Abstract
This study aimed at investigating the relationship between concentrations of macro and trace elements in blood serum, and fluids from small and large follicles (SFF and LFF, respectively), oviduct (OF), and uterus (UF) of female dromedary camels. Fluids from small (2–6 mm) and large follicles (7–20 mm), oviduct and uterus, and blood samples were collected from 19 camels. The results indicated that the concentrations of serum Mg, Fe, and Mn were significantly higher than their follicular fluid, OF, and UF concentrations. Levels of Zn, Fe, Cu, Cr, and Mn were significantly higher in SFF than in LFF. Se and Mo concentrations were higher in LFF. Co concentration was lower in serum than in reproductive tract fluids. Cr concentration was higher in UF and OF than in the serum, SFF, and LFF. High Ca concentration was observed for serum and SFF, followed by LFF. The concentration of Na was about 1.18-fold higher in SFF than in serum, OF, and LFF, and approximately 4.1-fold higher in serum than in UF. K was present in higher concentration in SFF than in serum and LFF; however, its concentration was low in UF and OF. In conclusion, this study shows the concentrations of certain elements in small and large follicular, uterine, and oviductal fluids, which may be low or high depending on their function in the development and growth of follicles. This information can support the development of new media for in vitro oocyte maturation and fertilization of female camels.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Camels are highly valued in the agricultural production systems of the Arabian subcontinent. They are a better source of milk and meat in the stressful environment, compared with other farm animals, which are harshly affected by high temperatures and scarcity of food and water (Saadeldin et al. 2018). Camels have adapted biologically to their environment and can tolerate a high dietary intake of salt, high, and low ambient temperatures, and do not develop hypertension or diabetes even after consuming eight times of salts more than sheep or cattle (Vito et al. 2009). Furthermore, camel milk has potential therapeutic effects, due to its antibacterial (Habib et al. 2013; Kanwar et al. 2015; Shori 2015), antiviral (Habib et al. 2013), antidiabetic, anti-aging (Choi et al. 2013), and anticarcinogenic (El-Redwan and Tabll 2007; Kanwar et al. 2015) properties.
Fluctuations in the biochemical constituents of blood serum can potentiality affect the biochemical components of follicular fluid (small follicular fluid—SFF, large follicular fluid—LFF), oviductal fluid (OF), and uterine fluid (UF)), thus indirectly influencing oocyte maturation, development and quality, and subsequently, the fertilization, and development of embryo in the reproductive tract (Sreenan and Morris 2007; Ur-Rahman et al. 2008; Ménézo et al. 2015). Ovarian follicles contain the oocyte, which is affected by the follicular fluid composition, as serum transudates can be altered by follicular metabolic activities. In addition to providing nutritional supplies to the developing oocyte, follicular fluid also sustains an environment suitable for oocyte development and maturation, and locally created constituents that share the metabolic activity of cumulus cells (Gerard et al. 2002).
This follicular fluid also contains several molecules that are involved in follicular cell development, proliferation, and differentiation (Ghoneim et al. 2013; Hamdi et al. 2018). The metabolic activity and barrier properties of the blood–follicle barrier have been shown to alter significantly during the follicular growth phases. Consequently, the biochemical components of follicular fluid also vary according to the phase of follicular development, in order to provide proper nutrients for normal oocyte development. Alterations in the follicular fluid metabolites can also influence oocyte competence within the follicles (Hugentobler et al. 2007; Ur-Rahman et al. 2008). Recently, it has been shown that low concentrations of OF and UF promote embryo development in in vitro serum-free cultures, where UF potentially has antioxidative activity, and OF provides a better control over embryo methylation (Hamdi et al. 2018).
Before focusing on the potential impacts of follicular metabolic molecules on oocyte competence, it is necessary to assess the physiological concentrations of certain minerals in fluids from small and large follicles, oviduct, and uterus, and then determine the extent of correlation between the serum and follicular fluid values of these metabolites. Therefore, the current study aimed at investigating the electrolytes and metabolites present in follicular fluids from small and large follicles, oviductal fluids, uterine fluids, and the blood serum of dromedary camels.
Materials and methods
Sample collection
Clinically healthy reproductive tracts were collected from 19 healthy, adult (6–11 years of age), non-pregnant female camels (Camelus dromedarius), immediately after slaughter in local slaughterhouses, during the breeding season (December–April). At the time of slaughter, blood samples (10 mL) from each animal were collected into non-heparinized tubes, kept at room temperature for 20 min to coagulate, and then centrifuged (3000 rpm for 15 min.). The serum was separated and stored at − 20 °C until analysis.
