Abstract
A total of 398 serum samples from free-range indigenous chickens originating from four villages in Southern Mozambique were tested for the presence of avian encephalomyelitis virus (AEV) and Pasteurella multocida (PM) antibodies through commercial enzyme-linked immunosorbent assay (ELISA) kits. AEV and PM antibodies were detected in all villages surveyed. The proportion of positive samples was very high: 59.5% (95% confidence interval (CI) 51.7–67.7%) for AEV and 71.5% (95% CI 67.7–77.3%) for PM. Our findings revealed that these pathogens are widespread among free-range indigenous chickens in the studied villages and may represent a threat in the transmission of AEV and PM to wild, broiler or layer chickens in the region. Further research is warranted on epidemiology of circulating strains and impact of infection on the poultry industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
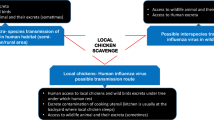
Indigenous chicken (Gallus gallus domesticus) rearing is a common practice in Mozambique. It provides eggs and poultry meat to most rural and many urban consumers. However, the productivity of indigenous chicken is hampered by several factors, including a variety of infectious diseases. Moreover, it is widely believed that indigenous chickens may act as potential reservoirs for important poultry diseases (Bouzoubaa et al., 1992).
Avian encephalomyelitis virus (AEV) is the aetiological agent of avian encephalomyelitis (AE) that is transmitted via the oral-faecal route (Tannock and Shafren, 1985). Based on the characteristics of the genome, AEV belongs to the genus Hepativirus within the family Picornaviridae (Bakhshesh et al., 2008). In young chickens, AEV induces neurological disorders such as tremors, paralysis, ataxia, and muscular distrophy. In older birds, the infection is mainly subclinical and may lead to reduction of egg production and hatchability (Tannock and Shafren, 1985; Berger, 1982; Meroz et al., 1990). Although AE is considered an important and ubiquitous disease of birds (Toplu and Alcigir, 2004; Welchman et al., 2009), literature on AEV infection of indigenous chickens in Africa is scarce and limited to one serological survey that detected AEV-specific antibodies in Zimbabwean indigenous chickens (Kelly et al., 1994). To our knowledge, there are no reports of the presence of AEV in either commercial or indigenous chickens in Mozambique.
Avian pasteurellosis (AP) also known as fowl cholera, avian cholera and avian haemorrhagic septicemia is an acute, fatal septicemic disease of various domestic and wild bird species, which is responsible for significant loss in poultry industry worldwide. Its causative agent, Pasteurella multocida, belongs to Pasteurellaceae family and is a Gram-negative, nonmotile, capsulated, nonspore forming rod-shaped bacterium (OIE, 2008). Strains of the bacteria are currently classified into five serogroups (A, B, D, E and F) based on capsular composition and 16 somatic serovars (1–16). Clinical signs of acute AP are inaptence, fever, ruffled feathers, oral mucus discharges, dyspnea and watery or yelowish diarrhoea (Rhoades and Rimler, 1990). Birds suffering from chronic form of the disease may show depression, conjunctivitis, dyspnea, lameness, torticollis, swelling of the wattles, sinuses, limb joints, footpads and sternal bursae (Christensen and Bisgaard, 2000). Although AP is considered a leading killer of domestic and wild birds (Rimler and Glisson, 1997), literature on the epidemiology of the disease in poultry in African countries is scanty, with few reports in indigenous chickens in Zimbabwe (Kelly et al., 1994), Tanzania (Muhairwa et al., 2001), Kenya (Mbuthia et al., 2008) and Ethiopia (Chaka et al., 2012). There are no published reports of this disease in poultry in Mozambique.
Unlike to what have been reported on broiler chickens (Frechaut et al., 2015; Muchanga et al., 2015), disease surveillance and control in indigenous poultry systems are very limited in Mozambique. This fact is due to lack of resources and also explains the paucity of information on the presence and prevalence of the above diseases in indigenous chickens. Therefore, this information can only readily be obtained through indirect serological studies on apparently healthy chickens. This study aimed to determine the presence of AEV and P. multocida antibodies in free-range indigenous chickens in Southern Mozambique.
Method and materials
The study site, shown in Fig. 1, consists of four administrative villages in the Mandlhakazi district, Soutern Mozambique: Chidenguele, Macuacua, Chizavane and Nwadjahane. Free-range indigenous chicken and broiler restocking development programs are being implemented in these villages with support of local government and nongovernment oraganizations.
The study was conducted between February and March 2016, using a cross-sectional design. Two sets of eligibility criteria for a villager to participate in the study were established: the presence of a high number of chickens, and willingness and commitment to the objective of the study. To avoid stress, chickens laying eggs and chicks younger than 3 weeks of age were excluded.
A total of 384 sera samples from indigenous chickens were collected. This was the calculated sample minimum size using the formula n = Z 2 PQ/L 2 and based upon a 50% prevalence (Thrufield, 2007).
