Abstract
The aim of the present work was to investigate in detail the influence of running-in with acetylacetone on the friction behavior of 5CB liquid crystal between steel surfaces. Friction tests were carried out using a sliding ball-on-disk tribometer. The coefficient of friction (COF) decreased significantly after a running-in process with acetylacetone. Tribochemical reactions between steel and acetylacetone generated Fe(acac)3 and groove-structured wear on the ball. Their synergistic effect is the reason for the ultralow friction, and without either one, the COF increases. Special attention was given to the rheological behavior of 5CB. Shear history has a significant influence on the viscosity and storage moduli of 5CB. In addition, the COF under the same low speed decreased by 37% after a friction test at higher speed. The possible mechanism is discussed, and an in-depth understanding of this tribological system is needed to develop possible future applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Approximately 23% (119 EJ) of the world’s total energy is consumed by friction and wear between moving components [1]. There is an increasing demand to minimize friction and wear so as to increase energy efficiency in various mechanical devices. It is estimated by Holmberg and Erdemir that energy losses due to friction and wear could potentially be reduced by 40% in 15 years if new technologies in surfaces, materials, and lubrication are used [1]. The industry is now in urgent need of new lubricating methods that can further reduce friction and wear.
Liquid crystals (LCs) are substances that exhibit a mesophase between liquid and crystalline solid within a certain range of temperature or concentration. The specific physical behavior endows liquid crystals with the fluidity of liquids and some of the ordered molecular arrangement of crystals [2]. In addition to their wide commercial use in liquid crystal display (LCD) [3], LCs are of great interest in tribology as lubricants [4,5,6,7] or additives [7,8,9,10] because of their encouraging lubricating properties. The encouraging lubricating properties of LCs are believed to be related to molecular orientation. For example, surface anchoring and electrical field-induced molecular orientation significantly influences the lubricating behavior of LCs [11, 12].
Despite the notable improvement in lubricating performance, lubricating with LCs, however, in most cases can only reduce the COF to a certain extent (by a factor of two or less) in research, and no ultralow friction was reported except by Eidenschink [13]. Unfortunately, Eidenschink’s pioneering findings did not attract enough attention from researchers for 10 years until 2009 [14]. Based on Eidenschink’s research, Amann and co-workers conducted a more in-depth study. Superlow friction (0.005) is achieved between steel surfaces in a reciprocating line-contact friction system when lubricated with specific types of LCs [14,15,16]. They found that tribochemical reactions between LC and steel during the running-in process decrease contact pressure dramatically and form a specific surface texture on wear areas, and these are important factors responsible for the ultralow friction [15, 17]. Simulations of the friction process show that molecular orientation plays an important role in reducing friction [18,19,20]. In addition, the potential technical application of these specific types of LCs in sintered bearings has shown lower friction and longer life time compared to standard oils [21].
In our prior work [22], the tribological performance of 4-Cyano-4′-pentylbiphenyl (5CB) LC was investigated after a running-in process with acetylacetone (Hacac). The COF between steel surfaces in a ball-on-disk sliding friction test decreased from 0.055 to 0.013. Tribochemical reactions between Hacac and steel surfaces lead to the generation of tris(acetylacetonato) iron(III) (denoted as Fe(acac)3), lower contact pressure, and an evenly distributed grooved structure on wear area of the ball. The results show that lower contact pressure cannot directly lead to the ultralow friction of 5CB. Therefore, the groove-structured wear and Fe(acac)3 are assumed to be the reasons for the encouraging tribological performance. This work aims at a deeper insight into the influence of the groove-structured wear and Fe(acac)3 on the friction behavior of 5CB. Combined with the dynamic rheology of 5CB, a possible mechanism for the ultralow friction is proposed.
