Abstract
In the current paper, trilayered films (abridged as APS-GO/PFPE) composed of graphene oxide (GO) and perfluoropolyethers (PFPE) were fabricated successfully on the silicon substrate pre-modified with a self-assembled monolayer of 3-aminopropyl triethoxysilane. The so-prepared films were characterized by a range of complementary techniques including Raman spectroscopy, contact angle measurements, X-ray photoelectron spectroscopy, and atomic force microscopy, to reveal the surface chemical compositions and surface morphologies. Furthermore, the microtribological behavior of the so-prepared films was studied on a ball-on-plate tribometer. The APS-GO/PFPE film showed better friction-reducing and wear-resisting properties as compared with the control samples, which was ascribed to the excellent wear-resisting properties of GO and the excellent lubricating nature and low shear strength of PFPE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Graphene oxide (GO) has attracted much attention in a wide range of research areas over the recent years because of its unique structure and remarkable physical and chemical properties [1–3]. In particular, the low-cost production potential, ease of processing, remarkable mechanical strength, and good solubility in both water and organic solvents make it an attractive candidate as a thin-film lubricant suitable for the basic building block of future micro-/nanoelectromechanical systems (MEMS/NEMS) and other micro-/nanodevices that experience contact stress [3–7]. Liang et al. [8] found that the GO film fabricated via electrophoretic deposition could reduce the friction and prolong the wear life of silicon. Furthermore, GO contains a large amount of oxygenous functional groups bonded covalently onto the surface, among which hydroxyl and epoxy groups are on the basal plane and hydroxyl and carboxylic acid groups are at the edges [9, 10]. Such oxygenous functional groups enable GO sheets to be firmly grafted onto certain solid substrates. For example, it has been found that GO can be successfully assembled onto substrate surfaces covalently decorated with amine groups through reactions between epoxy and carboxyl groups with the amine groups [11]. Based on these findings, several studies have focused on the design of GO-modified films and their tribological properties [12–14]. Ou et al. [12] reported that GO covalently assembled onto a silicon surfaces exhibited good tribological properties under a low applied load due to its strong bonding to the substrate surfaces and excellent mechanical properties. Wang et al. and Ou et al. reported that multiple-alkylated cyclopentane-coated GO films or octadecyltrichlorosilane-coated GO films presented excellent tribological properties under even high applied loads [13, 14]. In other words, the tribological properties could be improved by the multilayered structures of the GO-based films even under relatively high applied loads.
Due to its superior properties, such as chemical and thermal stability, low vapor pressure, and good lubricity, perfluoropolyether (PFPE) has been a potential candidate for tribological applications in MEMS/NEMS [15, 16]. We therefore hypothesized that the combination of the strong mechanical properties of GO and the excellent lubricating nature of PFPE could result in improved friction-reducing and wear-resisting properties. For example, Kozbial et al. [17] prepared graphene/PFPE multilayer films on SiO2/Si substrates in which graphene was deposited on a Cu substrate by chemical vapor deposition first and was then transferred onto a SiO2/Si substrate. Results showed that although the friction was reduced by such a system, the wear-reducing properties were not improved. The latter was attributed to the fact that the adhesion of the grapheme at the SiO2/Si interface was weak and “debonding” at the interface occurred under the external load regardless of the lubrication conditions.
Herein, to avoid such weak interfacial interaction, GO was covalently anchored to Si substrates pre-modified by 3-aminopropyl triethoxysilane (APS). The samples were further coated with PFPE by a simple immersion/drying process. The tribological properties of these trilayered films were investigated, and it was expected that the friction-reducing and wear-resisting properties would be improved due to the combination of the excellent mechanical properties of GO and the excellent lubricating nature of PFPE.
