Abstract
The study was conducted with the objective of isolation and molecular characterization of infectious bursal disease virus (IBDV) circulating in Ethiopia and to assess the immunogenicity of different commercially available live attenuated IBD vaccines and finally to select the appropriate vaccine strain for the existing IBDV. Outbreak samples collected from different poultry farms with IBD infection between 2013 and 2015 were used for the virus isolation and molecular characterization. IBD vaccine immunogenicity test was conducted using four different commercially available live attenuated IBD vaccine strains: namely D78, B2K, LC75, and EXTREM. Day-old Bowman brown chickens purchased from commercial farm in Debre Zeit were used for the experiment. Serum samples were collected at days 14 and 21 and screened for the presence of maternal IBDv antibodies. The screening test result revealed that most of the chickens from vaccinated progeny were positive at the age of day 14 with mean antibody titer of .42, but declined at day 21 to 0.049 below cut-off point (S/P < 0.3). Chickens were divided into five different groups (four vaccinal and one control) and vaccinated at the age of day 21 and boosted after 14 days. Serum samples were collected and all of them were challenged at their 42 days of age with locally isolated very virulent infectious bursal disease virus (vvIBDV). From four of the vaccine strains used for immunogenicity study, the intermediate plus strains (LC75 and EXTREM) found to be superior and efficiently cross protect against the challenge with locally isolated vvIBDV. The development of clinical signs was studied and post-mortem examinations were conducted both on dead and sacrificed birds. From a total of 25 tissue samples processed for virus isolation on chicken fibroblast cell culture, 95% (18/20) of bursa and 80% (4/5) of the spleen samples showed visible cytopathic effect (CPE). The positive samples were tested by PCR and 19 of them had the expected band (643 bp). Further 11 representative samples were sequenced and confirmed that the circulating virus among poultry population in the country is vvIBDV. The study has recommended to produce vaccine using intermediate plus strains to prevent and control currently circulating vvIBDV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious bursal disease, also known as Gumboro, is an economically important acute and highly contagious immunosuppressive infectious disease of young susceptible chicks and it affects the poultry industries worldwide (Toro et al. 2009; Rauf 2011). Infectious bursal disease (IBD) is caused by infectious bursal disease virus (IBDV) (Mahgoub et al. 2012; Muller et al. 2012), which belongs to a genus Avibirnavirus, of the family Birnaviridae (Fauquet et al. 2005). It is dsRNA, non-enveloped, icosahedral capsid with bi-segmented genome (Wu et al. 2007; Eterradossi and Saif 2008; Zhu et al. 2008). The larger segment A encodes four viral proteins designated as VP2, VP3, VP4, and VP5, and also the smaller segment B encodes only VP1 which has polymerase activity (Zhu et al. 2008). The two viral proteins, VP2 and VP3 are structural proteins which form the viral capsid. The epitopes responsible for the induction of neutralizing and protective antibodies are located on the VP2 protein. There are two serotypes of the virus: IBD virus serotype 1 and IBD virus serotype 2. IBD virus serotype 1 is an important pathogen of chickens (Van den berg et al. 2004).
Clinical disease occurs solely in chickens but turkeys, ducks, guinea fowl, and ostriches may be infected naturally and experimentally, as evidenced by serological response and isolation; however, the infections are apathogenic (OIE 2012). Depending on the virulence of the IBDV strain, age at the time of infection, presence of IBDV antibodies, and the genetic background of the infected chicken, infection with IBDV may induce a temporary or permanent destruction of bursa of Fabricius (BF) and other lymphoid tissues. Destruction of B cells and macrophages resulted in IBDV-induced immune suppression (Khatri et al. 2005).
IBD causes heavy economic losses in poultry industries due to immune suppression in subclinical cases (Jackwood and Sommer-Wagner 2010) and in acute cases; it is associated with high morbidity and mortality (Jackwood et al. 2009). The disease mainly affects young chickens of between 3 and 6 weeks of age, which is characterized by enlarged bursa of Fabricius, watery diarrhea, accumulation of urate in the urinary structure, and severe depression (OIE 2012).