Approximately 3 mL of blood was collected from each chicken into disposable syringes, left horizontally for 3 h, and then vertically for the serum to ooze out. Serum was collected in 2-mL cryovial tubes and kept at −20 °C until testing. Serum samples were analysed using commercial enzyme-linked immunosorbent assay (ELISA) kits for the presence of antibodies to AEV (ProFLOK® Avian Encephalomyelitis Virus Antibody Test Kit, Synbiotics Corp., San Diego, CA, item number 96-6518) and PM (ProFLOK® Pasteurella multocida Antibody Test Kit, Synbiotics Corp., San Diego, CA, item number 96-6527), according to the manufacturer instructions. These commercial kits are based on the principle of indirect ELISA. The sample and control OD values were read using an automated microplate reader (EL × 800, BIOTEK, Instruments Inc., Winooski, VT) at 405 nm. For each sample, the sample-to-positive (S/P) ratios were calculated from OD values by the formula:
S/P ratio = (ODsample − negative control mean OD)/(positive control mean OD − negative control mean OD).
All data were entered in MS Excel (Microsoft Corporation) spreadsheet and exported to STATA version 12.1® (Stata IC 12.1 for Windows), software for analysis. Descriptive statistics were based on frequencies and percentages for qualitative variables and means and confidence intervals for quantitative variables. Proportion of positive sample data was calculated using either Fisher’s exact test or the χ 2 test. Chi-square analysis was used to compare the association between dependent (seroconversion status: positive or negative) and independent variable (location). In all chi-square tests, a probability level of P < 0.05 was considered statistically significant.
Results
Table 1 shows the proportion of positive samples for AEV and PM antibodies in the different villages. The overall proportion of positive samples was 59.5% (95% confidence interval, CI 51.7–67.7%) for AEV and 71.5% (95% CI 67.7–77.3%) for PM. The proportion of positive cases for AEV ranged from 36% (95% CI 25.1–46.9%) (Chizavane) to 91.1% (95% CI 83.6–98.5%) (Macuacua) and it was significantly higher in Macuacua than in Chizavane (P = 0.05). The proportion of positive cases for PM had a narrowed variation and varied from 56.6% (95% CI 52.2–60.1%) (Nwdjahane) to 79.2% (95% CI 75.2–93.2%) (Macuacua) and did not differ significantly between villages.
Discussion
In the present study, the free-range indigenous chicken had never been vaccinated against AEV or AP. In unvaccinated flocks, positive serological results are clear evidence that the birds have been exposed to the infectious agents under investigation. Therefore, the presence of antibodies to AEV and PM was considered evidence of exposure to natural infection.
Our study reported an overall high proportion of positive samples of 59.5% for AEV and this constitutes the first documentation of AE infection in Mozambique. Our finding is in disagreement with that of Kelly et al. (1994), who reported a lower exposure of 11% in backyard chickens in Zimbabwe. This difference in the natural exposure rate could be due to the number of chickens sampled, environmental factors and the period that the studies were conducted. While high mortality is seen in chicks, AE is a subclinical disease for adult birds (Berger, 1982; Meroz et al., 1990). The disease higher exposure of the birds in the study area and the apparent absence of chick mortality could be due to the presence AEV of lower pathogenicity or genetic resistance among indigenous breeds of chickens in Mozambique, as reported elsewhere for other poultry viral diseases (Hassan et al., 2004). However, further studies are warranted to address these issues.
Our serological survey also revealed that PM is widespread among village chickens, with an overall proportion of positive cases of 71.7%. This is in agreement with surveys from Zimbabwe (52.1%; Kelly et al., 1994) and Ethiopia (62.2%, Chaka et al., 2012). The high proportion of serologic positive samples with absence of any clinical signs or significant mortality may imply that village chickens had been infected by less virulent strains as it has been described in Kenya (Mbuthia et al., 2008). Similarly to AEV, our findings are the first serologic evidence of PM in indigenous chickens in Mozambique.
Poor sanitary conditions, continuous exposure of chickens to range conditions and wild birds, nutritional deficiencies, the absence of vaccination and contact of chickens of one village with those in other villages characterize the free-range indigenous production system in Mozambique and other developing countries (Smith, 1992). Similar to what have been reported for other indigenous chicken pathogens, these factors can undoubtedly facilitate the spread and persistence of disease etiologic agents and may explain the relatively higher exposure rate for AEV and PM in the studied indigenous chickens (Júnior et al., 2017).
Conclusion
Our study has shown that AEV and PM are circulating among indigenous chicken in the investigated area. Therefore, the infected chickens may represent a threat in the transmission of those diseases to wild, broiler or layer chickens in that region. However, additional research is warranted to identify the circulating strains and the epidemiology of these diseases.
References
Bakhshesh M., Groppelli, E., Willcocks, M.M., Royall, E., Belsham, G.J., and Roberts, L.O., 2008. The picornavirus avian encephalomyelitis virus possesses a hepatitis C virus-like internal ribosome entry site element. Journal of Virology, 82, 1993–2003.
Berger, R.G., 1982. An in vitro assay for quantifying the virus of avian encephalomyelitis. Avian Diseases, 26, 534–541.