2 Experimental Section
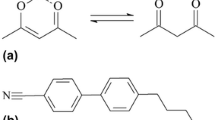
Commercially available LC 4-Cyano-4′-pentylbiphenyl (5CB, 99.5%, Shijiazhuang Huarui Scientific and Technological Co.) was used in friction tests. 5CB has rod-shaped molecules and exhibits a nematic phase within about 22–35 °C [23, 24]. Acetylacetone (Hacac, 99%, Chengdu Kelong Chemical Co., Ltd.) was used for the running-in process at the initial stage of the friction tests. Hacac exists as an equilibrium mixture of keto-enol tautomers, and its keto fraction is approximately 20% [25]. Figure 1 shows the chemical structure of 5CB and Hacac. All reagents were used without further purification.
The specimens were GCr15 steel balls (d = 6 mm, Ra = 25 nm) and disks (Ra < 100 nm). Before tests, the balls and disks were cleaned in an ultrasonic bath using acetone and ethanol for 15 min. Friction tests were carried out for 3 h in one direction plus 2 h in the reverse direction (COFs obtained in the reverse direction were negative) on a ball-on-disk tribometer (THT, Anton Paar, Switzerland) at 23–25 °C. Final COFs were obtained by averaging the absolute values measured in two reverse sliding directions to eliminate measurement errors [26, 27]. A normal load of 5 N was applied providing the initial maximum contact pressure of 1127 MPa. During friction tests, the disc rotated at 200 rpm with a track radius of 17.2 mm, corresponding to a linear speed of 360 mm/s. During the running-in process, Hacac was introduced multiple times (about 0.05 ml each time) between the ball and the disk to prevent its complete depletion [22]. The Fe(acac)3 generated from the reactions between Hacac and steel remained on the wear track after running-in. Approximately 0.03 ml of 5CB was applied as lubricant on the wear track of the disk after running-in.
A series of tests (running-in time was kept at 240 s, and the test duration was 5 h) were conducted to investigate the influence of Fe(acac)3 and the regular groove-structured wear on the COF of 5CB: (a) lubricating directly with 5CB after running-in with Hacac, denoted as acac-5CB; (b) washing out the Fe(acac)3 left on steel surfaces with acetone and ethanol repeatedly after running-in, and then lubricating with 5CB, denoted as acac-Washed; (c) lubricating with ethanol for 60 s after washing out Fe(acac)3, and then lubricating with 5CB, denoted as acac-Et60; and (d) lubricating with ethanol for 120 s after washing out Fe(acac)3, and then lubricating with 5CB, denoted as acac-Et120.
The topography and surface texture of the wear areas were investigated using an optical microscope (VHX-6000, Keyence). The autocorrelation function and 2D contour plots of the wear areas were obtained using an optical profiling system (white light vertical scanning interferometry, Wyko NT9300, Veeco). All friction surfaces were cleaned by sonication in acetone and then ethanol before any further investigation. The rheological behavior of 5CB was studied by a rotary rheometer (MCR302, Anton Paar) using cone-and-plate geometry with 20 mm diameter and 2°cone angle. The middle thickness of the sample between the peltier plate and cone was maintained at 0.105 mm in all measurements. As the rheological properties of LCs are dependent on shear history [2], the 5CB LC was gently added on the top of the plate, and then the cone was slowly lowered to the measuring position. The 5CB squeezed out between the cone and plate was then carefully removed. All measurements were carried out at 25 °C. The steady shear measurement was performed with shear rates varying from 1000 to 0.1 s−1. Before steady shear measurement, 5CB was allowed to rest for 30 min to relax from the deformation stress and attain temperature equilibrium. The influence of shear history on viscosity was studied by comparing the apparent viscosity of 5CB at shear rate of 0.1 s−1 before and after shearing the sample at a shear rate of 100 s−1. The measurement of the dynamic rheological behavior consisted of a steady shear for 120 s between two oscillatory time sweep measurements to study the influence of shear history on the storage moduli (G′) and loss moduli (G″) of 5CB. The oscillatory time sweep measurement was performed at an oscillatory strain of 1% and a shear frequency of 6.28 rad/s (1 Hz).
