Abstract
The Chinese highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) has caused a severe threat to the pig population in Southeast Asian countries. The purpose of this study was to investigate the efficacy of a type 2 PRRSV modified live vaccine (PrimePac™ PRRS, lineage 7) against a Thai HP-PRRSV (10PL01, lineage 8). Three-week-old PRRSV-free pigs were randomly assigned into three groups. Vaccinated challenged group (group 1, n = 16) was immunized with PrimePac™ PRRS vaccine at 3 weeks old. The unvaccinated challenged group (group 2, n = 16) was injected with PBS at 3 weeks old, and unvaccinated unchallenged group (group 3, n = 10) was served as a negative control. At 9 weeks old, all groups, except the negative control group, were challenged with the Thai HP-PRRSV. All pigs were monitored daily during 10 days post-infection (dpi) and were necropsied at 10 and 17 dpi. The results revealed that vaccinated challenged pigs showed significantly lower (p < 0.05) mean rectal temperatures, clinical respiratory scores, lung lesion scores, and levels of virus load in serum and lung tissue compared with the unvaccinated challenged pigs. Moreover, vaccinated challenged pigs exhibited PRRSV-specific serum neutralizing antibodies at the end of the experiment. Our findings indicated that the studied type 2 PRRSV vaccine provided partial protection against the Thai HP-PRRSV infection based on the body temperature, levels of viremia, and the severity of lung lesions. These results demonstrated that partial protection of PrimePac™ PRRS vaccine might be useful for controlling HP-PRRSV infection in the endemic area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porcine reproductive and respiratory syndrome (PRRS) has been recognized as a highly contagious swine disease, leading to massive economic losses about $664 million annually in the US swine industry (Holtkamp et al. 2013). PRRS is caused by PRRS virus (PRRSV) classified into type 1 (EU) and type 2 (US) genotypes. PRRSV belongs to the family Arteriviridae and genus Arterivirus (Cavanagh 1997; Meulenberg et al. 1997). The clinical outcomes of PRRSV infection are reproductive failure in breeding herd and respiratory disorder in nursery to finishing pigs. In 2006, massive outbreaks of highly pathogenic PRRSV (HP-PRRSV) with discontinuous 30 amino acid deletion in NSP 2 emerged in China resulting in high mortality and morbidity rates in infected pigs (Tian et al. 2007). After the first outbreak in China, HP-PRRSV rapidly spread to Southeast Asian countries including Vietnam, Laos, Cambodia, Philippines, Myanmar, and Thailand (Jantafong et al. 2015). Interestingly, recent surveillances of genetic diversity of PRRSV circulating in Thai swine industry showed that HP-PRRSV has been reported as the dominant virus in Thailand (Jantafong et al. 2015). Thus, it is speculated that HP-PRRSV could be a major threat to the Thai swine industry.
To protect pigs from HP-PRRSV, attenuated live vaccines based on highly pathogenic PRRSV strains provided completely protection against HP-PRRSV challenge in China (Leng et al. 2012; Yu et al. 2015). However, these vaccines were not allowed for use in other countries including Thailand. Therefore, commercially available type 2 PRRS vaccines, belonging to lineage 5 and lineage 8, were proven under the experimental condition and reported to be effective against HP-PRRSV (Do et al. 2015; Lager et al. 2014; Wei et al. 2013). Unfortunately, cross-protection between vaccine strains and field strains was limited (Do et al. 2015; Lager et al. 2014). In addition, the safety is of concern. It has been documented that lineage 5 and lineage 8 PRRSV vaccines are able to revert to virulence in many regions (Opriessnig et al. 2002; Shi et al. 2010). Interestingly, type 2 PRRS vaccine (PrimePac™ PRRS) belongs to lineage 7 proved to be more closely related to cluster of HP-PRRSV than type 2 PRRS vaccine derived from lineage 5 (Shi et al. 2010). It is possible that PrimePac™ PRRS vaccine could be used effectively to protect pigs against heterologous HP-PRRSV infection. Moreover, the efficacy of PrimePac™ PRRS has not been reported against HP-PRRSV previously.