2 Experimental Details
2.1 Materials
P-type polished single-crystal silicon (100) wafers (obtained from GRINM Semiconductor Materials Co., Beijing, China) were used as substrates. Graphite powders (purchased from Qingdao Hensen Graphite Co., China) were used for the preparation of GO. PFPE (formula HOCH2CF2O–(CF2–CF2O) m –(CF2O) n –CF2CH2OH, m and n are integers, MW3800, with the commercial name Zdol 3800) and methoxyperfluorobutane (HFE 7100) were provided by Aldrich Chem. Co. Ltd. APS (99 %) and octadecyltrichlorosilane (OTS) was purchased from Sigma-Aldrich. Multiply-alkylated cyclopentanes (MACs) were synthesized by the reaction of dicyclopentadiene with alcohols of various chain lengths [18]. All other reagents were analytical grade and were used as received. Deionized water was used for the preparation of all aqueous solutions and for rinsing.
2.2 Fabrication of the Films
First, GO was synthesized from graphite powders by the same procedure as shown in Ref. [13]. GO dispersion of 0.4 mg ml−1 was created by dispersing graphitic oxide in deionized water by ultrasonication for 30 min. Then the GO-based multilayer films were fabricated on the surface of silicon wafers through a multistep route. The silicon wafers were ultrasonicated in acetone followed by ethanol for 10 min. Then, they were hydroxylated in a freshly prepared piranha solution, a mixture of 7:3 (v/v) 98 % H2SO4 and 30 % H2O2 at 90 °C for 30 min. The piranha-treated silicon wafers were rinsed copiously with deionized water and were dried with N2 stream. These hydroxylated silicon wafers were then immersed in a freshly prepared 5 mM APS solution in mixed solvent of acetone and deionized water (the volume ratio was 5:1), and held for 30 min. These wafers were then taken out and were rinsed copiously with deionized water followed by drying in N2 stream, and the corresponding films were denoted by APS SAMs. Subsequently, the APS-assembled silicon wafers were kept in the prepared GO aqueous dispersion at 80 °C for 12 h and then ultrasonicated in deionized water and dried in N2 stream. The as-prepared films were denoted by APS-GO. At last, silicon wafers with APS-GO films and APS-assembled silicon wafers were immersed into a dilute solution of 1 mM PFPE in HFE 7100 and kept for 24 h. Then the samples were taken from the coating solution and were put in an oven maintained at 120 °C for 3 h, which allow the formation of PFPE films thereon (denoted by APS-GO/PFPE and APS-PFPE, respectively). APS-GO was dipped into MACs solution with a concentration of 0.05 % (w/v) for 120 s and withdrawn from the solution at a constant velocity to allow the formation of MACs films thereon, which were denoted by APS-GO/MACs. APS-GO was immersed into a solution of 5 mM OTS in toluene for 12 h. The sample was then heated in air at 80 °C for 1 h to facilitate the anchoring of OTS molecules, which was coded as APS-GO/OTS. All procedures mentioned above were carried out in a clean room at 20 °C and a humidity of 35 %.
2.3 Characterization of the Films
The as-prepared GO sheets were analyzed by a LabRAM HR800 Raman spectroscopy (HORIBA Jobin Yvon, France) using an Ar+ laser of 532 nm with a resolution of 1 cm−1. The thickness of the layers has been measured on a L116-E ellipsometer (Gaertner, USA), which was equipped with a He–Ne laser (632.8 nm) set at an incident angle of 50°. The results were shown in Table 1. The static water contact angles (WCA) on the samples were obtained using a DSA100 contact angle meter with a measurement error below 2° (Krüss, Germany). The water droplet volume used was about 5 μl. At least five repeat measurements were conducted in different regions on the surfaces of the films, and the averaged values were reported. The surface chemical compositions of the obtained films were examined with a PHI-5702 multifunctional X-ray photoelectron spectroscope (XPS, PerkinElmer, USA), using Mg Ka radiation as the excitation source, and the binding energy of contaminated carbon (C1s: 284.8 eV) was used as reference. The surface morphologies of the obtained films were examined with an atomic force microscope (AFM, Nanoscope IIIa, Digital Instrument, USA), using tapping scanning mode. The standard Nanoscope IIIa software was used to analyze all images.