3 Results and Discussion
The results of 5CB friction tests after running-in with Hacac for different times (ranging from 0 to 480 s) are shown in Fig. 2. Once Hacac was introduced on the disk, the generated Fe(acac)3 was dissolved by Hacac. As Hacac is a liquid with very low viscosity (0.7 mm2/s at 40 °C), excessive Hacac (and the Fe(acac)3 dissolved in it) left the wear track as a result of centrifugal force. So after application, only a small amount of Hacac was left on the wear track. The remaining Hacac reacted with steel, generating Fe(acac)3. Given the same amount of Hacac applied each time (0.05 ml) and the same rotation speed, Hacac left on the wear track was basically the same, and hence, the generated Fe(acac)3 was presumed to be basically the same despite the difference in running-in time. The 5CB shows a stable COF value of about 0.055 during the whole testing period. In contrast, COFs are much smaller when 5CB was used as a lubricant after the running-in process with Hacac. As shown in Fig. 2, the COFs are reduced notably as the running-in time increases from 0 s to 240 s and reaches its minimum value (0.013) at 240 s. The COF value is basically the same when the running-in time increased from 240 to 480 s.
By running-in with Hacac for 0, 60, 120, 240 and 480 s, the wear diameters on the balls were measured to be 239, 343, 374, 446 and 494 µm, corresponding to the contact pressure of 116, 54, 46, 32 and 26 MPa, respectively. Contact pressure between sliding surfaces is very important in determining the COF, as lower contact pressure may transform the system from boundary lubrication to elastohydrodynamic lubrication, which decreases the COF notably. In our prior study, however, friction tests under contact pressure as low as 9 MPa reveal that reducing the contact pressure alone does not reduce the COF for 5CB [22]. Actually, as a result of the tribochemical reactions, running-in for different times leads to different wear topography on the ball as well as different contact pressure. Few shallow furrows could be found on wear area of the ball when the running-in time was 120 s, and a regular grooved structure was observed when the running-in time reached 240 s (optical images and the 3D plots in Fig. 5 in our prior study [22]). Reduced contact pressure was unavailable in the generation of the regular groove-structured wear that relies on the rapid wear of the ball caused by tribochemical reactions. Considering the phenomena mentioned above, it is reasonable to assume that wear topography rather than reduced contact pressure is the reason for the reduced COF.
Figure 3 shows the COF in the first 450 s of the acac-Et60 friction test. It can be seen that there is an obvious difference in the COF for Hacac and ethanol, even though they are all low viscosity, volatile organic liquids. Hacac and ethanol were applied on wear track of the disk occasionally to prevent depletion. The diagram depicts the COF of Hacac decreasing quickly before it was replenished, and once Hacac was replenished, the COF was restored to about 0.2 and began to decline again. In contrast, the COF of ethanol increased quickly before ethanol was replenished, and once ethanol was replenished, the COF was restored to about 0.15 and began to increase again. The different lubricating performance is believed to stem from the generation of Fe(acac)3, which has been used as an additive in lubricating oils and reduces friction [28]. The increasing content of Fe(acac)3 in Hacac as a result of the consumption of Hacac and the generation of Fe(acac)3 is a possible reason for the decreasing COF. On the contrary, no tribochemical reactions occurred between ethanol and steel, and that is why its COF presents an opposite change compared to Hacac.
The wear morphologies after friction tests of acac-Et120, acac-Et60, acac-Washed and acac-5CB are shown in Fig. 4. Lubricating with ethanol after running-in evidently damages the evenly distributed groove-structured wear on the ball. As shown in Fig. 4a, b, the wear surfaces for acac-Et120 and acac-Et60 are rough and irregular, with apparent exfoliation. The wear of acac-Et120 is more severe as the time lubricating with ethanol was longer. The wear area of acac-Washed (Fig. 4c) is much better than acac-Et120 and acac-Et60. Just washing out Fe(acac)3 does not change the wear surface morphology obtained by running-in with Hacac before lubricating with 5CB, but compared to acac-5CB (Fig. 4d), the regular grooved structure becomes less obvious after lubricating with 5CB for acac-Washed. This result indicates that Fe(acac)3 has the effect of protecting sliding surface. The autocorrelation functions and 2D contour plots of the wear areas also indicate that the furrows on the wear surface of acac-5CB are more uniform and regular.