Induction of specific immune responses requires approximately 4 weeks after infection (Lopez and Osorio 2004; Loving et al. 2015; Yoon et al. 1994). Infection after adequate immune responses in vaccinated pigs (4–6 weeks post-vaccination) may be useful for assessment of vaccine efficacy. The goal of this study was to determine the efficacy of a type 2 PRRS modified live vaccine (PrimePac™ PRRS) against a Thai HP-PRRSV (10PL01) based on clinical, immunological, virological, and pathological parameters under an experimental condition.
Materials and methods
Viruses and cells
The Thai type 2 HP-PRRSV (10PL01) was isolated from an outbreak farm in Phitsanulok province (Ayudhya et al. 2012) and was kindly provided by the Chulalongkorn University, Veterinary Diagnostic Laboratory (CU-VDL). The virus was cultured and titrated in MARC-145 cells (Thanawongnuwech et al. 1998), and stored at − 80 °C until needed.
Experimental design
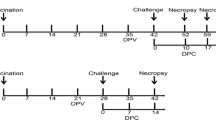
Forty-two 3-week-old castrated pigs from a PRRS-negative farm were allocated into three groups and identified by ear tag. Vaccinated challenged group (group 1, n = 16) was immunized with the assigned modified live PRRS vaccine (PrimePac™, Batch No. A600AD03) at 3 weeks old according to the manufacturer’s instructions. The unvaccinated challenged group (group 2, n = 16) was injected with a phosphate-buffer solution (PBS) at 3 weeks old, and unvaccinated unchallenged group (group 3, n = 10) was served as a negative control. Prior to vaccination, all serum samples were tested and found PRRSV seronegative using a commercial ELISA (IDEXX PRRS X3 antibody test, USA), and PRRSV genetic-free in sera using a modified qRT-PCR. At 6 weeks after vaccination, all groups except the negative control group (group 3) were intranasally challenged with 2 ml of viral suspension containing 104 TCID50/ml of the Thai HP-PRRSV lineage 8 (10PL01). Necropsy was performed on 10 days post-infection (dpi) and on 17 dpi or whenever found moribund or dead pigs. All methods and animal studies were conducted under the approval of Chulalongkorn University Animal Care and Use Committee, Chulalongkorn University (Animal Use Protocol No. 1431086).
Clinical examinations
Animals were monitored daily during the subsequent 10 dpi for physical condition, rectal temperatures, and clinical respiratory disease. The clinical respiratory scores were estimated using the previously described protocol (Sirisereewan et al. 2017). All pigs were given a score of clinical respiratory signs ranging from 0 to 3: 0 = normal; 1 = mild respiratory distress; 2 = moderate respiratory distress; 3 = severe respiratory distress with cyanosis.
Serological examinations
Blood and serum samples were collected at − 45, − 39, − 35, − 28, − 21, − 14, − 7, 0, 2, 4, 7, 10, 14, and 17 dpi. PRRSV-specific antibody response was tested using a commercial PRRSV ELISA test kit (IDEXX PRRS X3 Ab test, USA). Serum samples were considered positive for PRRSV antibody when the S/P ratio was greater than 0.4, according to manufacturer’s instruction.
Serum virus neutralization (SVN) test were performed with the challenged virus at 0 and 14 dpi, as previously described (Sirisereewan et al. 2017). Positive samples were considered to be positive for neutralizing antibodies (NAs) if the titer ≥ 1:2 (1log2). The presence of PRRSV was confirmed by IPMA assay as previously described (Thanawongnuwech et al. 1998).
Quantification of PRRSV RNA in the sera and lung tissues
RNA was extracted from serum samples and lung tissue using an RNA extraction test kit (NucleoSpin, Germany). Quantification of PRRSV RNA in sera was performed by a real-time PCR as previously described (Egli et al. 2001).
