Abstract
The objective of this study was to determine the prevalence of gastrointestinal nematode (GIN) infection in goat flocks on semi-arid rangelands of northeastern Mexico (25° N, 350–400 mm annual precipitation). The study included 668 pluriparous goats from 18 herds in five municipalities of Coahuila and Nuevo Leon, Mexico. Five genetic groups were considered (predominance of Boer, Nubian, Alpine, Saanen, and Toggenburg). Fecal samples were taken from the rectum of each animal to determine the number of eggs per gram (EPG) of GIN. The prevalence of flocks with GIN infections was 88.9%. Similar results were observed for the number of goats infected in the flocks. The Alpine breed presented the highest prevalence and highest EPG loads of GIN, whereas Boer and Nubian were the genetic groups with the lowest (P < 0.05) EPG. There was a negative effect of GIN infection on the live weight of goats (P < 0.05). The GIN genera found were Trichostrongylus spp. and Haemonchus spp. It was concluded that in the goat flocks of the semi-arid zones of Mexico was found a high prevalence of infections with gastrointestinal nematodes. The municipality and the breed of the animals were factors that showed influence on this prevalence and the level of infection of the goats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The arid and semi-arid zones of the world concentrate 64% of goats and are particularly important in areas of marginal agriculture (Lebbie 2004). In Mexico, these areas concentrate more than 33% of the national goat stock and produce 67% of milk and 39% of goat meat (SIAP 2016). The states of Coahuila, Nuevo León, and San Luis Potosí (located in the arid zones of Mexico) contribute almost to 20% of the national caprine population, being Coahuila the state with the largest population and production of goat meat and milk (SIAP 2016). The characteristics of the goat production systems in semi-arid zones of Mexico were recently described (Escareño et al. 2012; Salinas-González et al. 2016). Alpine, Saanen, Toggenburg, and Nubian were the dairy goat breeds exploited. The feeding system was based on browsing/grazing native vegetation and the main health problems in the flocks according to the farmer were brucellosis, pneumonia, and digestive problem. The gastrointestinal nematodes (GIN) affect the health and productivity of small ruminants on pasture worldwide (Torres-Acosta et al. 2012a; Yimer and Birhan 2016). In Mexico, the negative effects of gastrointestinal nematodes on goats have been documented in tropical and subtropical areas (Torres-Acosta et al. 2006, 2012a); however, in the arid and semi-arid zones of Mexico, there is limited information on internal parasites affecting small ruminants, and their effects of on goat production and productivity (Escareño et al. 2012; Salinas-González et al. 2016). The prevalence, genera, species, and severity of infections by gastrointestinal nematodes vary according to humidity, ambient temperature, rainfall, type of vegetation, and management practices carried out in each region (Dilgasa et al. 2015; Besier et al. 2016). Among the environmental factors associated with GIN infections are agro-ecological conditions, livestock practices, housing systems, deworming schedule, and grassland management (Ratanapob et al. 2012). In animals, the sex, age, physiological stage, body condition, and genotype are also risk factors associated with GIN infections (Adeyemi et al. 2017). Indirect measurements, such as body condition, show that in goats there is an inverse and significant relationship between body condition, egg per gram of feces (EPG), and adult GIN burden (Idika et al. 2012). Similar results have been reported through FAMACHA measurements, which is a consistent method evaluating the ocular conjunctiva color of the goats according to a color chart. The color is related to the level of anemia and measures indirectly the pathogenic effect of GIN presence in the goats (Torres-Acosta et al. 2012a). The GIN control is costly and not always effective because helminths have become resistant to most anthelmintic drugs (Tibbo et al. 2008; Torres-Acosta et al. 2012b). Torres-Acosta et al. (2014) and Medina-Pérez et al. (2015) have proposed selective deworming, which consists in deworming only goats and sheep that require it, considering the body condition score, FAMACHA© and the EPG of gastrointestinal nematodes. However, in order to implement selective deworming, the first requirement is to know the prevalence of parasitic diseases, the gastrointestinal nematode genus present and their dynamics throughout the year; it is also required to know the resistance/susceptibility against anthelmintic drugs from existing strains of GIN. The objective of the present study was to determine the prevalence of gastrointestinal nematode infection in goats on rangeland in semi-arid areas of northeastern Mexico.