The frictional coefficient (FC) and durability of the films were evaluated using an UMT-2MT tribometer (Bruker, USA) in the ball-on-plate reciprocating mode. Commercially available AISI-52100 steel balls with a diameter of 3 mm were used as the stationary upper counterparts, while the lower specimens were mounted onto a reciprocating table with a traveling distance of 5 mm. A sliding rate of 10 mm s−1 and the loads of 100–400 mN were used for all the measurements. The estimated contact areas and contact pressures for the various loads used were obtained using Hertz Formulas, as shown in Table 2. Plots of the FC versus time were recorded automatically, and at least three repeated measurements for each test condition were performed, and the average values were shown. The morphologies of worn surfaces were observed using a ContourGT-K0 3D noncontact interferometric microscope (Bruker, USA) after the wear test. All tests were conducted at 20 °C and at a humidity of 35 %.
3 Results and Discussion
Figure 1 shows the Raman spectrum of GO sheets on a bare Si substrate. Strong D (1354 cm−1) and G (1597 cm−1) peaks were characteristic of the sp 2 C=C network of GO [13, 19]. The G band was related to the in-plane vibration of the sp 2-hybridized carbon in a hexagonal lattice, whereas the D band corresponded to vibration of the disordered sp 3-hybridized carbon due to in-plane defects associated with vacancies and grain boundaries in GO [20, 21]. The intensity of the 2D band, which was weak and broad because of the disorder of the GO layers, originated from the two-phonon double-resonance process.
As a simple and useful tool, WCA measurement is usually used to gain insight into surface chemical components. As depicted in Fig. 2, the piranha-treated silicon substrate exhibited a low WCA value of approximately 2°, which was attributed to the abundantly generated surface hydroxyl groups. After assembling the APS onto the piranha-treated silicon substrate, the average WCA increased to 44°, which was related to the outer amine groups of APS SAM. When the GO was coated onto the APS SAM, the average WCA decreased to 34.8° due to the outer oxygenous groups on APS-GO. Once PFPE was coated onto APS-GO, the WCA values increased sharply to approximately 90°. The sharp increase suggested that the PFPE were adsorbed successfully on surface of APS-GO film, making it more hydrophobic because of the apolar –(CF2) n – (hydrophobic) groups.
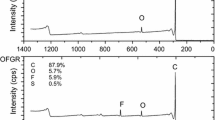
To demonstrate the actual formation of PFPE layer on GO, XPS spectra of the films were recorded, as presented in Fig. 3. Strong O1s [22] and C1s peaks were observed in the survey spectrum for the APS-GO film (Fig. 3a). C1s can be deconvoluted into four types of carbon bonds: C–C (284.8 eV), C–O (hydroxyl and epoxy groups, 286.7 eV), C=O (287.8 eV), and O–C=O (289.2 eV) [23, 24]. The wide scan spectrum from XPS for APS-GO/PFPE showed strong F1s peaks, suggesting successful deposition of PFPE molecules onto the surface of the APS-GO film (Fig. 3c). As presented in Fig. 3d, the existence of the five C1s peaks (C–C, C–O, O–C–F2, F–C–F, and O–C–F) also confirmed a successful formation of PFPE film [25–27].
In order to obtain morphological information about the fabricated films of APS-GO and APS-GO/PFPE, the surface topography was measured using AFM, as shown in Fig. 4. The GO sheets were uniformly distributed in the APS-GO film, with a root-mean-square (RMS) roughness of 1.14 nm (Fig. 4a, b). The film showed sheet-like characteristics, and some overlap of the sheets was observed. As shown in Fig. 4c, GO sheets were still faintly visible in APS-GO/PFPE film, which consisted of a fuzzy surface morphology. The phase map of the APS-GO/PFPE film is given here, as shown in Fig. 4d. It can be seen that there were sheet-like characteristics. The surface was smoother with a RMS roughness of 0.84 nm because of the PFPE coating (Fig. 4c). It demonstrates that the PFPE molecules were adsorbed uniformly on surface of the APS-GO film.