Isolation of peripheral blood mononuclear cell and flow cytometry
Peripheral blood mononuclear cells were isolated based on the previous protocols (Suradhat et al. 2015). In vitro activation was performed in a flat-bottomed 96-well plate by seeding 1 × 106 cells of peripheral blood mononuclear cells (PBMCs) into each well, and then cultured with 0.01 multiplicity of infection (m.o.i.) of the Thai prototype PRRSV of genotype 2 (01NP1) or only media or mock infected MARC-145 lysate for 48 h prior to harvesting for fluorescent staining and flow cytometric analyses. Details of the staining step and staining protocol for Treg, IL-10 and IFN-γ were described previously (Suradhat et al. 2016). All flow cytometric analyses were performed using the FC 500 MPL System (Beckman Coulter, CA, USA).
Data representing mean percentage (± SEM) of the cytokine-producing cells from pigs in the same group, were calculated (% cytokine-producing cells obtained from the PRRSV-cultured PBMC − % cytokine-producing cells obtained from the mock-cultured PBMC).
Pathologic examination
The gross lung lesions and histopathological changes were evaluated using the previously described protocol (Halbur et al. 1995). All lungs were scored to estimate the percentages of lungs affected by pneumonia, and the sections of lung tissue were scored ranging from 0 (normal) to 4 (severe) to estimate the severity of lung lesions.
Statistical analyses
Data were analyzed using analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests and Kruskal-Wallis followed by Dunn’s multiple comparison tests and pair t test. All statistical analyses were performed using GraphPad Prism for Windows (GraphPad Software Incorporated, San Diego, CA, USA).
Results
Clinical examination
Following HP-PRRSV challenge, all challenged pigs exhibited clinical signs related to HP-PRRSV infection including depression, anorexia, cough, and high fever (104–107 °F) starting from the first dpi (Fig. 1a). Vaccinated challenged pigs had lower mean rectal temperature compared to the unvaccinated challenged pigs at 9 and 10 dpi (p < 0.05). The clinical respiratory scores in vaccinated challenged pigs were significantly lower (p < 0.05) than those of the unvaccinated challenged pigs at 2, 5, 7, 8, 9, and 10 dpi (Fig. 1b). In addition, all challenged pigs exhibited transient decrease in the number of total white blood cells compared to the pre-infection levels. However, the leucocyte numbers rebounded to the pre-infection levels at 4 dpi in the vaccinated challenged pigs, but remained at lower levels in the unvaccinated challenged pigs (Fig. 1c). Pigs in the negative control group remained normal both rectal temperature and respiratory signs throughout the observation period.
Mean rectal temperature (a), mean respiratory score (b), total white blood cells (c) from the experimental pigs following HP-PRRSV challenge. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparison test. An asterisk indicates the difference (p < 0.05) between the group receiving MLV and PBS
Quantification of PRRSV RNA in sera and lung tissue
Following vaccination, viremia in pigs receiving MLV was sporadically detected in the serum at 3 days post-vaccination (dpv) to 21 dpv (Fig. 2a). In group-2 pigs receiving PBS, PRRSV were not detected in the serum until after challenged. Viremia was initially detected in all challenged pigs as early as 2 dpi and peaked at 4–7 dpi. Vaccinated challenged pigs exhibited lower levels (p < 0.05) of viremia in the serum compared to unvaccinated challenged pigs at 7 and 14 dpi and faster reduction of viremia was observed (Fig. 2a). HP-PRRSV was not detected in the negative control pigs throughout the observation period.
PRRSV genomic copies in the sera (a) and lungs (b) of experimental pigs. Pigs were immunized with MLV or PBS, and challenged with HP-PRRSV at 0 dpi. Lung samples were collected at 10 and 17 dpi. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparison test. An asterisk indicates the difference (p < 0.05) between the group receiving MLV and PBS
At first necropsy, levels of viral load in lung tissue had no significant differences in both challenged groups at 10 dpi. However, the vaccinated challenged pigs had lower levels of viral load in lung tissue (p < 0.05) at 17 dpi (Fig. 2b). HP-PRRSV was not detected in the lung tissue of negative control pigs throughout the experiment.