In addition to hygienic farm management and biosecurity, the current IBD control methods involve passive and active immunization (Fussell 1998). It has been shown that the timing of IBD vaccine administration in chicken progeny is pivotal. The optimal vaccination time depends upon the maternal-derived antibody (MDA) level of the chicks, the vaccine strain used, vaccine break through titer, and the IBDV field pressure (de Wit 2001). Vaccination in the presence of IBDV antibody levels above the breakthrough titer of the vaccine will lead to a significant delay in induction of immunity, and also IBD vaccine virus may even be completely neutralized by maternally derived antibodies (Moraes et al. 2005). In order to have chickens protected against IBDV field challenge, it is crucial to determine the optimal timing for IBD vaccine delivery. The optimal timing is often predicted based on serological data following detection of IBDV MDA by an ELISA system during the first few weeks post-hatch (Kouwenhoven and van den Bos 1994).
At present, Ethiopia is experiencing rapid growth in its poultry sector. This is being driven by increasing demand due to fast population growth, rising incomes, and the expanding middle class together with the fact that poultry products are among the cheapest sources of protein. But the sector is constrained by prevailing infectious diseases including IBD (Zeleke et al. 2005).
IBD first reported in Ethiopia in 2002 at privately owned commercial poultry farm (Zeleke et al. 2003). Subsequently, it is becoming among the most important disease for the juvenile poultry industry in the country (Mazengia 2012; Jenbreie et al. 2013; Jenberie et al. 2014). Research findings and case reports coming from various regions of the country indicated that episodes of IBD outbreaks in several commercial poultry farms, poultry breeding, and multiplication centers have wiped out large number of exotic chickens despite regular vaccination practices and improved biosecurity measures (Zeleke et al. 2005). Over the past few years, 25 to 75% of the deaths/losses in exotic and cross chickens have been associated with IBD (Zeleke et al. 2002; Zeleke et al. 2005; Woldemariam and Wossene 2007).
Therefore, this study was conducted with the objective to isolate and characterize the existing IBDV virus circulating in the country, to evaluate the efficacy of certain commercially available IBDV vaccines, to determine level of maternal antibodies before primary vaccination, and finally to propose appropriate vaccine and vaccination schedule.
Materials and methods
Study area
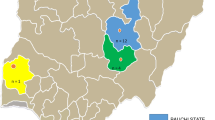
Clinical materials (bursa and spleen) were collected from IBD suspected chicken reared under semi-intensive and intensive poultry farms from Mekele (Tigray Regional State), Kombolcha and Bahir-dar (Amhara Regional State), Wollita and Dilla (Southern Nations and Nationalities of People’s Regional State), Bishoftu and Haramaya University (Oromia Regional State), and Addis Ababa (Fig. 1).
Study animals and study designs
Cross sectional study design was applied to isolate and molecularly characterize IBD on chickens of all ages and breeds reared under semi-intensive and intensive management systems that had experienced IBD outbreaks. Experimental study was implemented to determine MDA level and IBD vaccine immunogenicity using day-old Bowman brown breed chickens which were purchased from Debre Zeit poultry farm.
Sample collection and virus isolation
Bursa and spleen samples were collected aseptically from IBD suspected clinically sick and/or dead chickens following careful examination of cases for a period between 2013 and 2015. Samples were collected in two ways, i.e., either from clinically sick chickens suspected of IBD brought by the poultry owners or attendants to National Veterinary Institute (NVI) Research and Diagnostic Laboratory for disease diagnosis or through farm visit during outbreak reports. Samples were carefully labeled and either processed immediately or kept at −80 °C until processed. On the other hand, for IBDV vaccine immunogenicity experimental study, serum samples were collected and preserved at −20 °C until tested (OIE 2012).
The tissue samples were chopped into small pieces using a sterile scalpel blade and scissors and minced using a sterile mortar and pestle. A 10% (w/v) suspension of each sample was prepared in sterile phosphate buffer saline. The suspension was transferred into sterile centrifuge tube and centrifuged at 3000 rpm for 10 min. The upper aqueous phase (supernatant) fluid was harvested aseptically to sterile test tubes and filtered through a membrane of pore size 0.45 μm (Millipore, USA). The filtrate was inoculated into already prepared confluent primary chicken embryo fibroblast (CEF) cells with adsorption techniques (OIE 2012). Cultures were observed microscopically for up to 7 days for the presence of cytopathic effect (CPE) characteristic of IBDV. After 7 days, samples with no CPE were blindly passed further three times following two cycles of freeze-thawing. Samples which did not develop any CPE after the third blind passage were considered as negative, whereas samples revealed that characteristic CPE was considered as positive and kept at −20 °C for further analysis by molecular techniques (OIE 2012).