Camels were slaughtered for their meat, and not for the investigations; hence, ethical approval from the institutional ethical committee was not required. Female reproductive organs were removed from the body after slaughter, washed twice in 0.9% NaCl and blotted dry. Ovarian follicles were assessed using Vernier calipers and were classified as small (2–6 mm) or large (7–20 mm) depending on their diameter. Only the growing and dominant follicles were aspirated, while the atretic and cystic follicles (˃ 20 mm in diameter) were excluded (Tibary and Anouassi 1997; Swelum and Alowaimer 2015; Swelum et al. 2018). The total number of aspirated follicles was ≥ 114/follicular group.
Follicular fluids were aspirated from small to large follicles by means of a sterilized 22-gauge hypodermic needle and syringe. The follicular fluid was centrifuged at 1250×g at 4 °C for 10 min, after which the supernatant was isolated and stored at − 20 °C till further analysis.
Oviductal and uterine fluids (20–40 μL per animal) were collected by gentle aspiration using a fire-polished glass Pasteur pipette. Oviductal fluid was collected from the ampulla and isthmus, while uterine fluid was collected from the base of the two uterine horns. Collected fluid was centrifuged at 1250×g at 4 °C for 10 min; the supernatant was collected and filtered using a polyether sulfone syringe filter (low protein binding 0.2 μm), for removing any cells or debris. All samples were stored at − 20 °C until further analysis and processing. All procedures relating to sample collection were completed within 30 min of slaughter (reviewed by Pillai et al. 2017).
Estimation of elements
Macro and trace elements were evaluated by ion chromatography, approved by Analytical Data Services Ltd., UK (Brickfield Trading Estate, Brickfield Lane, Chandlers Ford, Hants, SO53 4DR, UK). The samples were diluted with deionized water before analysis, 100 and 200-fold for cations and anions, respectively, and then transferred to Dionex autosampler vials (0.5 mL).
The levels of Na and K in the different fluids (serum, SFF, LFF, OF, and UF) were evaluated using a flame photometer (Jenway PFP7 Industrial Flame Photometer, Garforth, Leeds Ls25 IDX, UK), while the concentration of Ca was assessed using an atomic absorption spectrophotometer (SpectrAA 5, Varian Techtron, Mulgrave, Victoria 3170, Australia). Trace elements, such as Zn, Mg, Fe, Se, Cu, Co, Cr, Mn, and Mo, were determined using the atomic absorption spectrophotometer.
Statistical analyses
The data were analyzed statistically using general linear models with one-way ANOVA in SPSS. The differences in concentrations of elements were determined using the post hoc Newman–Keuls test. Pearson’s correlation coefficients were calculated to determine the correlation between the concentration of an element in the serum and in fluids from different female reproductive organs. The strength of the linear association between the concentration of an element in the serum and in aforementioned fluids was evaluated by calculating the coefficient of determination. Bonferroni’s correction was applied, and a difference was considered significant at P < 0.05. Data are expressed as mean ± standard error.
Results
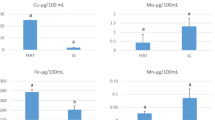
Table 1 shows the concentrations of macro and trace elements in the blood serum and small and large follicular, oviductal, and uterine fluids of studied animals. It is evident that the values of Mg, Fe, and Mn in the serum were significantly different (P < 0.05) from those in SFF, LFF, OF, and UF. The concentrations of Se, Fe, Cu, Cr, and Mn were higher (P < 0.05) in SFF than in LFF, while Mg and Fe had similar concentrations in UF and OF. SFF showed the highest Zn concentration, which was approximately 3-fold higher than its concentration in the serum, LFF, OF, and UF. No significant differences were observed in concentrations of Mg in SFF, LFF, OF, and UF; however, serum concentration of Mg was about 12-fold higher than its concentration in other fluids. Concentration of Fe was the highest in the serum and the lowest in LFF; however, its concentration in OF and UF was not significantly different. The highest level of Se was noted for LFF, followed by UF, SFF, and OF in decreasing order. Cu concentration in SFF was 5, 3, 2.5, and 2.5-fold higher (P < 0.05) than in OF, serum, LFF, and UF, respectively. The concentration of Co was generally low in the serum and all fluids; however, it had a higher concentration in LFF and UF, than in SFF, serum, and OF. In uterine and oviductal fluids, Cr concentration was higher (P < 0.05) when compared with that in the serum, SFF, and LFF. Mn concentration was the highest in the serum and significantly lower in other fluids. Mn concentration in LFF, UF, and OF was approximately 0.15%, 0.14%, and 10.7% of the serum concentration, respectively. The concentration of Mo in the serum, SFF, and LFF was significantly higher (P < 0.05) than that in UF and OF.
The concentration of Ca was higher in the serum and SFF than in LFF, while it was comparable in both UF and OF. The concentration of Na in SFF was about 1.18-fold higher than in the serum, OF and LFF, while the serum Na concentration was approximately 4.1-fold higher than the UF concentration. Similarly, higher (P < 0.05) concentration of K was observed in SFF than in the serum and LFF, while its concentration was low in both UF and OF.