Serological examinations
All pigs had no seroconversion to PRRSV based on the ELISA results at the time of vaccination. Seroconversion was first shown at 14 dpv in the group receiving MLV immunization. Following the HP-PRRSV challenge, vaccinated challenged pigs had significantly higher (p < 0.05) anti-PRRSV antibody titers than those of unvaccinated challenged pigs at 4 and 7 dpi (Fig. 3a). Pigs in the negative control group remained PRRSV seronegative throughout this experiment.
Mean S/P ratios (a) and mean SN antibody titers (b) on 0 and 14 dpi of the experimental pigs. Pigs were immunized with MLV or PBS, and challenged with HP-PRRSV at 0 dpi. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparison test and Kruskal-Wallis followed by Dunn’s multiple comparison tests. An asterisk indicates the difference (p < 0.05) between the group receiving MLV and PBS
HP-PRRSV-specific serum neutralizing antibodies were not detected in all pigs at the challenged day (0 dpi). Interestingly, SN titers were observed in pigs receiving MLV immunization (6/8) at 14 dpi and significantly increased compared to 0 dpi (Fig. 3b). Pigs in the negative control group had no neutralizing antibodies throughout this study.
Frequency of PRRSV-specific interferon-γ-secreting cells, IL-10, and Treg in PBMC
Following MLV vaccination and HP-PRRSV infection, MLV-immunized pigs induce the numbers of PRRSV-specific IL-10-producing cells starting from 7 dpv and, then, gradually declined at 28 dpv. MLV-immunized pigs significantly enhanced IL-10 production at 56 dpv or 14 dpi compared to the PBS-immunized pigs (Fig. 4a). The numbers of PRRSV-specific Treg in the MLV-receiving group were higher than those of the PBS-receiving group during the pre-challenged and post-challenged periods (Fig. 4b). However, the numbers of PRRSV-specific CD3+IFN-γ+ cells had no difference in MLV-immunized pigs and PBS-immunized pigs throughout the observation period (Fig. 4c).
% PRRSV-specific CD3+IL-10+ subpopulation (a), % PRRSV-specific CD3+IFN-γ+ subpopulation (b), and % PRRSV-specific CD4+ CD25+ FoxP3+ subpopulation (c) of the experimental pigs. Pigs were immunized with MLV or PBS, and challenged with HP-PRRSV at 0 dpi. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparison test. An asterisk indicates the difference (p < 0.05) between the group receiving MLV and PBS
Macroscopic lung lesions
The severity of PRRSV-induced lung lesions was characterized by multifocal, tan-mottled areas, with irregular and indistinct borders. The gross lung lesion scores had no significant differences among groups at 10 dpi. However, gross lung lesion scores were significantly lower in vaccinated challenged pigs compared to unvaccinated challenged pigs at 17 dpi (Fig. 5a). No macroscopic lung lesions were observed in the negative control pigs throughout the experiment.
Mean macroscopic lung lesion score (a) and microscopic lung lesion score (b) of the experimental pigs. Statistical analyses were performed using one-way ANOVA followed by Tukey’s multiple comparison test and Kruskal-Wallis followed by Dunn’s multiple comparison tests. An asterisk indicates the difference (p < 0.05) between the group receiving MLV and PBS
Microscopic lung lesions
Microscopic lung lesions were characterized by thickening of alveolar septa with infiltration of mononuclear cells, type II pneumocyte hyperplasia and hypertrophy, and accumulation of macrophages in alveolar spaces. At 10 dpi, all challenged pigs exhibited PRRSV-specific lung lesions by developing interstitial pneumonia in all challenged groups. However, there were no significant differences between the two challenged groups at 10 dpi. Interestingly, vaccinated challenged pigs exhibited lower microscopic lung lesions at 17 dpi compared to 10 dpi. In contrast, the microscopic lung lesions had no significant difference in unvaccinated challenged pigs between 10 and 17 dpi (Fig. 5b).