RNA extraction and reverse transcription
Extraction of RNA from 10% (w/v) tissue sample suspensions and/or cell culture homogenates was carried out using PureLink™ RNA Mini Kit (Life Technology, Carlsbad, USA) based on the manufacturer’s protocols. Accordingly, 400-μl tissue suspension was transferred in to 1.5-ml microcentrifuge tube and 400-μl lysis buffer was added to each tube, mixed by vortexing and incubated at 56 °C for 30 min. Four hundred microliters of 70% ethanol was added to the cell homogenate and vortexed to mix thoroughly; the homogenized suspension was transferred to spin cartridge with collection tube and centrifuged at 12,000 rpm for 15 s. The RNA bound to the membrane was eluted to clean tube by adding 40-μl RNAse-free water into collection tube and by centrifuging at 12,000 rpm for 2 min.
Complementary DNA (cDNA) was generated from RNA template using the reverse transcriptase RevertAid™ (Fermentas, Lithuania) by two-step cDNA synthesis method. Briefly, 10-μl volume reaction mix was prepared first from 3-μl RNase-free water, 1-μl Oligo(dT), and 1 μl of 10-Mm dNTPs and mixed by vortex, and then 5-μl template RNA (1 μg/μl) was added and incubated at 65 °C for 5 min and placed at +4 °C. Also, a 10-μl volume cDNA synthesis mix was prepared from 1-μl DEPC-treated water, 2-μl RT buffer, 4 μl of 25-mm MgCl2, 2 μl of 0.1-M DTT, and 1-μl superscript IIIRT enzyme. The two reaction combinations were added together and the reverse transcription reaction was performed at 55 °C for 50 min, and the reaction was terminated by heating at 85 °C for 5 min.
Polymerase chain reaction (PCR)
PCR amplification was performed as per the manufacturer’s protocol (Fermentas, Lithuania) on the partial sequence of VP2 gene of IBD virus using IBDV specific designed primers with NCBI Accession No: KC603937.1. Forward primer IBDV2 5′ TCACCGTCCTCAGCTTAC 3′ and reverse primer IBDV1 5′ TCAGGATTTGGGATCAGC 3′ were used. The amplification was carried out in a final reaction volume of 20 μl containing 5 μl of 5× PCR buffer with MgSO4, 1 μl of 10-mM dNTPs, 6-μl RNase-free water, 1-U Taq DNA polymerase, 2 μl of each primer (10 pmol/μl), and 3 μl of cDNA template. Touchdown PCR reaction was carried out for 1 cycle at 94 °C for 10 min for initial denaturation and 95 °C for 30 s, 55 °C for 45 s, 72 °C for 45 s for 35 cycles, and final extension at 72 °C for 10 min. The PCR products were visualized by 1.5% (w/v) agarose gel electrophoresis stained with gel red.
Sequencing and phylogenetic analysis
The PCR products were purified individually using Wizard® SV Gel and PCR product purification kit (Promega, Germany) following the manufacturer’s instruction, and the concentration of extracted DNA was determined with NanoDrop Spectrophometer (Thermo Scientific). The quantified DNA and IBDV VP2 gene specific forward and reverse sequencing primers were sent to the sequencing company (GATC Biotech AG, Germany). The nucleotide sequences obtained were assembled and edited using the SeqMan II (DNASTAR® Lasergene 8.0) software program (http://www.dnastar.com). The assembled sequences were aligned using ClustalW multiple alignment in the BioEdit software (Hall 1999). Phylogenetic trees were constructed using neighbor-joining method of analysis included in MEGA version 5.1: maximum composite likelihood model (Tamura et al. 2011), and confidence levels were assessed by 1000 bootstrap replications.