Tables 2 and 3 show associations between concentrations of ions in LFF, SFF, OF, UF, and the serum. The concentrations of Ca (P < 0.05) and Mn (P < 0.01) in LFF were significantly positively correlated with their serum concentrations. Conversely, a significant negative correlation was observed for SFF and serum levels of Se (P < 0.01) and Mn (P < 0.05). K concentration in OF was positively correlated (P < 0.05) with the serum concentration. Similarly, serum concentrations of Se and Mo (P < 0.05) were positively correlated with their UF (P < 0.05) and SFF (P < 0.05) concentrations, respectively. Mo concentrations in OF and blood serum were negatively correlated.
Discussion
The impact of macro and trace elements concentration in follicular fluid on oocyte quality and maturation is showed in Tables 4. The impact of macro and trace elements concentration in oviductal fluid on fertilization and early embryo development is showed in Table 5. The impact of macro and trace elements concentration in uterine and oviductal fluid on the embryo, early embryonic death and implantation is showed in Table 6.
Mo and Mn were the main ions in follicular fluids (SFF and LFF) and the blood serum. The concentrations of Se, Mn, and Cr were higher in UF than in OF, while Na and Mo concentrations were higher in OF than in UF. Ur-Rahman et al. (2008) found that Na concentration was significantly different in blood serum and small follicles in one-humped camels, and it was higher in large follicular fluid than in the small follicular fluid.
Previous studies have shown that Na levels are different, although not significantly, in fluids from large and small follicles in camels (Iwata et al. 2004; Ur-Rahman et al. 2008). Na was one of the main elements and was present in higher concentrations, in OF, SFF, LFF, and blood serum. It has previously been reported that Na tends to have a lower concentration in bovine uterine fluid (Hugentobler et al. 2007). It is known that sodium is associated with the development of blastocyst, as blastocyst expansion, resulting from an accumulation of fluids between the cells, is dependent on the pumping of Na into the blastocoel cavity by the Na+/K+-ATPase pump (Hobbs and Kaye 1986; Brison and Leese 1993). This state is observed during pregnancy; however, the findings of the present study reflect the concentration of Na during the breeding season, in non-pregnant females. This could explain the lower concentration of Na in UF than in OF, SFF, LFF, or blood serum observed in this study.
A higher K concentration in SFF and serum than in LFF was noted in the current study. The level of K in SFF was 3-fold higher than in OF and UF and 1.25-fold higher than in the serum. Leroy et al. (2004) and Iwata et al. (2004) have confirmed that K concentration is higher in SFF than in LFF in bovines, which is consistent with our results. In addition, Chang et al. (1976) suggested that higher K concentration in small follicular fluid can be attributed to glucose uptake, a mechanism that leads to the transfer of K from extracellular fluid to intracellular lumens. We found higher levels of K in SFF and serum, together with a lower K concentration in the uterine fluid.
Ca levels in the present study were higher in the serum than in the other fluids examined, which is in concordance with the findings of Ur-Rahman et al. (2008). However, no significant differences were recorded in the Ca levels of UF and OF. Higher Ca concentration in the serum can be explained by the fact that serum is the main pool of several elements that are exploited by different tissues of the body. Ca is also essential for normal development, expansion, and functioning of the granulosa cells (Leung and Steele 1992). Furthermore, many previous studies have demonstrated that higher concentration of Ca at the site of fertilization (oviduct) is essential for sperm viability, as it allows the binding of oviductal proteins to the spermatozoa (Bavister 2000; Lapointe and Sirard 1996). Studies have also shown that Ca is indispensable for sperm capacitation, acrosome reaction, and processes associated with fertilization in mammals, humans in particular (Stock and Fraser 1989). The high SFF Ca concentration, compared with LFF, OF, and UF concentrations, recorded in the present study, could be related to oocyte development and maturation occurring in the follicles. However, as the size of follicles increases, the concentration of Ca decreases. It was interesting to note that the serum Ca concentration recorded in this study (3.65 nmol/L) was lower than that previously reported (13.3 nmol/L) by Ur-Rahman et al. (2008), which might be due to variations in nutritional status, age, and genetic makeup.
The present study showed that serum concentrations of Mg, Fe, and Mn were significantly higher than their SFF, LFF, OF, and UF concentrations. Ménézo et al. (2015) stated that the levels of Ca, Mg, Na, Cl, and PO4 are not taken into account during in vitro maturation (IVM), in vitro fertilization (IVF), or embryo culture in humans and other animals, and their influence on oocyte maturation and preimplantation embryo growth has not been examined.