Discussion
In this study, we hypothesized that the studied commercial type 2 vaccine was able to provide protection against the Thai HP-PRRSV challenge. Our results demonstrated that the newly introduced commercial type 2 PRRS MLV was partly efficacious in protecting growing pigs against the Thai HP-PRRSV challenge. This finding is consistent with the previous studies that current commercial PRRS MLV vaccines had variations on their efficacy against the HP-PRRSV challenges depending on various factors (Do et al. 2015; Lager et al. 2014; Wei et al. 2013). It indicates that a PRRSV controlled strategy still requires the better efficacious vaccine and vaccination strategies against the HP-PRRSV infection.
PRRSV-induced immunosuppression causes porcine respiratory disease complex (PRDC) recognized as a major complication of PRRSV infection. Unfortunately, current commercial MLV vaccines could not provide complete protection against field strains of PRRSV. However, there are many advantages of PRRSV vaccination in PRRSV endemic areas. Evidently, PRRSV vaccination was able to reduce disease severity, virus shedding, and some clinical outcomes (Charoenchanikran et al. 2016; Do et al. 2015; Lager et al. 2014; Martelli et al. 2009; Park et al. 2014; Savard et al. 2016; Wei et al. 2013; Zuckermann et al. 2007). Thus, preventive vaccination programs seem meaningful and easy to use in PRRSV-positive herds in order to stabilize the herd immunity. They may also minimize the chances of having PRDC induced by PRRSV. Moreover, receiving PRRSV vaccine did improve growth performance in fattening pigs under the field condition (Lyo et al. 2016). On the contrary, strict farm managements are effective methods involving biosecurity, herd health monitoring, or depopulation and repopulation, etc. However, those are time-consuming and may have a negative economic impact. Nevertheless, effective PRRS control strategies should combine both PRRS vaccination programs and farm management in order to reduce the virus load and transmission within and among herds, particularly in PRRSV endemic areas.
According to the previous study, ORF5 sequence similarity between the PRRSV field strain and PRRSV vaccine strain was suggested for a better protective efficacy of the selected MLV vaccine (Labarque et al. 2004). However, vaccination with two genetically distant PRRS MLV vaccines, lineage 5 and 8, did not provide significant differences for protective efficacy against the HP-PRRSV belonging to lineage 8 (Do et al. 2015). It indicated that having the same genetic lineage was not a reliable parameter to guarantee the vaccine protection. In this study, we examined a newly introduced type 2 PRRSV vaccine of lineage 7 demonstrating that the studied type 2 PRRSV vaccine of lineage 7 was able to provide partial protection against the Thai HP-PRRSV. This studied vaccine might become a vaccine of choices for controlling HP-PRRSV in endemic areas.
In this study, immunization with the MLV vaccine could not elicit PRRSV-specific cellular immunity and neutralizing antibodies prior to the HP-PRRSV challenge. No upregulation of PRRSV- specific IFN-γ-secreting cells possibly caused by the induction of IL-10 and Treg following the MLV immunization. Evidently, a reduction of IL-10 and Treg induced by the heterologous prime-boost immunization strategy could elicit PRRSV-specific IFN-γ-secreting cells prior to HP-PRRSV exposure (Sirisereewan et al. 2017). In this study, neutralizing antibodies were detected in the vaccinated pigs at 14 dpi, probably associated with cross-neutralizing antibody. Induction of cross-neutralizing antibody achieved by the MLV immunization might associate with cross-neutralizing epitopes located in the first 60 amino acids of the GP5 gene (Kim et al. 2013). Moreover, neutralizing antibody did play a major role on viremic reduction (Lopez and Osorio 2004; Yoon et al. 1996). In this study, reduction of HP-PRRSV viremia might be influenced by inducing PRRSV-specific neutralizing antibodies. According to the previous study, the levels of viremia correlated with the severity of interstitial pneumonia (Han et al. 2013; Johnson et al. 2004), thus reducing the levels of viremia resulting in reduction of lung lesions and lung viral load in the late phase of infection.
