 2017.
Wirth, T., Falush, D., Lan, R., Colles, F., Mensa, P., Wieler, L.H., Karch, H., Reeves, P.R., Maiden, M.C., Ochman, H., Achtman, M., 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 60, 1136–1151.
Zankari, E., Hasman, H., Kaas, R.S., Seyfarth, A.M., Agersø, Y., Lund, O., Larsen, M.V., Aarestrup, F.M., 2013. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J Antimicrob Chemother. 68, 771–777.
Funding
This work was supported by the Hatay Mustafa Kemal University Scientific Research Fund under Grant number of 18 M 014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by Animal Ethic Committee of Hatay Mustafa Kemal University with decision number of 2017/4–5.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslantaş, Ö. High occurrence of CMY-2-type beta-lactamase-producing Escherichia coli among broiler flocks in Turkey. Trop Anim Health Prod 52, 1681–1689 (2020). https://doi.org/10.1007/s11250-019-02167-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02167-8