The tribological performance of the films was tested on a ball-on-plate microtribometer under different conditions, as shown in Fig. 5. The APS SAM was worn out as soon as the steel ball slid on it under a load of 100 mN (Fig. 5a). Once GO was assembled, the resulting APS-GO film exhibited improved tribological properties and had a much lower FC of 0.17 and a much longer anti-wear life (>3600 s). This may be ascribed to disordered structures of the GO sheets (Fig. 4a). In other words, during the tribological tests, overlapped GO sheets tended to glide, just like the graphite lubricant [14]. However, when the applied load rose to 200 mN (Fig. 5b), the APS-GO film showed a short lubricating life of <1600 s, suggesting that the APS-GO film was an effective lubricant film only at relatively low applied loads. Compared with the APS SAM and APS-GO films, the APS-GO/PFPE film exhibited both reduced FC values and an extending wear life at all of the experimental conditions with the FC remaining similar for all normal loads tested. It can be seen that it had both reduced FC values of 0.09 and an anti-wear life of over 1 h even under a load of 400 mN (Fig. 5d). Based on these results, it can be concluded that the PFPE outerlayer played an important role in improving the tribological behaviors. The improvement may be attributed to the excellent lubricating properties of PFPE and the bound and unbound (i.e., mobile) system of the film [15, 28]. After rinsing with HFE 7100, the thickness of the APS-GO/PFPE film (59 Å) decreased to about 46 Å (Table 1), which demonstrated that there was a bound and mobile system for the tested unrinsed APS-GO/PFPE film. The bound phase can be used as the protective interface, and the mobile phase can diffuse to a contact region where the lubricant is lost and protect it, resulting in excellent tribological properties [26, 28, 29]. However, the APS-PFPE film exhibited a higher FC and limited load-carrying capacity characterized by a short anti-wear life of 800 s, which is much shorter than that of APS-GO/PFPE film, under a load of 400 mN (Fig. 5d). The obvious improvement was ascribed to the composition difference between APS-GO/PFPE and APS-PFPE films. In other words, the sublayer of overlapped GO sheets endowed the outer layers with better load-carrying capacity.
In our previous work, the trilayered films of APS-GO/MACs and APS-GO/OTS were also fabricated and the wear resistant performance was found to be good at an applied load of 200 mN. While, in the present study, raising the applied load to 300 mN, APS-GO/MACs and APS-GO/OTS films only exhibited an antiwear life of ~1300 and 750 s, respectively (Fig. 5c), which was much shorter than that of APS-GO/PFPE. It further confirms that APS-GO/PFPE film exhibited excellent tribological properties.
Figure 6 presents the topography of the worn film surfaces under different conditions to further show the anti-wear properties of the films. All of the surface components of the visible wear tracks should be Si for that all of the thicknesses of the coated films are below 6 nm. Due to poor anti-wear ability, the silicon surface with the APS SAM film showed a deep wear groove with significant accumulation of particles at the boundaries of the wear path after sliding against the steel ball for 300 s under an applied load of 100 mN (Fig. 6a). Once a GO layer was deposited on the silicon surface, the scratch was nearly completely inhibited, even after sliding for 3600 s under 100 mN (results not shown). The scratch was still inhibited to some degree in the case of the APS-GO surface showing a shallow groove under 200 mN after sliding for 1500 s (Fig. 6b). When PFPE was coated onto the APS-GO surface, the silicon surface with the APS-GO/PFPE trilayered film (Fig. 6c) showed no wear scar even after sliding for 3600 s under 400 mN. However, there was an obvious wear path on the silicon surface with the APS-PFPE film (Fig. 6d) after sliding for 900 s under 400 mN. These results further confirmed the synergistic effect of the immobilized GO component and PFPE lubricant.