Morphology and autocorrelation function (perpendicular to the sliding direction) of the wear area on the ball after friction tests with different treatment conditions after running-in with Hacac: a acac-Et120, b acac-Et60, c acac-Washed and d acac-5CB. The background color images are the 2D contour plots of the corresponding wear surface
The surface bearing curve of wear areas on the ball after friction tests are shown in Fig. 5. Compared to acac-5CB, washing out the generated Fe(acac)3 makes the slope of the bearing area curve less gentle (Washed in Fig. 5), whereas lubricating with ethanol makes the slope much steeper (Et-60 and Et-120 in Fig. 5). The height distribution of acac-5CB is more concentrated than the other three, with more than 91% of the surface height distributed within a range of ± 100 nm (acac-5CB in Fig. 5). Considering that a height that is too small or too high will be influenced by a few abnormal high peaks or deep valleys, it can be said that the height distribution of acac-5CB is very uniform.
Figure 6 depicts the COFs of acac-Et-120, acac-Et-60, acac-Washed and acac-5CB. As shown in the diagram, just by removing the Fe(acac)3 on sliding surfaces, the COF of acac-Washed increased to 0.029 from 0.013. The obvious increment in COF verifies our prior assumption that Fe(acac)3 does reduce friction. To further prove the benefit of Fe(acac)3 in reducing friction, Fe(acac)3 and 5CB were washed out after the friction test of acac-5CB from sliding surfaces and approximately 0.05 ml of ethanol solution that contained 0.03 g/ml Fe(acac)3 was applied on the wear track. A few minutes later, the ethanol volatilized completely and only Fe(acac)3 was left on the wear track. About 0.03 ml of 5CB was then applied on the wear track. The COF was measured to be 0.016, which is a little higher than acac-5CB but is much smaller than acac-washed. As for acac-Et60 and acac-Et120, the regular grooved structure on wear area of the ball was removed by lubricating with ethanol, increasing the COF. Corresponding to their wear morphology, the COF of acac-Et120 is 0.057, which is higher than 0.042 for acac-Et60.
Except for different wear morphologies, the other conditions of acac-Washed, such as the lubricant, contact pressure (lubricating with ethanol after running-in has little effect on the contact pressure) and sliding speed, are identical to those of acac-Et-120 and acac-Et-60. However, the COF of acac-Washed is smaller than that of acac-Et-120 and acac-Et-60, indicating that the regular groove-structured wear on the ball is beneficial for reducing friction as well. The results above demonstrate that both the groove-structured wear on the ball and the Fe(acac)3 induced by tribochemical reactions are beneficial in reducing friction, and without either one, the COF increases. In other words, the ultralow friction of 5CB is a result of synergy between the groove-structured wear and Fe(acac)3.
The rheological behavior of lubricants is crucial for determining their lubricating performance. Flow of LC causes molecular realignment (shear alignment), which results in shear thinning [29]. The dependence of the apparent viscosity on shear rate in Fig. 7 reveals the typical shear thinning behavior of 5CB.
Figure 8 depicts the influence of shear history on the apparent viscosity of 5CB. First, the viscosity of 5CB was measured at a shear rate of 0.1 s−1, and then the viscosity of the same sample was measured at shear rates of 100 and 0.1 s−1, successively. The subsequent measurement was made immediately after the previous one to make sure that there was no time for 5CB to relax from the deformation stress. Under the same shear rate, the viscosity of 5CB at the first and the third stages of the measurement is very different. The viscosity of 5CB changed from about 85 mPa·s to 53 mPa·s after being sheared at 100 s− 1, a reduction of 37.6%, indicating that under the same shear rate, the viscosity of 5CB is deeply influenced by its shear history.