In our study, Fe had a significantly higher concentration in serum than in other fluids. Further, it was shown that a significantly higher concentration of Fe was found in SFF and OF, than in LFF and UF. This difference in the Fe concentration might be related to its function at these sites. Fe plays an important role in the synthesis of nucleic acids and proteins, cellular respiration, electron transport, and cell proliferation and differentiation (Lieu et al. 2001), all of which are closely related to hormone secretion, development of oocytes and zygote, and metabolism occurring in the small follicular and oviductal fluids (Kolesarova et al. 2011).
Zinc, which is the second most abundant transition metal after iron, is also of importance. It attenuates toxicity induced by Cu and/or Fe and is involved in many significant metabolic responses that are a prerequisite for cell growth and development pathways. In the present study, we found a higher level of Zn in SFF than in other fluids. This might be due to the metabolic activities occurring during oocyte development, which produce free radicals than can be scavenged upon by zinc, thus reducing the oxidative stress. In addition, it acts as a co-factor for at least 200 enzymes, including zinc superoxide dismutase and carbonic anhydrase, both of which are present in oviductal fluid.
Zinc can scavenge on oxidative agents by capturing hydroxyl and superoxide radicals, through its participation in metallothioneins and metal-response element-binding transcription factors (Ménézo et al. 2013). It is involved in the regulation of the one-carbon cycle and consequently in methylation and imprinting during oocyte maturation and embryo development (Ménézo et al. 2013). Recently, Junior et al. (2018), in their study characterizing the main proteomics of ovarian follicular fluid during different physiological stages in locally adapted “Canindé” goats, found that zinc-alpha-2-glycoprotein (AZGP1) was one of the most abundant proteins. AZGP1 is associated with the activation of β3-adrenoceptors, resulting in an increase in intracellular cAMP (Russell et al. 2004). This unique feature of zinc is essential during sperm capacitation and acrosome reactions and may be associated with Ca signaling pathways (Qu et al. 2007).
Magnesium concentrations in SFF, LFF, OF, and UF were found to be similar in the present study, ranging from 0.07 to 0.11 mg/dL, which is in concordance with the results of Grippo et al. (1992) and Kenny et al. (2002). In the present study, the concentration of Mg was higher in serum than in other fluids. Jordan et al. (1983) proposed that magnesium is required for embryonic and fetal development, as fetal malformations were found to be associated with magnesium shortages.
Se, a micro mineral, plays a significant role as an antioxidant in different tissues and is the main structural component of many antioxidant enzymes, including glutathione peroxidase and thioredoxin reductase, which decrease the amount of reactive oxygen species (ROS) (Ramos et al. 2013). In the present study, a higher concentration of Se was observed in SFF, LFF, and UF; however, its concentration was the lowest in OF. It has previously been reported that Se influences processes occurring during pregestation and gestation stages, and can improve the activity of thioredoxin reductases (TrxRs), which are selenoproteins involved in reducing and maintaining the levels of a small antioxidative protein known as thioredoxin (Ufer and Wang 2011; Mistry et al. 2012).
Cu is considered to be one of the most essential elements, indispensable to the activities of two enzymes that are vital for immune responses, i.e., copper/zinc-superoxide dismutase and ceruloplasmin (Hussein and Staufenbiel 2012). The concentration of Cu in SFF was about 3-fold higher than in other fluids in the present study. Thus, we can presume that Cu is important for the proper functioning of the thyroid gland and secretion of its hormones. These thyroid hormones play a vital function in the development of follicles. No previous studies have been conducted concerning this element.
Co is essential for several metabolic activities, such as cell division, synthesis of thymine for DNA synthesis, formation of red blood cells, growth, and reproduction (Kumar et al. 2011). We found low concentrations of Co in all fluids, as well as in the serum, ranging from 0.8 in UF to 0.2 mg/dL in OF. It is possible that Co has a vital role in later stages of follicular and zygote development. Hence, higher concentration of Co was recorded in UF and LFF, when compared with other fluids.
Cr is essential for carbohydrate, protein, and lipid metabolism pathways. It is a biologically active component of chromodulin, an oligopeptide potentiating the influence of insulin, where it acts as an insulin cofactor and facilitates the binding of insulin to its receptors on the cell membrane. The effects of Cr on reproduction in cattle have been given little consideration (Pechova and Pavlata 2007). Our study shows that concentration of Cr is higher in both UF and OV, and it is lowest in LFF No data were available about this element, for comparison with other animals.
The concentration of Mo was found to be higher in serum and LFF. According to Phillipo et al. (1987), Mo has negative effects on reproductive aspects, such as delayed puberty, reduced fertility, and increased incidence of anovulation in cows. Mn concentration was significantly different in all fluids, and serum Mn concentration was about 6.6, 3.6, 6.9, and 9.3 times higher than its LFF, SFF, UF, and OF concentrations, respectively.