4 Conclusions
Trilayered APS-GO/PFPE films were fabricated successfully on a silicon substrate via a simple self-assembly method. Bilayered films of APS-GO and APS-PFPE and trilayered films of APS-GO/MACs and APS-GO/OTS were also prepared for comparison. The results show that, compared to control samples, the APS-GO/PFPE film provided much better friction-reducing and wear-resisting properties. This result was attributed to the synergistic effect of the immobilized GO component as the load-carrying layer and the PFPE component as the lubrication layer, resulting in the best friction-reducing and anti-wear properties. It is expected that the present study would expand the scope of GO-based lubrication films, thus having great potential in MEMS/NEMS applications.
References
Yin, Z.Y., Sun, S.Y., Salim, T., Wu, S.X., Huang, X.A., He, Q.Y., Lam, Y.M., Zhang, H.: Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes. ACS Nano 4, 5263–5268 (2010)
Zhou, M., Zhai, Y., Dong, S.J.: Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 81, 5603–5613 (2009)
Zhu, Y.W., Murali, S., Cai, W.W., Li, X.S., Suk, J.W., Potts, J.R., Ruoff, R.S.: Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924 (2010)
Dikin, D.A., Stankovich, S., Zimney, E.J., Piner, R.D., Dommett, G.H.B., Evmenenko, G., Nguyen, S.T., Ruoff, R.S.: Preparation and characterization of graphene oxide paper. Nature 448, 457–460 (2007)
Mkhoyan, K.A., Contryman, A.W., Silcox, J., Stewart, D.A., Eda, G., Mattevi, C., Miller, S., Chhowalla, M.: Atomic and electronic structure of graphene-oxide. Nano Lett. 9, 1058–1063 (2009)
Ko, J.H., Kwon, S., Byun, I.S., Choi, J.S., Park, B.H., Kim, Y.H., Park, J.Y.: Nanotribological properties of fluorinated, hydrogenated, and oxidized graphenes. Tribol. Lett. 50, 137–144 (2013)
Schlüter, B., Mülhaupt, R., Kailer, A.: Synthesis and tribological characterization of stable dispersions of thermally reduced graphite oxide. Tribol. Lett. 53, 353–363 (2014)
Liang, H.Y., Bu, Y.F., Zhang, J.Y., Cao, Z.Y., Liang, A.M.: Graphene oxide film as solid lubricant. ACS Appl. Mater. Interfaces 5, 6369–6375 (2013)
Wei, Z., Barlow, D.E., Sheehan, P.E.: The assembly of single-layer graphene oxide and graphene using molecular templates. Nano Lett. 8, 3141–3145 (2008)
Yang, H.F., Li, F.H., Shan, C.S., Han, D.X., Zhang, Q.X., Niu, L., Ivaska, A.: Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J. Mater. Chem. 19, 4632–4638 (2009)
Li, P.F., Xu, Y., Cheng, X.H.: Chemisorption of thermal reduced graphene oxide nano-layer film on TNTZ surface and its tribological behavior. Surf. Coat. Technol. 232, 331–339 (2013)
Ou, J.F., Wang, J.Q., Liu, S., Mu, B., Ren, J.F., Wang, H.G., Yang, S.R.: Tribology study of reduced graphene oxide sheets on silicon substrate synthesized via covalent assembly. Langmuir 26, 15830–15836 (2010)
Wang, Y., Pu, J.B., Xia, L., Ding, J.N., Yuan, N.Y., Zhu, Y.Y., Cheng, G.G.: Fabrication and tribological study of graphene oxide/multiply-alkylated cyclopentanes multilayer lubrication films on Si substrates. Tribol. Lett. 53, 207–214 (2014)
Ou, J.F., Wang, Y., Wang, J.Q., Liu, S., Li, Z.P., Yang, S.R.: Self-assembly of octadecyltrichlorosilane on graphene oxide and the tribological performances of the resultant film. J. Phys. Chem. C 115, 10080–10086 (2011)