IBD vaccine immunogenicity experimental study
Experimental animal and study design
Day-old Bowman brown chickens obtained from a commercial hatchery were used for IBD vaccine immunogenicity trial. All animal experiment works were conducted according to National Veterinary Institute and Addis Ababa University College of Veterinary Medicine and Agriculture Animal Research Ethical Guidelines. IBD vaccine immunogenicity test was conducted using four different live attenuated commercially available IBD vaccine strains: namely, classical (D78), invasive intermediate (B2K), and intermediate plus (ETREM and LC75).
Before grouping and start of the experiment, blood samples were collected from wing vein using a 3-ml syringe at ages of 14 and 21 days, and the sera were checked for maternal-derived IBDV antibody level by flock screening enzyme-linked immunosorbent assay (ELISA) test. Those IBDV-specific antibody negative chickens were randomly assigned into five groups (30 chickens per group). Each of four groups in the experiment was immunized at ages of days 21 and 35 in intraoral route, and the fifth group (control group) remained unvaccinated and serves as challenge control. One week after the last vaccination (at day 42), birds in each group were challenged using vvIBDV local isolate (0.2 ml of 105.4 TCID50) in intraoral route. All birds were monitored for overt signs of disease and mortality over 21 days post-challenge.
Serological test
The serum samples collected were screened by ELISA as described by the manufacturer (Carnegie Campus, UK). Briefly, already diluted 50-μl serum samples and controls were added into the appropriate wells and incubated at 37 °C for 30 min with gentle agitation. The plates were washed four times with wash buffer, and then 50 μl of conjugate was added into each well and incubated at 37 °C for 30 min. Again, the plates were washed four times, and 50-μl substrate was added per well, incubated at 37 °C for 15 min, and finally the reaction was stopped with 50 μl stop solution. The test result was read by using microtitre plate reader at 550 nm absorbance. The percentage positivity (pp) for test samples in relation to the negative and the positive controls was calculated as per the formula. The cut-off value provided by the manufacturer was used to determine the pp.
Results
Outbreak investigation
A total of 60 chickens, 21 from Bishoftu, eight from Bahirdar, five from Addis Ababa, five from Kombolcha, ten from Tigray, five from Dilla, four from Haramaya, and one from Wollita, were examined and representative bursa and spleen samples were collected. The diseased chickens showed clinical signs of severe depression, watery diahorrea, ruffled feather, and reduced feed and water intake. Also during post-mortem examination, different lesions were recorded like enlarged hemorrhagic bursa, accumulation of urate crystals in the kidney, and hemorrhage in thigh and pictorial muscle. The age of the chickens varied from 35 to 56 days. Morbidity and mortality varied from one flock to another, and up to 60% mortality was recorded in a flock. Vaccination history showed that most of flocks were vaccinated with different IBD vaccines which were either locally produced at NVI or imported ones.
IBD virus isolation and characterization
From a total of 25 tissue samples (spleen and bursa) processed for virus isolation on chicken fibroblast cell culture, 95% (18/20) of bursa and 80% (4/5) of the spleen samples were positive and developed visible CPE. CPE was seen as small round cells, reflective, and later detached from the wall of cell culture flask.
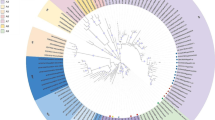
Totally, 20 cell culture suspensions were tested with RT-PCR for IBDV genome amplification, and 18 samples showed the needed PCR product and the remaining two samples were negative. The sequence result showed that the IBDV strain circulating in Ethiopia is the very virulent IBD virus (vvIBDV) and also isolates from the same area clustered together (Fig. 2).
Phylogenetic tree analysis of sequence data. Phylogenetic tree analysis of 25 IBD viruses based on nucleotide sequences of hyper variable coding (VP2) gene partial sequence. IBDV field isolates, classical/attenuated vaccine strains, and reference sequences of classical and very virulent strains retrieved from the GenBank database were included in the analysis. The current 11 field isolates are marked with colored circle. Infectious bursal disease virus isolates grouped phylogenetically into classical virulent (CV) and very virulent (vv) strains
Experimental studies
IBDV maternal-derived antibody (MDA) detection
Chickens sera collected at days 14 and 21 were screened for maternal antibody, and the result showed that the S/P ratio was 0.42 ± 0.14, and 0.049 ± 0.0009, respectively (Table 1). It showed a significant reduction of IBDV MDA on day 21-examined serum than on day 14 (p < 0.05). Based on the result, the experimental IBDV vaccine administration schedules were determined as day 21 for primary and day 35 for booster vaccination.