In addition, as shown in Fig. 9, not only is the viscosity of 5CB affected by shear history but also the storage moduli (G′) and loss moduli (G″). At the first stage of the measurement, the G′ of 5CB is negligible, consistent with the literature [30]. However, after shearing at 100 s−1, the G′ of 5CB rose by three orders of magnitude, and there was basically no change in G″. The result indicates that G′ of 5CB is no longer negligible under the conditions of shear. Moreover, the shear rates in friction tests are much higher than in rheology measurements due to the thinner film thickness, and thus G′ of 5CB in friction tests could be more prominent. The rise of G′ reflects the internal structure change, which may affect friction behavior of 5CB.
The molecules of nematic LC tend to align along a common direction, the so-called director \(\vec {n}\). The orientation of the director is influenced by many factors, such as temperature, shear, surface conditions (anchoring), electric and magnetic fields, etc. In rheology measurements and friction tests, shear flow and surface anchoring are the main factors influencing director orientation of LC. LC molecules will be subject to torque in a shear flow, and hence, the director orientation will be disturbed and fluctuate. Nematic LCs can be divided into two groups according to the tumbling parameter λ, used to describe the torque equilibrium of \(\vec {n}\) under steady shear flow. For LCs with |λ| < 1, no torque equilibrium for \(\vec {n}\) can be reached, so \(\vec {n}\) keeps rotating continuously in shear flow, and this type of LC is designated as flow tumbling. Whereas for LCs with |λ| ≥ 1, the torque disappears when \(\vec {n}\) is at an angle θ relative to the flow direction, and this type of LC is designated as flow aligning [31]. The value of θ can be calculated by the following equation [17, 32]:
where α2 and α3 are Leslie coefficients of LC. For 5CB, λ > 1 [33] and the angle θ between the \(\vec {n}\) and the flow direction is about 13° in a steady shear flow [17]. The shear thinning behavior of LC is believed to result from the shear alignment, which induces a possibly better orientational order [29, 34]. The orientation change of \(\vec {n}\) in shear flow causes the LC molecules to be subjected to a restoring force (restore \(\vec {n}\) to the orientation without shear flow), which is the reason for the sharp increase in G′ after shear [35].
The lubricating performance of a lubricant is closely related to its viscosity. In addition, director orientation plays an important role in determining friction of LC lubricants. For example, an electric field can affect the friction of LC by influencing the director orientation [12, 36]. As shear has a significant influence on 5CB viscosity, director orientation and storage moduli (elastic moduli), an experiment was designed to investigate the influence of shear history on the COF of 5CB. The 5CB left on the wear track after the acac-5CB friction test was first allowed to rest for 30 min to eliminate the influence of previous shear, and then friction tests were carried out at 50, 400 and 50 rpm successively. As shown in Fig. 10, after the friction test at 400 rpm, the COF at 50 rpm dropped from about 0.046 to 0.029, a decrease of 37%, and the COF of 0.029 lasted for the test time of 60 min. The result suggests that friction of 5CB is deeply influenced by its shear history. It has been reported in the literature that the low friction of LC is attributed to the flow alignment at high shear rates [6]. The molecular orientation deduced by shear at high shear rates is believed to change some properties of the boundary film formed by 5CB molecules, and these changes may avoid intimate contact of the sliding surfaces [36]. The result means that when LCs are used as lubricants, we can obtain lower COFs at low sliding speeds by prelubricating the friction pairs at high sliding speed, and the lower COF lasts for a long time under low sliding speeds.
Other than flow alignment, surface anchoring affects the molecular orientation and hence the viscosity of LC. The surface with microgrooves forms uniform planar anchoring in which \(\vec {n}\) is parallel to the surface and to a certain in-plane direction (along the microgroove direction) [37, 38]. Thus, the grooved structure generated by running-in with Hacac very likely induces uniform planar anchoring of 5CB. The flow alignment and uniform planar anchoring streamline the 5CB molecules along the flow direction, and they can more readily pass each other, so the COF decreases. That may be the reason for the ultralow friction of 5CB after running-in with Hacac. The grooved structure was damaged by lubrication with ethanol after running-in, and thus, the uniform planar anchoring of 5CB was altered to other anchoring conditions, for instance, random planar anchoring (\(\vec {n}\) parallel to the surface with arbitrary angle) or homeotropic anchoring (\(\vec {n}\) perpendicular to the surface). It can be assumed that the increment of COF (Fig. 4) after damaging the wear surface on the ball (Fig. 6) is caused by changing the 5CB anchoring conditions that make 5CB molecules less streamlined.