Jonathan, L.Y., Harikumar, V., Satyanarayana, N., Sinha, S.K.: Localized lubrication of micromachines: a feasibility study on Si in reciprocating sliding with PFPE as the lubricant. Wear 270, 19–31 (2010)
Numata, T., Nanao, H., Mori, S., Miyake, S.: Chemical analysis of wear tracks on magnetic disks by TOF–SIMS. Tribol. Int. 36, 305–309 (2003)
Kozbial, A., Li, Z.T., Iasella, S., Taylor, A.T., Morganstein, B., Wang, Y.J., Sun, J.N., Zhou, B., Randall, N.X., Liu, H.T., Li, L.: Lubricating graphene with a nanometer-thick perfluoropolyether. Thin Solid Films 549, 299–305 (2013)
Venier, C.G., Casserly, E.W.: Lubricants comprising novel cyclopentanes cyclopenta-dienes, cyclopentenes and mixtures thereof and methods of manufacture. U.S. Patent 4, 929,782 (1990)
Li, X.S., Cai, W.W., An, J.H., Kim, S., Nah, J., Yang, D.X., Piner, R., Velamakanni, A., Jung, I., Tutuc, E., Banerjee, S.K., Colombo, L., Ruoff, R.S.: Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324, 1312–1314 (2009)
Kang, H., Kulkarni, A., Stankovich, S., Ruoff, R.S., Baik, S.: Restoring electrical conductivity of dielectrophoretically assembled graphite oxide sheets by thermal and chemical reduction techniques. Carbon 47, 1520–1525 (2009)
Ferrari, A.C., Meyer, J.C., Scardaci, V., Casiraghi, C., Lazzeri, M., Mauri, F., Piscanec, S., Jiang, D., Novoselov, K.S., Roth, S., Geim, A.K.: Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401 (2006)
Stankovich, S., Dikin, D.A., Piner, R.D., Kohlhaas, K.A., Kleinhammes, A., Jia, Y., Wu, Y., Nguyen, S.T., Ruoff, R.S.: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007)
Yang, D.X., Velamakanni, A., Bozoklu, G., Park, S., Stoller, M., Piner, R.D., Stankovich, S., Jung, I., Field, D.A., Ventrice, C.A., Ruoff, R.S.: Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and micro-Raman spectroscopy. Carbon 47, 145–152 (2009)
Xu, Y., Bai, H., Lu, G., Li, C., Shi, G.: Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 130, 5856–5857 (2008)
Miller, B.P., Theodore, N.D., Brukman, M.J., Wahl, K.J., Krim, J.: A nano-to macroscale tribological study of PFTS and TCP lubricants for Si MEMS applications. Tribol. Lett. 38, 69–78 (2010)
Eapen, K.C., Patton, S.T., Zabinski, J.S.: Lubrication of micro electromechanical systems (MEMS) using bound and mobile phases of FomblinZdol®. Tribol. Lett. 12, 35–41 (2002)
Minn, M., Satyanarayana, N., Sinha, S.K., Kondo, H.: Microtribological study of perfluoropolyether with different functional groups coated on hydrogen terminated Si. Appl. Surf. Sci. 258, 2350–2355 (2012)
Renner, D.W., Irving, D.L., Kingon, A.I., Krim, J., Padgett, C.W.: Multiscale analysis of liquid lubrication trends from industrial machines to micro-electrical–mechanical systems. Langmuir 23, 9253–9257 (2007)
Choi, J.H., Kawaguchi, M., Kato, T.: Nanoscale lubricant with strongly bonded phase and mobile phase. Tribol. Lett. 15, 353–358 (2003)
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (Grant Nos. 51102028 and 51105352) and the Priority Academic Program Development of Jiangsu Higher Education Institutions on Renewable Energy Material Science and Engineering for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Ji, H., Li, L. et al. Trilayered Film with Excellent Tribological Performance: A Combination of Graphene Oxide and Perfluoropolyethers. Tribol Lett 60, 41 (2015). https://doi.org/10.1007/s11249-015-0618-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-015-0618-y