Mn2+ is an abundant, naturally occurring, crucial trace mineral that is essential for normal mammalian physiological processes, such as those associated with normal growth and development of bones, connective tissues, and cartilage (Hurley 1981) and the reproductive system (Greger 1998). The results of the present study further suggest that Mn, through its ability to stimulate cell development and division, may contribute to events leading to enhanced oocyte competence, and embryonic growth and development. Trace elements greatly influence animal health, productivity, and reproductive physiology, and their scarcity can lead to depressed reproductive efficiency, and subsequent economic losses to the dairy industry (Kumar et al. 2011).
The significant positive correlation between Ca and Mn levels in LFF blood serum, found in the present study, show that the ion levels in large follicular fluid are dependent on their concentrations in blood. This further suggests that Ca and Mn have an important role in the development of follicles, and their concentration in the blood may determine the physiological status of animals. A strong negative correlation was found for the concentration of Se in SFF and blood serum, while a positive correlation was observed for Se levels in UF and blood serum. This shows that Se may benefit the capitation of spermatozoa, and also the early stages of embryonic development and implantation. Previous reports on uterine and oviductal pH (Hugentobler et al. 2004), and on energy substrates (Salleh et al. 2005) of these reproductive tract fluids support the conclusion that OF and UF formation operate under different mechanisms (Hugentobler et al. 2007). An unexpected result of this study was that the serum Mo concentration was positively and negatively correlated with its SFF and OF levels, respectively. No previous studies have investigated the role of Mo in the reproductive tract and the maturation of oocytes. This provides scope for future studies on the biological role of Mo and its effects on reproduction.
Iron concentration in OF was positively correlated with its serum concentration. Fe plays a significant role in modulating the functions of physiological pathways in various cells of the body. This correlation demonstrates the importance of Fe in OF, where it elevates the antioxidant enzymes activities and prevents the increase in lipid peroxidation, in a dose-dependent manner during spermatozoa maturation (Murugan et al. 2002; Massányi et al. 2003). Furthermore, K concentration in OF was positively correlated with its serum concentration. Ion diffusion across epithelial cells of the reproductive tract is certainly related to physiological responses and hormone regulation in female animals.
In conclusion, the results of the present study depict the comparative levels of some macro- and microelements in small and large follicular, uterine, and oviductal fluids and blood serum of female dromedary camels. This knowledge improves our understanding of the in vivo environment during oocyte maturation and early embryonic development, which can lead to the enhancement of in vitro culture media, consequently improving in vitro embryo production. The energy and pH sources of the gametic microenvironment, the embryonic alterations during the early reproductive stages, and the culture media for in vitro embryo production should reflect the in vivo physiological conditions more closely. For example, the fact that Na, K, Ca, Fe, Cu, Mo, Se, and Zn levels are higher in the small follicular fluid and blood serum than in other fluids provides information on the in vivo ion levels and the resulting physiological conditions. The discrepancies regarding the functions of trace elements can pave the way for future research investigating the biological role of trace minerals and their relation with oocyte competence and embryonic growth and development. Further studies are required to evaluate the relationship between the concentrations of macro and trace elements and the quality of granulosa cells and oocyte.
References
Anchordoquy, J.P., Anchordoquy, J.M., Sirini M.A., Mattioli G., Picco S.J., and Furnus C.C., 2013. Effect of different manganese concentrations during in vitro maturation of bovine oocytes on DNA integrity of cumulus cells and subsequent embryo development, Reproduction in Domestic Animal, 48(6), 905–11. doi: https://doi.org/10.1111/rda.12184
Anchordoquy, J.P., Anchordoquy, J.M., Sirini, M.A, Testa J.A., Peral-García, P., and Furnus, C.C., 2015. The importance of manganese in the cytoplasmic maturation of cattle oocytes: blastocyst production improvement regardless of cumulus cells presence during in vitro maturation, Zygote, 1, 139–48.
Bavister, B.D., 2000. Interactions between embryos and the culture milieu, Theriogenology, 53, 619–26.
Bedwal R.S. and Bahuguna A., 1994. Zinc, copper and selenium in reproduction, Experientia, 50, 626–640.
Bi, C.M., Zhang, Y.L., Liu, F.J., Zhou, T.Z., Yang, Z.J., Gao, S.Y., Wang, S.D., Chen, X.L., Zhai, X.W., Ma, X.G., Jin, L.J. and Wang S., 2013. The effect of molybdenum on the invitro development of mouse preimplantation embryos, Systems Biology in Reproductive Medicine, 59(2), 69–73.
Brison, D.R. and Leese, H.J., 1993. Role of chloride transport in the development of the rat blastocyst, Biology of Reproduction. 48, 692–702.