4 Conclusion
In this study, the ultralow friction behavior of 5CB liquid crystal achieved by running-in with acetylacetone was investigated. Shear history has a significant effect on the viscosity, storage moduli (G′) and COF of 5CB. We obtain lower COF at low sliding speeds by prelubricating the friction pairs at high sliding speed, and the lower COF lasts for a long time under low sliding speeds. The groove-structured wear generated during the running-in process very likely induces uniform planar anchoring of 5CB along the grooves, and hence, under the combined action of shear alignment, the molecules of 5CB become more streamlined along the flow direction and can more readily pass each other, so the COF decreases.
In summary, both the Fe(acac)3 and groove-structured wear on the ball induced by the tribochemical reactions between Hacac and steel surfaces are beneficial for reducing friction, and without either one, friction will increase notably. So, the ultralow friction of 5CB liquid crystal achieved by running-in with acetylacetone is a result of the combined action of Fe(acac)3 and the groove-structured wear. The mechanism for the beneficial effect of Fe(acac)3 that protects the wear surface and reduces friction needs more investigation. In addition, in situ observation of the molecular orientation of 5CB during friction should be carried out in future work to prove our assumption directly. An in-depth understanding of this tribological system is needed for development and possible future applications.
References
Holmberg, K., Erdemir, A.: Influence of tribology on global energy consumption, costs and emissions. Friction. 5(3), 263–284 (2017). https://doi.org/10.1007/s40544-017-0183-5
Prost, J.: The Physics of Liquid Crystals, vol. 83. Oxford University Press, Oxford (1995)
Bahadur, B.: Liquid Crystals: Applications and Uses, vol. 1. World Scientific, Singapore (1990)
Tadokoro, C., Nihira, T., Nakano, K.: Minimization of friction at various speeds using autonomous viscosity control of nematic liquid crystal. Tribol. Lett. 56(2), 239–247 (2014). https://doi.org/10.1007/s11249-014-0404-2
Sulek, M.W., Wasilewski, T.: Antiseizure properties of aqueous solutions of compounds forming liquid crystalline structures. Tribol. Lett. 18(2), 197–205 (2005). https://doi.org/10.1007/s11249-004-1776-5
Mori, S., Iwata, H.: Relationship between tribological performance of liquid crystals and their molecular structure. Tribol. Int. 29(1), 35–39 (1996). https://doi.org/10.1016/0301-679X(95)00032-Y
Carrion, F.J., Martinez-Nicolas, G., Iglesias, P., Sanes, J., Bermudez, M.D.: Liquid crystals in tribology. Int. J. Mol. Sci. 10(9), 4102–4115 (2009). https://doi.org/10.3390/ijms10094102
Ghosh, P., Upadhyay, M., Das, M.K.: Studies on the additive performance of liquid crystal blended polyacrylate in lubricating oil. Liq. Cryst. 41(1), 30–35 (2013). https://doi.org/10.1080/02678292.2013.831132
Ważyńska, B., Tykarska, M., Okowiak-Chinalska, J.: The estimation of abilities of liquid-crystalline compounds dissolved in paraffin oil for accumulation on solid surface. Mol. Cryst. Liq. Cryst. 546(1), 163/[1633]-1168/[1638] (2011). https://doi.org/10.1080/15421406.2011.571951
Kupchinov, B.I.: Tribology and the liquid-crystalline state. J. Frict. Wear 30(3), 169–171 (2009). https://doi.org/10.3103/s1068366609030039