Materials and methods
Study area
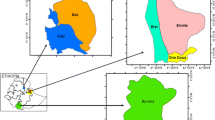
The study was carried out in 18 goat flocks on semi-arid rangelands of northeastern Mexico from June to November 2016. The herds are located in the municipalities of Arteaga, General Cepeda, Ramos Arizpe, and Saltillo, belonging to the state of Coahuila, and in the municipality of Galeana, in the state of Nuevo Leon (Fig. 1). Altitude ranged between 1390 and 1680 m above sea level, with an extremely dry climate, with an average annual temperature of 14–19 °C, irregular rains of 350–450 mm annually, with higher precipitation in June–September. The vegetation is forest, spineless parvifolio scrub and thorny scrub, characteristic of the Chihuahua desert (INEGI 2003).
Animals and their management
Of 2699 adult female goats with different levels of Alpine, Boer, Nubian, Saanen, and Toggenburg breeds, 668 animals were sampled. The inclusion criteria considered that the herds were greater than 100 goats and had not received anthelmintic treatment in the last 6 months prior to the visit. The goats were grazed from 10:00 to 17:00 h daily and penned in rustic material installations near the houses where the herdsmen and family live. The natural vegetation community where the goats grazed was composed of grasslands with browse, Opuntia, trees like Prosopis and Acacia and some shrubs like Fluorencia and Larrea. In times of extreme drought, in addition to pastures, goats are fed with agricultural wastes, such as corn stubble, oat straw or bean straw. In some farms, it is common to manage goats along with sheep and sometimes also with cattle.
Measurements
Weight of animals
The individual goats were weighed the day of the visit on a digital electronic weighing platform with a capacity of 250 kg and a sensitivity of at least 25 g.
Collection and examination of fecal samples
Fecal samples were taken from each goat directly from the rectum, using polyethylene bag. The samples were identified and refrigerated until their processing in the Animal Production Laboratory of the Universidad Autonoma Agraria Antonio Narro in Saltillo, Coahuila, Mexico. Fecal samples were processed using the modified McMaster technique and the EPG of GIN was determined according to Rodríguez et al. (1994). The McMaster technique is used to determine the number of eggs per gram of feces. Briefly, 2 g of feces macerated is dissolved in 28 ml of a sugar solution (density of 1.27). The McMaster chamber has two compartiments. In each compartment is deposited 0.15 ml of the mixture. After 7 min, the eggs of the GIN float and are counted using a microscope (×10). Finally, the number of EPG results from multiplying the total number of eggs counted in both compartments by 50.
Fecal cultures and larvae identification
In order to identify the genus of GIN in the flocks, a fecal pool coproculture from each flock was carried out using the Corticelli Laí technique (Corticelli and Lai 1963). Coproculture consists in placing a pool of goat feces in a petri dish sprayed every day with water and maintained in incubation at 27 °C for 5 days in order to allow eggs to hatch and to develop the larval stage. On day 6, the L3 larvae were harvested. The identification of the larvae was performed using the keys of identification described by Bowman and Lynn (1999). The keys consider mainly the larvae head shape and length of the tail.
Statistical analysis
Prevalence and 95% confidence intervals were calculated according to Thrusfield (2005). The effect of the municipality and breed of goats on the prevalence of GIN infection was compared by contingency tables. The live weight and EPG count were determined using a linear model using the PROC GLM of SAS (SAS 2004). The data of EPG were log transformed (ln (n + 1)) prior to the analysis.
Results
The results of the present study showed that 88.9% of the goat flocks of semi-arid zones of northeastern Mexico presented infections with GIN. The prevalence of GIN infection for municipalities is shown in Table 1. The two goat flocks from Ramos Arizpe were not infected with GIN. GIN infections were observed in more than 80% of the sampled animals. According to the infection level, the EPG count of gastrointestinal nematodes showed values from 0 to 11,600 EPG. In relation to the genus of GIN, in Arteaga and Galeana municipalities, only larvae of Trichostrongylus were found, whereas, in General Cepeda and Saltillo municipalities, larvae of Trichostrongylus (96.0 and 96.4%) and Haemonchus (4.0 and 3.6%) were found.