It should be noted that this is the first experimental challenge study evaluating the commercial type 2 PRRSV vaccine (PrimePac™ PRRS), of lineage 7, against the Thai HP-PRRSV challenge (lineage 8). The vaccine efficacy was assessed based on clinical, virological, pathological, and immunological parameters and the vaccine, therefore, could be used in the field to control HP-PRRSV infection. Unfortunately, current commercial PRRSV vaccines still have the limitation against the HP-PRRSV as mentioned previously. Effective prevention and control of PRRSV must employ both vaccine and herd management implementation.
Conclusion
The modified live PRRSV vaccine (PrimePac™ PRRS) definitely could provide partial protection against the Thai HP-PRRSV challenge. Vaccinated challenged pigs showed significantly lower levels of rectal temperature, clinical respiratory scores, lung lesion scores, levels of HP-PRRSV viremia, and levels of HP-PRRSV load in the lung tissue compared to the unvaccinated challenged pigs. In addition, vaccinated challenged pigs exhibited PRRSV-specific serum neutralizing antibodies at the end of the experiment consistent with the presence of viremic reduction.
References
Ayudhya, S.N., Assavacheep, P., Thanawongnuwech, R., 2012. One world—one health: the threat of emerging swine diseases. An Asian perspective. Transboundary and Emerging Diseases, 59 Suppl 1, 9–17.
Cavanagh, D., 1997. Nidovirales: a new order comprising coronaviridae and arteriviridae. Archives of Virology, 142, 629–633.
Charoenchanikran, P., Kedkovid, R., Sirisereewan, C., Woonwong, Y., Arunorat, J., Sitthichareonchai, P., Sopipan, N., Jittimanee, S., Kesdangsakonwut, S., Thanawongnuwech, R., 2016. Efficacy of Fostera® PRRS modified live virus (MLV) vaccination strategy against a Thai highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection. Tropical Animal Health and Production, 48, 1351–1359.
Do, D.T., Park, C., Choi, K., Jeong, J., Nguyen, T.T., Nguyen, K.D., Vo, D.T., Chae, C., 2015. Comparison of two genetically distant type 2 porcine reproductive and respiratory syndrome virus (PRRSV) modified live vaccines against Vietnamese highly pathogenic PRRSV. Veterinary Microbiology, 179, 233–241.
Egli, C., Thur, B., Liu, L., Hofmann, M.A., 2001. Quantitative TaqMan® RT-PCR for the detection and differentiation of European and North American strains of porcine reproductive and respiratory syndrome virus. Journal of Virological Methods, 98, 63–75.
Halbur, P.G., Paul, P.S., Frey, M.L., Landgraf, J., Eernisse, K., Meng, X.J., Lum, M.A., Andrews, J.J., Rathje, J.A., 1995. Comparison of the pathogenicity of Two US Porcine Reproductive and Respiratory Syndrome Virus Isolates with that of the Lelystad Virus. Veterinary Pathology, 32, 648–660.
Han, K., Seo, H.W., Oh, Y., Kang, I., Park, C., Chae, C., 2013. Comparison of the virulence of European and North American genotypes of porcine reproductive and respiratory syndrome virus in experimentally infected pigs. The Veterinary Journal, 195, 313–318.
Holtkamp, D., Kliebenstein, J., Neumann, E., Zimmerman, J.J., Rotto, H., Yoder, T., Wang, C., Yeske, P., Mowrer, C., Haley, C., 2013. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on U.S. pork producer. Journal of Swine Health and Production, 21, 72–84.
Jantafong, T., Sangtong, P., Saenglub, W., Mungkundar, C., Romlamduan, N., Lekchareonsuk, C., Lekcharoensuk, P., 2015. Genetic diversity of porcine reproductive and respiratory syndrome virus in Thailand and Southeast Asia from 2008 to 2013. Veterinary Microbiology, 176, 229–238.
Johnson, W., Roof, M., Vaughn, E., Christopher-Hennings, J., Johnson, C.R., Murtaugh, M.P., 2004. Pathogenic and humoral immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) are related to viral load in acute infection. Veterinary Immunology and Immunopathology. 102, 233–247.