IBDV antibody detection after immunization
Antibody titer, S/P ratio, results on days 35 and 42 before challenge, and also S/P ratio and percent protection on day 63 (post-challenge) along the treatment groups were measured and compared. The results showed that the mean S/P ratio obtained from chickens vaccinated with D78 was significantly low (S/P ratio 0.470 at day 35 and 0.43003 at day 42), whereas the remaining three vaccines gave comparable antibody titers (Table 2). The S/P ratio for chickens vaccinated with D78 and B2K at day 63 was lower than challenge control group (2.58887 for D78, 2.68563 for B2K), but group LC75 induced significantly higher S/P ratio (3.20194).
Vaccine efficacy test result
There was no any clinical sign observed in LC75- and EXTREM-vaccinated groups, whereas in D78- and B2K-vaccinated groups, clinical sickness was recorded with the rate of 23.3 and 10%, respectively. 100% morbidity was observed in non-vaccinated challenge control group. There was no any mortality on vaccinated groups, but the challenge virus induced 60% (n = 18/30) mortality in control groups starting from day 4 post-challenge (Table 3).
The sick birds showed a pathognomonic clinical signs of IBD: intense prostration, in appetence, watery faces, pasting vents and anorexia, ruffled feathers, and depression. Necropsy was done on dead and scarified birds and the result showed presence of hemorrhage on pictorial and thigh muscle, enlarged blood-stringed bursa, and urate in kidney. The proportion of pathological lesions observed among different groups ranges from 10% for EXREM and LC75 to 40% for D78 and 50% of non-vaccinated control groups.
Discussion
This study demonstrated the incidence of IBDV from clinically diseased chickens reared under different production systems in Ethiopia. It is in agreement with previous studies, which reported about the existence of IBD virus in commercial chickens and as it becomes a serious threat on the juvenile poultry industry in Ethiopia. Some of those reports were supported by virus isolation and molecular analysis, and some of the remaining reports were based on serological analysis (Zeleke et al. 2005; Tesfaheywet and Getnet 2012; Mazengia 2012; Jenbreie et al. 2013; Jenberie et al. 2014).
In the present study, the molecular analysis revealed the existence and circulation of vvIBDV in Ethiopian chicken population. This result is in agreement with the previous report of Jenberie et al. (2014) who reported the existence of vvIBDV in Ethiopian chickens.
The occurrence of IBD was demonstrated in different poultry farms despite vaccination and improved biosecurity measures regularly practiced in the farms, which is in agreement with the report of different researchers (Zeleke et al. 2005; Jenberie et al. 2014). The occurrence of IBD is usually influenced by certain factors like host’s immune status, host range, and strain variations. Although vaccination of chickens has remained as the principal method to control the disease (Okwor et al. 2013; El-mahdy et al. 2013), some important factors determine the success of vaccination including the time of vaccination, vaccine type, maternal antibodies in the chicks, and pathogenicity of the offending virus (Hair-Bejo et al. 2004). The timepoint of vaccination is crucial as persisting MDA neutralizes the vaccine. The titer of MDA may also vary considerably within a flock, and it has to be taken into consideration that vvIBDV will break through immunity provided by highly attenuated vaccine strains.