Casslen B., and Nilsson B., 1984. Human uterine fluid, examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin, glucose, and urea, American Journal of Obstetric and Gynecology, 150, 877–81.
Chang, S.C.S., Jones, J.D., and Ellefson R.D., 1976. The porcine ovarian follicle: I. Selected chemical analysis of follicular fluid at different developmental stages, Biology of Reproduction, 15, 321–328.
Choi, S.K., Park, K.D, Kim, D.A, Lee, D.W., and Kim, Y.J., 2013. Preparation of camel milk liposome and its anti-aging effects, Journal of Society of Cosmetic Scientists of Korea, 40(2), 155–161.
Corrah, L., 1996. Trace mineral requirement of grazing cattle, Animal Feed Science and Technology, 59, 61–70.
El-Redwan, R.M. and Tabll A., 2007. Camel lactoferrin markedly inhibits hepatitis C virus genotype 4 infection of human peripheral blood leukocytes, Journal of Immunoassay and Immunochemistry, 28(3), 267–277.
Gerard, N., Loiseau, S., Duchamp, G., and Seguin, F., 2002. Analysis of the variations of follicular fluid composition during follicular growth and maturation in the mare using proton nuclear magnetic resonance (1H NMR), Reproduction, 124, 241–248.
Geshi, M., Takenouchi, N., Yamauchi, N. and Nagai T., 2000. Effects of sodium pyruvate in nonserum maturation medium on maturation, fertilization, and subsequent development of bovine oocytes with or without cumulus cells, Biology of Reproduction, 63, 1730–1734.
Ghoneim, I.M., Waheed, M.M., El-Bahr, S.M., Alhaider A.K., and Al-Eknah, M.M., 2013. Comparison of some biochemical and hormonal constituents of overs follicles and preovulatory follicles in camels (Camelus dromedarius), Theriogenology, 79, 647–652.
Greger, J.L., 1998. Dietary standards for manganese: overlap between nutritional and toxicological studies, Journal of Nutrition, 128, 368S–371S.
Grippo, A.A., Henault, M.A., Anderson, S.H., and Killian, G.J., 1992. Cation concentrations in fluid from the oviduct ampulla and isthmus of cows during the estrous cycle, Journal of Dairy Science., 75, 58–65.
Habib, H.M., Ibrahim, W.H., Schneider-Stock, R., and Hassan, H.M. (2013). Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities, Food Chemistry, 141(1), 148–152
Hamdi, M., Lopera-Vasque, R., Maillo, V., Sanchez-Calabuig M.J., Nunez, C., Gutierrez-Adan A., and Rizos D., 2018. Bovine oviductal and uterine fluid support in vitro embryo development. Reproduction, Fertility and Development, https://doi.org/10.1071/RD17286
Hobbs, J.G., and Kaye, P.L., 1986. Glycine and Na+ transport in preimplantation mouse embryos, Journal of Reproduction and Fertility, 77, 61–6.
Hugentobler, S.A., Morris, D.G., and Kane M.T., 2004. In situ oviduct and uterine pH in cattle, Theriogenology, 61, 1419–27.
Hugentobler, S.A., Morris, D.G., Sreenan, J.M., and Diskin, M.G., 2007. Ion concentrations in oviduct and uterine fluid and blood serum during the estrous cycle in the bovine, Theriogenology, 68, 538–548.
Hurley, L., 1981. Teratogenic aspects of manganese, zinc and copper nutrition, Physiological Reviews, 61, 249–295
Hussein, H.A., and Staufenbiel, R., 2012. Variations in copper concentration and ceruloplasmin activity of dairy cows in relation to lactation stages with regard to ceruloplasmin to copper ratios, Biological trace element research, 146(1), 47–52
Iwata, H., Hashimoto, S., Ohota, M., Kimura, K., Shibano, K., and Miyake, M., 2004. Effects of follicle size and electrolytes and glucose in maturation medium on nuclear maturation and developmental competence of bovine oocytes, Reproduction. 127, 159–164.
Jeon, Y., Yoon, J.D., Cai, L., Hwang S.U., Kim, E., Zheng, Z., Lee, E., Kim, D.Y., and Hyun, S.H., 2014. Supplementation of zinc on oocyte in vitro maturation improves preimplatation embryonic development in pigs, Theriogenology, 82(6), 866–874.
Jordan, E.R., Chapman, T.E., Holtan, D.W., and Swanson, L.V., 1983. Relationship of dietary crude protein to composition of uterine secretions and blood in high-producing postpartum dairy cows, Journal of Dairy Science, 66, 1854–62
Junior, A.R.P., Tilburg, M.E., Lobo, M.D.P., Monterio-Moeriro, A.C.O., Melo, C.H.S., Souza-Fabjan, J.M.G., Araugo, A.A., Melo, L.M., Teixeria, D.I.A., Moura, A.A. and Freitas, V.J.F., 2018. Proteomic analysis of follicular fluid from tropically adapted goats. Animal Reproduction Journal, 188, 35–44. doi: https://doi.org/10.1016/j.anireprosci.2017.11.005.