In relation to the goat breeds, Table 2 shows that goats with a predominance of the Nubian breed showed the least GIN infection prevalence. Alpine breed had the highest worm EPG counts compared to Boer and Nubian breeds (P < 0.05). However, the Alpine breed was similar to Saanen and Toggenburg (P > 0.05). Nubian and Boer breeds showed similar counts of EPG (P > 0.05).
The frequency distribution of individual mean fecal egg counts of Alpine, Toggenburg, Saanen, Boer, and Nubian goats is shown in Fig. 2. In the study, most Nubian and Boer goats (70.3 and 70.0% respectively) had < 501 EPG and lesser had > 1000 EPG (16.5 and 14.1% respectively). Large proportions of Alpine, Toggenburg, and Saanen goats had EPG counts > 1000 (32.2, 32.0, and 19.9% respectively).
There was similar live weight between goats according to municipalities and breeds. However, there was difference between the live weight of goats with positive GIN infection compared to goats with negative infection (P < 0.05) (Table 3).
Discussion
The information about GIN in grazing goats in the semi-arid and arid zones of Mexico is limited (Aguilar-Caballero et al. 2009). This study clearly showed a high prevalence of GIN infections in the goat flocks of the present study. The prevalence of infected goats was very similar among flocks except in two herds. The prevalence of herd infected with gastrointestinal nematodes in the tropical, subtropical, and semi-arid conditions of the world is close to 90% (Torres-Acosta et al. 2012a). In this study, it was observed that goats in the municipality of Ramos Arizpe were free of GIN. These results could be explained by the fact that it is the municipality with the lowest rainfall (350 mm) and scarce and low vegetation. Under those conditions, the larvae emerging from the feces have no possibilities to survive because their exposition to the sun radiation and any chance to migrate to the plants because the soil is dry, so, the goat infection rate is low or null (Yimer and Birhan 2016).
In tropical conditions, the GIN genera involved are Haemonchus, Trichostrongylus, and Oesophagpstomum (Torres-Acosta et al. 2012a; Besier et al. 2016). In the present study, Trichostrongylus was the main genus followed by Haemonchus. It corresponds to the temperature prevalent during the study (Besier et al. 2016). The biological cycle of gastrointestinal nematodes such as Haemonchus contortus and Trichostrongylus colubriformis is influenced by the climatic conditions and vegetation of the region, and the host immunity to GIN (Molento et al. 2016). The development of larvae in both nematodes requires high levels of relative humidity, which is common during the rainy season (O’Connor et al. 2006). The present study was performed during the months of high rainfall of the region (June–November; 70% of the annual rainfall). These low rainfall values in sub-arid and arid areas are enough for the development of GIN (Khadijah et al. 2013a); the fecal and soil moisture are risk factors associated with the larval development. In those conditions, gastrointestinal nematodes have developed strategies that allow them to take full advantage of low rainfall rates to survive during the recurrent droughts every year (Khadijah et al. 2013b, c; Wang et al. 2014). Due to the anatomical and structural characteristics of their sheath that protects them from the environment, the L3 larvae are able to survive during the dry period withstanding solar radiation and the lack of environmental humidity (Lee 2012; Besier et al. 2016; Molento et al. 2016). For this reason, the eggs that are released to the external environment develop and undergo hypobiosis until the environmental conditions are favorable for their final development and these can be ingested later by the hosts (Gibbs 1986).