Tadokoro, C., Araya, S., Watanabe, M., Okubo, H., Nakano, K., Sasaki, S.: Synergy of two fatty acids as additives on lubricity of a nematic liquid crystal 5CB. Lubr. Sci. 30(3), 83–90 (2018). https://doi.org/10.1002/ls.1406
Gao, Y., Xue, B., Ma, L., Luo, J.: Effect of liquid crystal molecular orientation controlled by an electric field on friction. Tribol. Int. 115, 477–482 (2017). https://doi.org/10.1016/j.triboint.2017.06.021
Eidenschink, R., Konrath, G., Kretzschmann, H., Rombach, M.: Unusual lift by shearing mesogenic fluids. molecular crystals and liquid crystals science and technology. Section A. Mol. Cryst. Liq. Cryst. 330(1), 327–334 (1999). https://doi.org/10.1080/10587259908025606
Amann, T., Kailer, A.: Ultralow friction of mesogenic fluid mixtures in tribological reciprocating systems. Tribol. Lett. 37(2), 343–352 (2009). https://doi.org/10.1007/s11249-009-9527-2
Li, K., Amann, T., Walter, M., Moseler, M., Kailer, A., Rühe, J.: Ultralow friction induced by tribochemical reactions: a novel mechanism of lubrication on steel surfaces. Langmuir. 29(17), 5207–5213 (2013). https://doi.org/10.1021/la400333d
Amann, T., Kailer, A.: Analysis of the ultralow friction behavior of a mesogenic fluid in a reciprocating contact. Wear. 271(9–10), 1701–1706 (2011). https://doi.org/10.1016/j.wear.2010.12.012
Sengupta, A., Herminghaus, S., Bahr, C.: Liquid crystal microfluidics: surface, elastic and viscous interactions at microscales. Liq Cryst. Rev. 2(2), 73–110 (2014). https://doi.org/10.1080/21680396.2014.963716
Li, K., Amann, T., List, M., Walter, M., Moseler, M., Kailer, A., Rühe, J.: Ultralow friction of steel surfaces using a 1,3-diketone lubricant in the thin film lubrication regime. Langmuir. 31(40), 11033–11039 (2015). https://doi.org/10.1021/acs.langmuir.5b02315
Amann, T., Kailer, A., Oberle, N., Li, K., Walter, M., List, M., Rühe, J.: Macroscopic superlow friction of steel and diamond-like carbon lubricated with a formanisotropic 1,3-diketone. ACS Omega. 2(11), 8330–8342 (2017). https://doi.org/10.1021/acsomega.7b01561
Walter, M., Amann, T., Li, K., Kailer, A., Ruhe, J., Moseler, M.: 1,3-Diketone fluids and their complexes with iron. J. Phys. Chem. A. 117(16), 3369–3376 (2013). https://doi.org/10.1021/jp400980y
Amann, T., Kailer, A., Beyer-Faiß, S., Stehr, W., Metzger, B.: Development of sintered bearings with minimal friction losses and maximum life time using infiltrated liquid crystalline lubricants. Tribol. Int. 98, 282–291 (2016). https://doi.org/10.1016/j.triboint.2016.02.023
Chen, H., Xu, C., Xiao, G., Chen, Z., Yi, M.: Ultralow friction between steel surfaces achieved by lubricating with liquid crystal after a running-in process with acetylacetone. Tribol. Lett. 66(2) (2018). https://doi.org/10.1007/s11249-018-1020-3
Mansare, T., Decressain, R., Gors, C., Dolganov, V.: Phase transformations and dynamics of 4-cyano-4′-pentylbiphenyl (5cb) by nuclear magnetic resonance, analysis differential scanning calorimetry, and wideangle X-ray diffraction analysis. Mol. Cryst. 382(1), 97–111 (2002). https://doi.org/10.1080/713738756
Tsai, T.R., Chen, C.Y., Pan, C.L., Pan, R.P., Zhang, X.C.: Terahertz time-domain spectroscopy studies of the optical constants of the nematic liquid crystal 5CB. Appl. Opt. 42(13), 2372–2376 (2003). https://doi.org/10.1364/AO.42.002372