Kim, W.I., Kim, J.J., Cha, S.H., Wu, W.H., Cooper, V., Evans, R., Choi, E.J., Yoon, K.J., 2013. Significance of genetic variation of PRRSV ORF5 in virus neutralization and molecular determinants corresponding to cross neutralization among PRRS viruses. Veterinary Microbiology, 162, 10–22.
Labarque, G., Reeth, K.V., Nauwynck, H., Drexler, C., Van Gucht, S., Pensaert, M., 2004. Impact of genetic diversity of European-type porcine reproductive and respiratory syndrome virus strains on vaccine efficacy. Vaccine, 22, 4183–4190.
Lager, K.M., Schlink, S.N., Brockmeier, S.L., Miller, L.C., Henningson, J.N., Kappes, M.A., Kehrli, M.E., Loving, C.L., Guo, B., Swenson, S.L., Yang, H.C., Faaberg, K.S., 2014. Efficacy of Type 2 PRRSV vaccine against Chinese and Vietnamese HP-PRRSV challenge in pigs. Vaccine, 32, 6457–6462.
Leng, X., Li, Z., Xia, M., He, Y., Wu, H., 2012. Evaluation of the efficacy of an attenuated live vaccine against highly pathogenic porcine reproductive and respiratory syndrome virus in young pigs. Clinical and Vaccine Immunology, 19, 1199–1206.
Lopez, O.J., Osorio, F.A., 2004. Role of neutralizing antibodies in PRRSV protective immunity. Veterinary Immunology and Immunopathology. 102, 155–163.
Loving, C.L., Osorio, F.A., Murtaugh, M.P., Zuckermann, F.A., 2015. Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus. Veterinary Immunology and Immunopathology. 167, 1–14.
Lyo, K.S., Choi, J.Y., Hahn, T.W., Park, K.T., Kim, H.K., 2016. Effect of vaccination with a modified live porcine reproductive and respiratory syndrome virus vaccine on growth performance in fattening pigs under field conditions. Journal of Veterinary Medical Science, 78, 1533–1536.
Martelli, P., Gozio, S., Ferrari, L., Rosina, S., De Angelis, E., Quintavalla, C., Bottarelli, E., Borghetti, P., 2009. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine, 27, 3788–3799.
Meulenberg, J.J.M., Den Bestern, A.P., De Kluyver, E., Van Nieuwstadt, A., Wensvoort, G., Moormann, R.J.M., 1997. Molecular characterization of Lelystad virus. Veterinary Microbiology, 55, 197–202
Opriessnig, T., Halbur, P.G., Yoon, K.J., Pogranichniy, R.M., Harmon, K.M., Evans, R., Key, K.F., Pallares, F.J., Thomas, P., Meng, X.J., 2002. Comparison of Molecular and Biological Characteristics of a Modified Live Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Vaccine (Ingelvac PRRS MLV), the Parent Strain of the Vaccine (ATCC VR2332), ATCC VR2385, and Two Recent Field Isolates of PRRSV. Journal of Virology, 76, 11837–11844.
Park, C., Seo, H.W., Han, K., Kang, I., Chae, C., 2014. Evaluation of the efficacy of a new modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Fostera PRRS) against heterologous PRRSV challenge. Veterinary Microbiology, 172, 432–442.
Savard, C., Alvarez, F., Provost, C., Chorfi, Y., D’Allaire, S., Benoit-Biancamano, M.O., Gagnon, C.A., 2016. Efficacy of Fostera PRRS modified live virus vaccine against a Canadian heterologous virulent field strain of porcine reproductive and respiratory syndrome virus. Canadian Journal of Veterinary Research, 80, 1–11.
Shi, M., Lam, T.T., Hon, C.C., Murtaugh, M.P., Davies, P.R., Hui, R.K., Li, J., Wong, L.T., Yip, C.W., Jiang, J.W., Leung, F.C., 2010. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. Journal of Virology, 84, 8700–8711.