Kanwar, J.R., Roy, K., Patel, Y., Zhou, S.F., Singh, M.R., Singh, D., Nasir, M., Sehgal, R., Sehgal, A., Singh, R.S., et al., 2015. Multifunctional iron bound lactoferrin and nanomedicinal approaches to enhance its bioactive functions, Molecules 20(6), 9703–9731.
Kenny, D.A., Humpherson, P., Leese, H.J., Mooris, D., Tomos, A., Diskin, M.G., et al., 2002. Effect of elevated systemic concentrations of ammonia and urea on the metabolite and ionic composition of oviductal fluid in cattle, Biology of Reproduction, 66, 1797–804.
Kolesarova, A., Capcarova, M., Medvedova, M., Sirotkin, A.V., Kovacik, J., 2011. In vitro assessment of iron effect on porcine ovarian granulosa cells: secretory activity, markers of proliferation and apoptosis, Physiological Research, 60, 503–510
Kumar, S., Pandey, A.K., Razzaque, W.A.A, Dwived, D.K., 2011. Importance of micro minerals in reproductive performance of livestock, Veterinary World, 4(5), 230–233
Lapointe, S., and Sirard, M.A., 1996. Importance of calcium for the binding of oviductal fluid proteins to the membranes of bovine spermatozoa, Molecular Reproduction and Development, 44, 234–40.
Leroy, J.L., Vanholder, T., Delanghe, J.R., Opsomer, G., Vansoom, A., Bols, P.E.,and de Kruif, A., 2004. Metabolite and ionic composition of follicular fluid from different sized follicles and their relationship to serum concentrations in dairy cows, Animal Reproduction Science, 80, 201–211.
Leung, P.C.K., and Steele, G.L., 1992. Intracellular signaling in the gonads, Endocrine Reviews, 13, 476–497.
Lieu, P.T., Heiskala, M., Peterson, P.A., and Yang, Y., 2001. The roles of iron in health and disease. Molecular Aspects of Medicine, 22, 1–87.
Massányi, P., Trandžík, J., Nad, P., Koréneková, B., Skalická, M., Toman, R., Lukáč, N., Strapák, P., Halo M., and Turčan J., 2003. Concentration of copper, iron, zinc, cadmium, lead, and nickel in boar semen and relation to the spermatozoa quality. Journal of Environmental Science and Health, Part A: Toxic/ Hazardous Substances and Environmental Engineering, 38(11), 2643–2651.
Ménézo, Y., Lichtblau, I., and Elder, K., 2013. New insights into human pre-implantation metabolism in vivo and in vitro. Journal of Assisted Reproduction and Genetics 30, 293–303.
Ménézo, Y., Guérin, P., and Elde K., 2015. The oviduct: a neglected organ due for re-assessment in IVF. Mini Review. Reproductive BioMedicine Online (2015).
Mistry, H.D., Pipkin, F.B., Redman, C.W.G., and Poston, L., 2012. Selenium in reproductive health. American Journal of Obstetrics and Gynecology, 206, 21–30.
Mitchell, L.M., Robinson, J.J., Watt, R.G., McEvoy, T.G., Ashworth, C.J., Rooke, J.A., and Dwyer, C.M., 2007. Effects of cobalt/vitamin B12 status in ewes on ovum development and lamb viability at birth. Reproduction, Fertility and Development, 19, 553–562.
Murugan, M.A., Gangadharan, B., and Mathur, P.P., 2002. Antioxidative effect of fullerenol on goat epididymal spermatozoa. Asian Journal of Andrology., 4, 149–152.
Nandi, S., Girish Kumar, V., Manjunatha, B.M., Ramesh, H.S., Gupta, P.S., .2008. Follicular fluid concentrations of glucose, lactate and pyruvate in buffalo and sheep, and their effects on cultured oocytes, granulosa and cumulus cells. Theriogenology, 69(2), 186–96.
Pechova, A., and Pavlata L. (2007). Chromium as an essential nutrient: a review. Veterinarni Medicina, 52(1), 1–18.
Phillipo, M., Humphries, W.R., Atkinson, T., Henderson, G.D., and Gaythwaite, P.H., 1987. The effect of molybdenum and iron on copper status, puberty, fertility and oestrus cycles in cattle. The Journal of Agricultural Science, 109, 326–336.
Pillai, V.V., Weber, D.M., Phinney, B.S., and Selvaraj, V., 2017. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS ONE, 12(11), e0188105.
Qu, A.F., Ying, X., Guo, W., Guo, Q., Chen, G., Liu, Y., and Ding, Z., 2007. The role of Zn-a glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction, 134, 569–576.