Previous observations in the field and experimental studies had indicated that high MDA at the time of vaccination may interfere with the vaccine response and neutralize the vaccine virus (Hair-Bejo et al. 2004; Moraes et al. 2005). A delayed or prevented immune response may subsequently lead to being susceptible to IBDV challenge (de Wit and van Loon 1998). But information had not been available so far about the real influence of MDA on the outcome of the IBDV vaccine response in Ethiopia. During this experimental study, MDA titer ration was performed prior to vaccination at day 14 and day 21 and the result showed that the mean S/P ratio was 0.42 ± 0.14 and 0.049± 0.0009, respectively. The result recoded at day 21 was below the cut-off point (S/P < 0.03), and hence the experimental IBDV vaccine administration schedules were determined at days 21 and 35 for primary and booster vaccination date, respectively. This result agreed with finding of Kumar et al. (2000) who considered 21-days old as the ideal age for vaccination, since maternal antibodies were not detectable anymore and could not interfere with the replication of the vaccine virus. Although there is variability in the persistence of maternal antibodies in the progeny, antibody levels at the first day of age can be known, and it is thus possible to estimate antibody half-life and establish the most appropriate period for prime vaccination (Alam et al. 2002).
The efficacy of some commercial IBD vaccines (D78, B2K, LC75, and IBD EXTREM) used in field was determined post-vaccination in vitro by measuring the antibody level with ELISA and in vivo by challenge with vvIBDV. The highest antibody mean S/P ratio was detected in EXTREM vaccinated group at day 35 (S/P = 0.64414); at day 42, highest antibody level was recorded in B2K group with mean S/P ratio of 0.67037, but at day 63, the highest mean antibody titer was recorded for LC75 (S/P = 3.20194). In contrary, D78 induced the lowest mean antibody titer throughout the experimental period with S/P ratio of 0.4700, 0.43003, and 2.58867 at days 35, 42, and 63, respectively. This result was in line with Abd El-Aziz (2000) who stated as vaccination with intermediate plus strain resulted in better immune response. But it is in contrary with the study findings of El-mahdy et al. (2013) who reported highest mean antibody titer for D78-vaccinated group in Egypt.
All birds were observed for 2 weeks post-challenge, and signs, lesions, and mortalities were recorded. Death was not recorded in all vaccinated groups, and also no clinical signs were observed in LC75- and EXTREM-vaccinated groups, but 23.3 and 10% morbidity was observed in D78- and B2K-vaccinated groups, respectively. This finding showed that intermediate plus strains (LC75 and EXTREM) conferred relatively better protection than classical (D78) and invasive intermediate (B2K) vaccine strains against the currently circulating vvIBD virus in the country. This agrees with findings of Hassan et al. (2004) who reported that white leghorn chickens vaccinated with a live intermediate vaccine were fully protected when challenged 10 days later with vvIBDV. Van den Berg (2000) also demonstrated that chickens vaccinated with intermediate or intermediate plus vaccines were fully protected from challenge with vvIBDV strains. According to Abdel-Alim and Saif (2001), vvIBDV is antigenically related to attenuated vaccine types (70 to 80% homology), and the immunity induced by these vaccines protected 100% against challenge with standard vvIBDV. In the contrary, pathological lesions were observed in all groups with the rate of 50, 40, 33.3, 10, and 10% for non-vaccinated control, D78, B2K, LC75, and EXTREM groups, respectively. The findings showed that the current vaccines do not protect birds fully from lesion development, and such birds may serve as a source of infection.
In conclusion, this study demonstrated that IBD is one of the major poultry diseases causing very high morbidity and mortality in chickens and prevalent in different production systems in Ethiopia. It is confirmed that very virulent infectious bursal disease virus (vvIBDV) pathotype is circulating in the country. The IBDV MDA screening test result revealed that most of the chickens from vaccinated progeny were positive at their age of day 14, but dramatically declined at day 21. Certainly this study showed that determining level of MDA is a useful tool to estimate the optimal vaccination time in order to induce protective immunity in a timely manner. It is also verified that intermediate plus IBDV vaccine strains (LC75 and EXTREM) are appropriate candidate for IBD control in the country.
References
Abdel-Alim, G. A. U. and Saif, Y. M. 2001. Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Disease, 45, 646–654.
Alam, J., Rahman, M.M., Sil B.K., Khan M.S.R., and Giasuddin, Sarker, M.S.K. 2002. Effect of maternally derived antibody on vaccination against infectious bursal disease (Gumboro) with live vaccine in broiler. International Journal of Poultry Science, 14, 98–101.
Abd El-Aziz 2000. Evaluation of different infectious bursal disease vaccines. Assiut Veterinary Medical Journal, 44, 242–54.