Sato, K., Kammori, O.: Studies of the direct dissolution of metal in a β-diketone reagent. Bull. Chem. Soc. Jpn. 42(10), 2778–2790 (1969). https://doi.org/10.1246/bcsj.42.2778
Burris, D.L., Sawyer, W.G.: Addressing Practical Challenges of Low Friction Coefficient Measurements. Tribol. Lett. 35(1), 17–23 (2009). https://doi.org/10.1007/s11249-009-9438-2
Li, J., Zhang, C., Sun, L., Luo, J.: Analysis of measurement inaccuracy in superlubricity tests. Tribol. Trans. 56(1), 141–147 (2013). https://doi.org/10.1080/10402004.2012.732200
Kalatain, E., Vilenkin, A.: Additives used in lubricating oils for foreign aviation gas turbine engines (review of patents). Chem. Technol. Fuels Oils. 10(2), 162–166 (1974). https://doi.org/10.1007/BF00717145
Bailey, C., Fodor-Csorba, K., Gleeson, J.T., Sprunt, S.N., Jákli, A.: Rheological properties of bent-core liquid crystals. Soft Matter. 5(19), 3618 (2009). https://doi.org/10.1039/b907261f
Rai, P.K., Denn, M.M., Maldarelli, C.: Interfacial tension of liquid crystalline droplets. Langmuir. 19(18), 7370–7373 (2003). https://doi.org/10.1021/la0343730
Rienäcker, G., Hess, S.: Orientational dynamics of nematic liquid crystals under shear flow. Physica A 267(3–4), 294–321 (1999). https://doi.org/10.1016/s0378-4371(98)00669-4
Forster, D.: Microscopic theory of flow alignment in nematic liquid crystals. Phys. Rev. Lett. 32(21), 1161–1164 (1974). https://doi.org/10.1103/PhysRevLett.32.1161
Mather, P.T., Pearson, D.S., Burghardt, W.R.: Structural response of nematic liquid crystals to weak transient shear flows. J. Rheol. 39(3), 627–648 (1995). https://doi.org/10.1122/1.550715
Ruths, M., Steinberg, S., Israelachvili, J.N.: Effects of confinement and shear on the properties of thin films of thermotropic liquid crystal. Langmuir. 12(26), 6637–6650 (1996). https://doi.org/10.1021/la960412e
Pleiner, H., Brand, H.: Light scattering in nematic liquid crystals in the presence of shear flow. J. Phys. Lett. 44(1), 23–31 (1983). https://doi.org/10.1051/jphyslet:0198300440102300
Nakano, K.: Scaling law on molecular orientation and effective viscosity of liquid-crystalline boundary films. Tribol. Lett. 14(1), 17–24 (2003). https://doi.org/10.1023/a:1021710131040
Ermakov, S.F., Myshkin, N.K.: Tribological properties of liquid-crystal nanomaterials. In: Liquid-Crystal Nanomaterials, pp. 21–99. Springer (2018)
Chung, D.-H., Fukuda, T., Takanishi, Y., Ishikawa, K., Matsuda, H., Takezoe, H., Osipov, M.A.: Competitive effects of grooves and photoalignment on nematic liquid-crystal alignment using azobenzene polymer. J. Appl. Phys. 92(4), 1841–1844 (2002). https://doi.org/10.1063/1.1493658
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant no. 51575285), Shandong Provincial Natural Science Foundation, China (Grant nos. ZR2016EEP15, ZR2017LEE014), and Shandong Provincial Science and Technology Development Project, China (Grant nos. 2017GGX30118, 2017GGX30136).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Xu, C., Xiao, G. et al. Effect of Running-In Induced Groove-Structured Wear and Fe(acac)3 on Ultralow Friction When Lubricating with 5CB Liquid Crystal. Tribol Lett 67, 43 (2019). https://doi.org/10.1007/s11249-019-1157-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-019-1157-8