Ramos, G.B., Sia, A.J., Callejas, N.A.N., Revilla, C.J.P., Alfonso, N., and Sia, S.G., 2013. Pregestational and gestational maternal selenium – supplement: influence on ethanol – induced dysmorphogenesis in murine postimplantation embryos. Asian journal of experimental biological sciences, 4, 361–368.
Russell, S.T., Russell, T.P., Zimmerman, B.A., and Domin, M.J., 2004. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein, Biochimica et Biophysica Acta, 1636, 59–68.
Saadeldin, I.M., Swelum, A., Alzahrani F.A., and Alowaimer, A. N., 2018. The current perspectives of dromedary camel stem cells research. Review Article, International Journal of Veterinary Science and Medicine, 6, S27–S30.
Salleh, N., Baines, D.L., Naftalin, R.J., and Milligan, S.R., 2005. The hormonal control of uterine fluid secretion and adsorption. The Journal of Membrane Biology, 206, 17–28.
Shori, A.B., 2015. Camel milk as a potential therapy for controlling diabetes and its complications: a review of in vivo studies. Journal of Food and Drug Analysis, 23(4), 609–618.
Sreenan, J.M., and Morris, D.G., 2007. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Molecular Reproduction and Development, 74, 445–454. doi:https://doi.org/10.1002/MRD.20607.
Stock, C.E., and Fraser, L.R., 1989. Divalent cations, capacitation and the acrosome reaction in human spermatozoa. Journal of Reproduction and Fertility, 87, 463–78.
Swelum, A.A. and Alowaimer, A.N., 2015. The efficacy of controlled internal drug release (CIDR) in synchronizing the follicular wave in dromedary camels (Camelus dromedarius) during the breeding season. Theriogenology, 84, 1542–1548.
Swelum, A.A., Saadeldin, I., Moumen, A., Ba-Awadh, H. and Alowaimer, A.N., 2018. Efficient follicular wave synchronization using a progesterone-releasing intravaginal device (PRIDΔ) in Camelus dromedaries. Theriogenology 118, 203–211.
Tareq, K.M., Akter, Q.S., Khandoker, M.A., and Tsujii, H., 2012. Selenium and vitamin E improve the in vitro maturation, fertilization and culture to blastocyst of porcine oocytes. Journal of Reproduction and Development 58(6), 621–8.
Tibary, A., and Anouassi, A., 1997. Reproductive physiology in the female camelidae. In: Theriogenology in camelidae: anatomy, physiology, pathology and artificial breeding. IAVH II. Rabat, Morocco. 169-241.
Tuormaa, T. E., 2000. Chromium selenium copper and other trace minerals in health and reproduction. Journal of Molecular Medicine, 15, 145–157.
Ufer, C., Wang, C.C., 2011. The roles of glutathione peroxidases during embryo development. Frontiers in Molecular Neuroscience, 4, 1–14.
Ur-Rahman, Z., Bukhari, S.A., Ahmad, N., Akhtar, N., Ijaz, A., Yousaf, M.S. and Ha, I.U., 2008. Dynamics of follicular fluid in one-humped camel (Camelus dromedarius). Reproduction in Domestic Animal, 43, 664–671.
Vito, L., Vincenzo, T., Marco, D., Mohamed, H., Mabrouk, M.S., Giovanni, M.L., and Cataldo, D., 2009. A survey of chemical and nutritional characteristics of halophytes plants used by camels in Southern Tunisia. Tropical Animal Health and Production 41, 209–215.
Whitaker, M., 2006. Calcium at fertilization and in early development. Physiological Reviews. 86(1), 25–88.
Zhang, Y.L., Liu, F.J., Chen, X.L., Zhang, Z.Q., Shu, R.Z.,Yu, X.L., Zhai, X.W., Jin, L.J., Ma, X.G., Qi, Q., and Liu, Z.J,. 2013. Dual effects of molybdenum on mouse oocyte quality and ovarian oxidative stress. Systems Biology in Reproductive Medicine, 59(6), 312–318.
Acknowledgments
We would like to thank the DSR and RSSU (Researchers Support and Services Unit) of King Saud University for their technical support.
Funding
This study was supported by King Saud University, Deanship of Scientific Research (DSR), Research Group #RG-1438-066.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Camelids
Guest Editor: Bernard Faye
Rights and permissions
About this article
Cite this article
Swelum, A.AA., Saadeldin, I.M., Abdelnour, S.A. et al. Relationship between concentrations of macro and trace elements in serum and follicular, oviductal, and uterine fluids of the dromedary camel (Camelus dromedarius). Trop Anim Health Prod 52, 1315–1324 (2020). https://doi.org/10.1007/s11250-019-02137-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02137-0