Susan S. El-mahdy, Hayam, Farouk, Abd El-Wanis, N. A. and Hamoud, M. 2013.Comparative studies between different commercial types of live infectious bursal disease [IBD] vaccine strains in Egypt. American Journal of Research Communication, 1, 113–129.
Eterradossi, N., and Saif, Y.M. 2008. Infectious Bursal Disease. In: Saif, Y.M., Fadly, A.M., Glisson, J.R., Mcdougald, L.R., Nolan, L.K., Swayne, D.E. Diseases of poultry. 12th ed. Ames: Iowa State University Press;. p.185-208.
Fauquet, C., Mayo, M., Maniloff, J., Desselberger, U., and Ball, L. 2005. Taxonomy classification and nomenclature of viruses. 8th Eds. ICTV Report. Academic Press Elsevier, San Diego, pp. 566–567.
Fussell, L. W. 1998. Poultry industry strategies for control of immunosuppressive diseases. Poultry Science, 77, 1193–1196.
Hair-Bejo, M., Saline, S., Hafiza H, and Julaida S. 2004. In ovo vaccination against IBD in broiler chickens. Veterinary journal of Malaysia, 12, 63–69.
Hall, T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–8.
Hassan, M., Afify, M., and Aliy, M. 2004. Genetic resistance of Egyptian chicken to infectious bursal disease and Newcastle disease. Tropical Animal Health and Production, 36,1–9.
Jackwood, D. and Sommer-Wagner E. 2010. Detection and characterization of infectious bursal disease viruses in broilers at processing. Preventive Veterinary Medicine, 97, 45–50.
Jackwood, D.J., Sommer-Wagner, S.E., Stoute, A.S., Woolcock, P.R., Crossley, B.M., Hietala, S.K. and Charlton, B.R. 2009. Characteristics of a very virulent infectious bursal disease virus from California. Avian Disease, 53, 592–600.
Jenberie, S., Lynch, S.E., Kebede, F., Christley, R.M., Gelaye, E., Negussie, H., Asmare, K, Ayelet, G. 2014. Genetic characterization of infectious bursal disease virus isolates in Ethiopia. Acta Tropica, 130, 39–43.
Jenbreie, S., Ayelet, G., Gelaye, E., Kebede, F., Stacey E. Lynch and Negussie, H. 2013. Infectious bursal disease: seroprevalence and associated risk factors in major poultry rearing areas of Ethiopia. Tropical Animal Health and Production, 45, 75–79.
Khatri, M., Palmquist, J., Cha, R. and Sharma J. 2005. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Research, 113, 44–50.
Kouwenhoven, B. and van den Bos J. 1994. Control of very virulent infectious bursal disease (Gumboro disease) in the Netherlands with more virulent vaccines. In Proc. First International Symposium on infectious bursal disease and chicken infectious anaemia, 21–24 June, Rauischholzhausen (E. Kaleta, ed.). World Veterinary Poultry Association, Giessen, Germany, 262–271.
Kumar, K., Singh, K.C.P. and Prasad, C.B. 2000. Immune responses to intermediate strain IBD vaccine at different levels of maternal antibody in broiler chickens. Tropical Animal Health and Production, 32, 357–360.
Mahgoub, H., Bailey, M. and Kaiser, P. 2012. An overview of infectious bursal disease. Archives of Virology, 157(11), 2047–57
Mazengia, H. 2012. Review on major viral diseases of chickens reported in Ethiopia. Journal of Infectious Diseases and Immunity, 4,1–9.
Moraes, H.L.S., Salle, C.T.P., Nascimento, V.P., Salle, F.O., Rocha, A.C.G.T., Souza, G.F., Furian, T.Q. and Artencio, J.O. 2005. Infectious bursal disease: evaluation of maternal immunity and protection by vaccination of one-day old chicks against challenge with very virulent virus isolate. Brazilian Journal of Poultry Science, 7, 51–57.
Muller, H., Mundt, E., Eterradossi, N. and Islam, M. 2012. Current status of vaccines against infectious bursal disease. Avian Pathology, 41,133–39.