Sirisereewan, C., Nedumpun, T., Kesdangsakonwut, S., Woonwong, Y., Kedkovid, R., Arunorat, J., Thanawongnuwech, R., Suradhat, S., 2017. Positive immunomodulatory effects of heterologous DNA vaccine- modified live vaccine, prime-boost immunization, against the highly-pathogenic PRRSV infection. Veterinary Immunology and Immunopathology, 183, 7–15.
Suradhat, S., Wongyanin, P., Kesdangsakonwut, S., Teankum, K., Lumyai, M., Triyarach, S., Thanawongnuwech, R., 2015. A novel DNA vaccine for reduction of PRRSV-induced negative immunomodulatory effects: A proof of concept. Vaccine, 33, 3997–4003.
Suradhat, S., Wongyanin, P., Sirisereewan, C., Nedumpun, T., Lumyai, M., Triyarach, S., Chaturavittawong, D., Paphavasit, T., Panyatong, R., Thanawongnuwech, R., 2016. Transdermal delivery of plasmid encoding truncated nucleocapsid protein enhanced PRRSV-specific immune responses. Vaccine, 34, 609–615.
Thanawongnuwech, R., Halbur, P.G., Ackermann, M.R., Thacker, E.L., Royer, R.L., 1998. Effects of low (modified-live virus vaccine) and high (VR-2385)-virulence strains of porcine reproductive and respiratory syndrome virus on pulmonary clearance of copper particles in pigs. Veterinary Pathology, 35, 398–406.
Tian, K., Yu, X., Zhao, T., Feng, Y., Cao, Z., Wang, C., Hu, Y., Chen, X., Hu, D., Tian, X., Liu, D., Zhang, S., Deng, X., Ding, Y., Yang, L., Zhang, Y., Xiao, H., Qiao, M., Wang, B., Hou, L., Wang, X., Yang, X., Kang, L., Sun, M., Jin, P., Wang, S., Kitamura, Y., Yan, J., Gao, G.F., 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One, 2, e526.
Wei, Z., Zhang, J., Zhuang, J., Sun, Z., Gao, F., Yuan, S., 2013. Immunization of pigs with a type 2 modified live PRRSV vaccine prevents the development of a deadly long lasting hyperpyrexia in a challenge study with highly pathogenic PRRSV JX143. Vaccine, 31, 2062–2066.
Yoon, I.J., Joo, H.S., Goyal, S.M., Molitor, T.W., 1994. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest, 6, 289–292.
Yoon, S.L., Wu, L.L., Zimmerman, J.J., Hill, H.T., Platt, K.B., 1996. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral immunology, 9, 51–63.
Yu, X., Zhou, Z., Cao, Z., Wu, J., Zhang, Z., Xu, B., Wang, C., Hu, D., Deng, X., Han, W., Gu, X., Zhang, S., Li, X., Wang, B., Zhai, X., Tian, K., 2015. Assessment of the safety and efficacy of an attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus. Clinical and Vaccine Immunology, 22, 493–502.
Zuckermann, F.A., Garcia, E.A., Luque, I.D., Christopher-Hennings, J., Doster, A., Brito, M., Osorio, F., 2007. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Veterinary Microbiology, 123, 69–85.
Acknowledgements
We thank the staff of Veterinary Diagnostic Laboratories and the graduate students in Veterinary Pathobiology Program, Faculty of Veterinary Science, Chulalongkorn University, for their kindly supported.
Funding
The study was financially supported by The 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), the Chulalongkorn University Graduate Scholarship to commemorate the 72nd Anniversary of the His Majesty King Bhumibol Adulyadej (C.S.), and the MSD animal health Ltd. (Thailand) study number RES_58_056_31_003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sirisereewan, C., Woonwong, Y., Arunorat, J. et al. Efficacy of a type 2 PRRSV modified live vaccine (PrimePac™ PRRS) against a Thai HP-PRRSV challenge. Trop Anim Health Prod 50, 1509–1518 (2018). https://doi.org/10.1007/s11250-018-1589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1589-4