In relation to goat genetic group, all of them are exploited for milk production; the Alpine, Saanen, and Toggenburg goats are high milk producers and exploited in indoor conditions and consequently are more sensitive to GIN infections. In dairy goats, the volume of milk produced, management and the physiological stage of the animals have been associated with the severity of infections with GIN, with negative effects on milk production and animal health (Chartier et al. 2000). Unlike the Boer and Nubian goats, they are tropical-like breeds which are exploited under browsing/grazing vegetation. In that condition, the goats have developed an immunological memory to control GIN infections. This could explain the difference in parasite burden between the Alpine breed in comparison to Nubian and Boer goats when exposed to GIN under extensive grazing. There were differences in susceptibility to GIN infections between breeds. Alpine goats are recognized as highly sensitive to GIN infections when compared to Italian native (Alberti et al. 2012) or Saanen goats (Alberti et al. 2014). In the present study, numerical differences for EPG of GIN were found between Alpine goats compared to Saanen and Toggenburg, although this was not significant. Information on the Toggenburg breed and gastrointestinal parasitism were not found in the literature, probably because this breed is normally exploited under confinement systems. For Nubia and Boer goat breeds, information about parasitism has been obtained from the humid and sub-humid tropics, where these animals present parasite burden close to those observed in the present study (Torres-Acosta et al. 2014). According to the infection level, the variability in EPG of GIN in each breed shows the chance to control the GIN infection using Target Selective Treatment Scheme (Medina-Pérez et al. 2015) or segregate the goats resistant to GIN according to the EPG count looking for to avoiding negative effects on the health and production of the flocks (Torres-Acosta et al. 2014). The infection with GIN showed a negative effect on the live weight of the goats. Artificial and natural infection with GIN decreases the liveweight gain and the milk production in goats (Torres-Acosta et al. 2014; Besier et al. 2016). This condition could affect negatively the incomes of the farmers and the sustainability of the flock. So, it is important to know the health and production status of the herds to establish a GIN sustainable-control program because the semi-arid zone of México concentrate more than 33% of the national goat stock and produce 67% of milk and 39% of goat meat of the country.
Conclusions
The present study showed a high prevalence of goat flocks infected with gastrointestinal nematodes in semi-arid zones of northeastern Mexico. There was difference in the prevalence and level of GIN infection of the goats according to the municipality and the breed of the goats. The knowledge about the gastrointestinal nematode genus prevalence and parasite dynamic during the year is important to develop strategies that allow appropriate control of these parasites in the semi-arid zones of northeastern Mexico. This control would reduce the productivity losses of goats and consequently could improve the living conditions of peasants in these rural communities.
References
Adeyemi, M.T., Morenikeji, O.A, Emikpe, B.O. and Jarikre, T.A., 2017 Interactions between gastrointestinal parasitism and pneumonia in Nigerian goats. Journal of Parasitic Diseases, 1–8. https://doi.org/10.1007/s12639-017-0878-6
Aguilar-Caballero, A.J, Torres-Acosta, J.F.J. y Cámara-Sarmiento, R., 2009 Importancia del parasitismo gastrointestinal en ovinos y situación actual de la resistencia antihelmíntica en México. Avances en el control de la parasitosis gastrointestinal de ovinos en el trópico, Universidad Autónoma Chapingo, México 1–11
Alberti E,G, Zanzani, S.A., Ferrari, N., Bruni, G. and Manfredi, M.T., 2012. Effects of gastrointestinal nematodes on milk productivity in three dairy goat breeds, Small Ruminant Research, 106:S12-S17
Alberti, E.G., Zanzani, S.A., Gazzonis, A.L., Zanatta, G., Bruni, G., Villa, M. and Manfredi, M.T., 2014. Effects of gastrointestinal infections caused by nematodes on milk production in goats in a mountain ecosystem: Comparison between a cosmopolite and a local breed, Small Ruminant Research, 120:155–163
Besier, R.B., Kahn, L.P., Sargison, N.D. and Van Wyk, J.A., 2016. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. In: Haemonchus contortus and haemonchosis-Past, present and future trends. Gasser, R.B. and Von Samson-Himmelstjerna G. (Eds), Advances in parasitology, 93:95–143
Bowman, D.D. and Lynn, R.C., 1999. Diagnosis parasitology. In: Bowman, D.D, Lynn, R.C. (eds). Georgis’ parasitology for veterinarians, 7th edn. WB Saunder Company, Philadelphia, pp 361–367
Chartier, C., Etter, E., Hoste, H., Pors, I., Mallereau, M.P., Broqua, C. and Masse, A., 2000. Effects of the initial level of milk production and of the dietary protein intake on the course of natural nematode infection in dairy goats, Veterinary Parasitology, 92:1–13
Corticelli, B. y Lai, M., 1963. Ricerche sulla tecnica di coltura delle larve infestive degli strongili gastrointestinali del bovino, Acta Medica Veterinaria, año 9, Fasc V/VI
Dilgasa, L., Asrade, B. and Kasaye, S., 2015. Prevalence of gastrointestinal nematodes of small ruminants in and around Arsi Negele town, Ethiopia, American-Eurasian Journal of Scientific Research, 10:121–125
Gibbs, H.C., 1986. Hypobiosis in parasitic nematodes-an update, Advances in Parasitology, 25:129–174.