Bouzoubaa, K., Lemainguer, K. and Bell, J.G., 1992. Village chickens as a reservoir of Salmonella Pullorum and Salmonella Gallinarum in Morocco. Preventive Veterinary Medicine 12, 95–100.
Chaka, H., Goutard, F., Bisschop, S.P.R. and Thompson, P.N. 2012. Seroprevalence of Newcastle disease and other infectious diseases in backyard chickens at markets in Eastern Shewa zone, Ethiopia. Poultry Science, 91, 862–869.
Christensen, J.P., Bisgaard, M., 2000. Fowl cholera. Revue Scientifique et Technique, 19, 626–637.
Frechaut, E., Muchanga, E., Taunde, P., Nhambirre, O., Pondja, A. and Bila, C.G., 2015. Evaluation of serum antibodies titers against Newcastle Disease in Maputo and Matola Regions, Mozambique. International Journal of Poultry Sciences, 14, 622–624.
Hassan, M.K., Afify, M.A. and Aliy M.M., 2004. Genetic resistance of Egyptian chicken to infectious bursal disease and Newcastle disease. Tropical Animal Health and Production, 36, 1–9.
Júnior, A.M., Taunde, P., Zandamela, A.F., Júnior, A.P., Chilundo, A., Costa, R.. and Bila, C.G., 2017. Serological Screening Suggests Extensive Presence of Mycoplasma gallisepticum and Mycoplasma synoviae in Backyard Chickens in Southern Mozambique. Journal of Veterinary Medicine, 2743187.
Kelly, P.J., Chitauro, D., Rohde, C., Rukwava, J., Majok, A., Davelaar, F. and Mason P.R., 1994. Diseases and management of backyard chicken flocks in Chitungwiza, Zimbabwe. Avian Diseases, 38, 626–629.
Mbuthia, P.G., L.W. Njagi, P.N. Nyaga, L.C. Bebora, U. Minga, Kamundia, J. and Olsen, J.E., 2008. Pasteurella multocida in scavenging family chickens and ducks: Carrier status, age susceptibility and transmission between species. Avian Pathology, 37, 51–57.
Meroz, M., Elkin, N., Hadash, D., and Abrams, M., 1990. Egg drop associated with avian encephalomyelitis virus. Veterinary Record, 127, 532.
Muchanga, E., Frechaut, E., Taunde, P., Nhambirre, O., Pondja, A. and Bila, C.G., 2015. Vaccination against Bursal Disease Virus (IBDV) in Mozambique: Are we doing enough? International Journal of Poultry Sciences, 14, 644–646.
Muhairwa, A. P., M.M.A. Mtamboa, J.P. Christensen and Bisgaard M.P., 2001. Occurrence of Pasteurella multocidaand related species in village free–ranging chickens and their animal contacts in Tanzania. Veterinary Microbiology, 78, 139–153.
OIE; 2008. Fowl Cholera. In: Manual of diagnostic tests and vaccines for terrestrial animals 2016. http://www.oie.int/international–standard–setting/terrestrial–manual/access–online. Accessed 15 Apr 2016.
Rhoades, K.R. and Rimler, R.J.B., 1990. Virulence and toxicology of capsular serogroups D Pasteurella multocida strains isolated from avian hosts. Avian Diseases, 34, 384–388.
Rimler, R.B. and Glisson J.R., 1997. Fowl cholera. In: Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R. and Saif, Y.M. (eds). Poultry Diseases (Mosby–Wolfe, London).
Smith, A.J., 1992. Integration of poultry production into the agricultural systems. In: The tropical agriculturist: poultry, (The Technical Centre for Agricultural and Rural Development). Macmillan, London: 179–191.
Tannock, G.A. and Shafren, D.R., 1985. A rapid procedure for the purification of avian encephalomyelitis viruses. Avian Diseases, 29, 312–321.
Thrufield, M.V., 2007. Survey. In: Veterinary Epidemiology. Blackwell, pp. 228–246.
Toplu, N. and Alcigir, G., 2004. Avian encephalomyelitis in naturally infected pigeons in Turkey. Avian Pathology, 33, 381–386.
Welchman, B., Cox, W.J., Gough, R.E., Wood, A.M., Smyth, V.J., Todd, D., and Spackman, D., 2009. Avian encephalomyelitis virus in reared pheasants: a case study. Avian Pathology 38, 251–256.
Acknowledgements
We wish to express our gratefulness to the Fundo Nacional de Investigação, Mozambique (Grant FV5/2014), and Centre for Coordination of Agricultural Research and Development for Southern Africa (CCARDESA) (Grant CPRJ/INT/WB/CFP1/14/03) for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Taunde, P., Timbe, P., Lucas, A.F. et al. Serological evidence of avian encephalomyelitis virus and Pasteurella multocida infections in free-range indigenous chickens in Southern Mozambique. Trop Anim Health Prod 49, 1047–1050 (2017). https://doi.org/10.1007/s11250-017-1304-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1304-x