OIE 2012. Manual of diagnostic tests and vaccines for terrestrial animals. Newcastle disease: (chapter. 2.3.14, pp: 576–89) and Infectious bursal disease: (chapter. 2.3.12: pp: 549–65). World Animal Health Information Database. Version 2. World Animal Health Information Database. Paris, France: World Organization for Animal Health. http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home
Okwor, E.C., Eze, D.C., Anyanwu, M.U., Okpe, C.B., and Eze P.C. 2013. Effects of mixed vaccinations against Newcastle disease and infectious bursal disease on immune response feed consumption and weight gain in broilers. Journal of Agriculture and Veterinary Science, 6, 63–68.
Rauf, A. 2011. Persistence, distribution and immunopathogenesis of infectious bursal disease virus in chickens, the Ohio State University, Pp. 98.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. and Kumar, S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Tesfaheywet, Z. and Getnet, F. 2012. Seroprevalence of infectious bursal disease in chickens managed under backyard production system in Central Oromia, Ethiopia. MSc Thesis, Department of Parasitology and Pathology, College of Veterinary Medicine, Haramaya University, Ethiopia, pp 35–50.
Toro, H., van Santen, V.L., Hoerr, F.J. and Breedlove, C. 2009. Effects of chicken anemia virus and infectious bursal disease virus in commercial chickens. Avian Disease, 53, 94–102.
Van den Berg, T.P. 2000. Acute infectious bursal disease in poultry: a review. Avian Pathology, 29, 175–194.
Van den Berg, T., Morales, D., Eterradossi, N., Rivallan, G., Toquin, D., Raue, R., Zierenberg, K., Zhang, M., Zhu, Y., Wang, C., Zheng, H., Wang, X., Chen, G., Lim, B. and Muller, H. 2004. Assessment of genetic, antigenic, and pathotypic criteria for the characterization of IBDV strains. Avian Pathology, 33, 470–476.
de Wit , J.J. 2001. Gumboro disease: estimation of optimal time of vaccination by the Deventer formula. Annual report and proceedings of COST Action 839: immunosuppressive viral diseases in poultry, pp. 170–178.
de Wit, J.J. and van Loon A.A.W.M. 1998. Gumboro-vaccinatie. Tijdschriftvoor Diergeneeskunde, 123, 7–10.
Woldemariam, S. and Wossene, A. 2007. Infectious bursal disease (Gumboro disease): case report at Andasa poultry farm, Amhara region. Ethiopian Veterinary Journal, 11, 141–150.
Wu, C., Rubinella, P. and Lin T. 2007. Molecular detection and differentiation of infectious bursal disease virus. Avian Diseases, 51, 515–526.
Zeleke, A., Yami, M., Kebede, F., Melese, N., Senait, B., Gelaye, E., Sori, T., Ayelet, G. and Berhanu B. 2002.Gumboro: an emerging disease threat to poultry farms in Debre Zeit. Ethiopian Veterinary Journal, 6, 1–7.
Zeleke, A., Yami, M., Kebede, F., Melese, N. and Senait, B., 2003. Gumboro: an emerging disease threat to poultry farms in Debre Zeit. Proceedings of the 17th Annual Conference of Ethiopian Veterinary Association, Addis Ababa, Ethiopia.
Zeleke, A., Gelaye, E., Sori T., Ayelet, G., Sirak, A. and Zekarias, B. 2005. Investigation on infectious bursal disease outbreak in Debre Zeit, Ethiopia. International Journal of Poultry Science, 4, 504–506.
Zhu, L., Wu, S., Zhang, G. and Zhu, G. Q. 2008. The cellular receptor for infectious bursal disease. African Journal of Biotechnology, 7, 4832–4835.
Acknowledgements
The authors would like to thank the National Veterinary Institute Ethiopia for providing all the necessary supports including the funding. Alebachew Belay is highly appreciated for his professional support during laboratory work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors’ declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Mekuriaw, A., Bitew, M., Gelaye, E. et al. Infectious bursal disease: outbreak investigation, molecular characterization, and vaccine immunogenicity trial in Ethiopia. Trop Anim Health Prod 49, 1295–1302 (2017). https://doi.org/10.1007/s11250-017-1328-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1328-2