Escareño, L., Salinas-Gonzalez, H., Wurzinger, M., Iñiguez L., Sölkner, J. and Meza-Herrera, C., 2012. Dairy goat production systems Status quo: Perspectives and challenges, Tropical Animal Health & Production, 45: 17–34
Idika, I.K., Iheagwam, C.N., Ezemonye, C.N. and Nwosu, C.O., 2012. Gastrointestinal nematodes and body condition scores of goats slaughtered in Nsukka, Nigeria, Nigerian Veterinary Journal, 33:440–447
INEGI., 2003. Instituto Nacional de Estadística Geografía e Informática. Registro Nacional de Información Geográfica (RNIG). Aguascalientes, Agsc. México
Khadijah, S., Kahn, L.P., Walkden-Brown, S.W., Bailey, J.N. and Bowers, S.F., 2013a. Soil moisture modulates the effects of the timing and amount of rainfall on faecal moisture and development of Haemonchus contortus and Trichostrongylus colubriformis to infective third stage larvae, Veterinary Parasitology, 196: 347–357
Khadijah, S., Kahn, L.P., Walkden-Brown, S.W., Bailey, J.N. and Bowers, S.F., 2013b. Effect of simulated rainfall timing on faecal moisture and development of Haemonchus contortus and Trichostrongylus colubriformis eggs to infective larvae, Veterinary Parasitology, 192:199–210
Khadijah, S., Kahn, L.P., Walkden-Brown, S.W., Bailey, J.N. and Bowers, S.F., 2013c. Soil moisture influences the development of Haemonchus contortus and Trichostrongylus colubriformis to third stage larvae. Veterinary Parasitology 196:161–171
Lebbie, S.H.B., 2004. Goats under household conditions, Small Ruminant Research, 51:131–136
Lee, D.L., 2012. Life cycle. In: Lee DL Editor. The biology of nematodes. Taylor and Francis, New York, 141–161
Medina-Pérez, P., Ojeda-Robertos, N.F., Reyes-García, M.E., Cámara-Sarmiento, R. and Torres-Acosta, J.F.J., 2015. Evaluation of a targeted selective treatment scheme to control gastrointestinal nematodes of hair sheep under hot humid tropical conditions, Small Ruminant Research, 127:86–91
Molento, M.B., Buzatti, A. and Sprenger, L.K., 2016. Pasture larval count as a supporting method for parasite epidemiology, population dynamic and control in ruminants, Livestock Science, 192:48–54
O’Connor, L.J., Walkden-Brown, S.W. and Kahn, L.P., 2006. Ecology of the free-living stages of major trichostrongylid parasites of sheep, Veterinary parasitology, 142: 1–15
Ratanapob, N., Arunvipas, P., Kasemsuwan, S., Phimpraphai, W. and Panneum, S., 2012. Prevalence and risk factors for intestinal parasite infection in goats raised in Nakhon Pathom Province, Thailand, Tropical Animal Health Production, 44:741–745
Rodríguez, V.R., Domínguez, A.J. y Cob, G.L., 1994. Técnicas Diagnósticas de Parasitología Veterinaria. Universidad Autónoma de Yucatán. Mérida, ISBN:968-6843-60-4.
Salinas-González, H., Valle-Moysen, E.D., De Santiago-Miramontes, M.A., Veliz-Deras, F.D., Maldonado-Jáquez, J.A., Vélez-Monroy, L.I., Torres-Hernández, D., Isidro-Requejo, L.M. and Figueroa-Viramontes, U., 2016. Descriptive analysis of goat production units in the southwest of the Laguna region, Coahuila, Mexico, Interciencia, 41:763–768
SAS (Statistical Analysis System) SAS Institute Inc., (2004) SAS/ACCESS® 9.1 for windows. Cary, N. C. SAS Institute, Inc
SIAP., 2016. Servicio de Información Agroalimentaria y Pesquera. SAGARPA. Estados Unidos Mexicanos https://www.gob.mx/cms/uploads/attachment/file/165999/caprino.pdf. (Accessed 03 May 2017)
Thrusfield, M., 2005. Veterinary epidemiology. 2nd ed. Blackwell Science Ltd, United Kingdom.
Tibbo, M., Aragaw, K., Philipsson, J., Malmfors, B., Näsholm, A., Ayalew, W. and Rege, J.E.O., 2008. A field trial of production and financial consequences of helminthosis control in sheep production in Ethiopia, Preventive Veterinary Medicine, 84:152–160
Torres-Acosta, J.F.J., Jacobs, D.E., Aguilar-Caballero, A.J., Sandoval-Castro, C., Cob-Galera, L. and May-Martínez, M., 2006. Improving resilience against natural gastrointestinal nematode infections in browsing kids during the dry season in tropical Mexico, Veterinary Parasitology, 135:163–173
Torres-Acosta, J.F.J., Sandoval-Castro, C.A., Hoste, H., Aguilar-Caballero, A.J., Cámara-Sarmiento, R. and Alonso-Díaz, M.A., 2012a. Nutritional manipulation of sheep and goats for the control of gastrointestinal nematodes under hot humid and subhumid tropical conditions, Small Ruminant Research, 103:28–40
Torres-Acosta, J.F.J., Molento, M. and Mendoza de Gives, P., 2012b. Research and implementation of novel approaches for the control of nematode parasites in Latin America and the Caribbean: Is there sufficient incentive for a greater extension effort? Veterinary Parasitology, 186:132–142
Torres-Acosta, J.F.J., Pérez-Cruz, M., Canul-Ku, H.L., Soto-Barrientos, N., Cámara-Sarmiento, R., Aguilar-Caballero, A.J., Lozano-Argáes, I., Le-Bigot, C. and Hoste, H., 2014. Building a combined targeted selective treatment scheme against gastrointestinal nematodes in tropical goats, Small Ruminant Research, 121:27–35
Wang, T., Van Wyk, J.A., Morrison, A. and Morgan, E.R., 2014. Moisture requirements for the migration of Haemonchus contortus third stage larvae out of faeces, Veterinary Parasitology, 204:258–264
Yimer, A. and Birhan, E., 2016. Prevalence and identification of gastrointestinal nematodes of small ruminants in northern Ethiopia, Middle-East Journal of Scientific Research, 24:2602–2608
Acknowledgments
The first author, Raquel Olivas Salazar received a scholarship from SEP-MEXICO (DSA/103.5/16/5852) to undergo her Ph.D. studies at Universidad Autónoma de Sinaloa.
Funding
The authors are grateful to PROMEP-SEP-MEXICO for The financial support (Project: Alimentación y Salud de Pequeños Ruminates en Zonas Áridas. IDCA23812, Clave: UAAAN-CA-29, Cuerpo Académico en Producción Animal y Biotecnología).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Olivas-Salazar, R., Estrada-Angulo, A., Mellado, M. et al. Prevalence of gastrointestinal nematode infections in goat flocks on semi-arid rangelands of northeastern Mexico. Trop Anim Health Prod 50, 807–813 (2018). https://doi.org/10.1007/s11250-017-1499-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1499-x