Abstract
Mice are the most widely used mammalian animal model worldwide. Their use presents many advantages, including our ability to manipulate their genome. Unfortunately, transgenic mice often need to be introgressed to transfer the transgene of interest in a specific mouse line. This time-consuming process can be shortened using the speed congenics technique. However, the need for a panel of informative markers to evaluate the proportion of donor and receiver genomes in different individuals produced at each generation hinders the utilisation of speed congenics. In this study, we present 255 microsatellites and 10 RFLPs which can be used in 18 marker panels, allowing the easy and fast introgression of genes of interest from three mouse lines commonly used for transgenesis (C57BL/6, 129/Sv and FVB) to six mouse lines relevant for biomedical research (BALB/c, C3H, DBA/1, DBA/2, SJL and SWR/J). In addition, our markers analysis confirmed a recently described lack of isogeny in well-established inbred mouse lines available from commercial breeders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mice are the most widely used mammalian animal model worldwide. Their use presents many advantages, including our ability to manipulate their genome. Although transgenic mice are a crucial tool in biological and clinical studies, they are mostly generated in a few specific lines (C57BL/6, 129/Sv and FVB) or in mixed backgrounds, which is a drawback. This is because the two techniques traditionally used to transfer new genes into the murine genome are applied with more or less success and ease depending on the chosen mouse line. The FVB mouse line is particularly well suited for transgenic mice production via pronuclear injection, as its eggs have a large and prominent pronucleus and are remarkably resistant to lysis (Taketo et al. 1991; Auerbach et al. 2003). Additionally, commercially available embryonic stem cells derived from C57BL/6 or 129/Sv blastocysts make it relatively easy to generate transgenic chimeras by injecting modified ES cells into receiver blastocysts (Schuster-Gossler 2001).
It is well documented that the phenotype resulting from a mutation depends on the inbred strain in which it is maintained (Doetschman 2009; Heiman-Patterson et al. 2015; Li et al. 2002; Montagutelli 2000). As a consequence, most researchers will need to introgress (genetically transfer) their transgene of interest into a specific mouse line, creating a congenic line. This is traditionally accomplished by backcrossing the gene donor to the recipient strain for ten generations and selecting for progeny carrying the gene of interest at each generation. Homozygote founders are subsequently produced by intercrossing N10 individuals. The whole process takes approximately 2.5 to 3 years (Markel et al. 1997), which often limits its usefulness and feasibility, given the pace of research. Alternatively, a marker-assisted selection technique known as “speed congenics” can be applied. The speed congenics method evaluates the proportion of the genome coming from the donor and receiver strains in a series of individuals at each generation (starting from N2). The “best” individual (i.e., the individual with the highest proportion of the receiver genome) is then used as the progenitor for the next generation. This strategy enables the establishment of a congenic line in as few as five generations of backcrossing and can be realized within ~ 15 to 18 months (Markel et al. 1997, Wakeland, 1997). Moreover, Ogonuki et al. (2009) described a high-speed congenic strategy using first-wave round spermatids retrieved from immature males and injected into receiver oocytes. This technique reduces the generation time to 40 days, and the speed congenics process to approximately half a year. Grove et al. (2016) used a different approach involving the female germline. They transferred blastocysts obtained from superovulated pre-pubertal females into the uteri of pseudopregnant recipient mice. This technique reduces the generation time to 7 weeks and allows the generation of a congenic line within 10 months.
The need for a panel of informative markers to evaluate the proportion of donor and receiver genomes in different individuals produced at each generation hinders the utilisation of speed congenics. Each donor/receiver strain combination requires a different, specific set of markers.
Currently, single nucleotide polymorphisms (SNPs) are the most widely used markers. Nevertheless, most SNP-based assays for speed congenics are based on array technologies, which require specialised equipment; therefore, most research labs outsource speed congenics SNP genotyping. These arrays have the downside to be designed to genotype a fixed number of individuals at once. If this number is different from the number of offspring to test, it will result in empty wells and increase the cost per sample. A new mouse SNP genotyping assay based on high-throughput sequencing has recently been described (Andrews et al. 2021). It has the advantage of being compatible with many strain combinations commonly used for speed congenics and is more cost effective than array-based assays. However, it uses next-generation sequencers, which are not accessible for every laboratory. An alternative solution is to use another marker type like microsatellites. Microsatellites are abundant, ubiquitous, highly polymorphic, easy to automate, codominant, universal, robust, reliable and reproducible markers (Grover and Sharma 2016). Microsatellite typing is also very flexible. Once a microsatellite is homozygous for the receiver strain it can simply be removed from the assay for the following generations. The number of markers to genotype, and consequently the hands-on time and the costs, will hence decrease during the introgression process. The Mouse Genome Informatics webpage describes many murine microsatellites. Nevertheless, the generation of an informative marker panel evenly distributed throughout the murine genome might be a lengthy task, especially if it involves a less well-described mouse line, or if the donor and receiver lines are phylogenetically close.
This paper describes (1) 18 panels of informative markers that allow the introgression of genes of interest (from C57BL/6, 129/Sv or FVB to BALB/c, C3H, DBA/1, DBA/2, SJL or SWR/J); (2) pooling strategies to reduce genotyping costs; (3) analyses of the genotypes of nine commonly used mouse lines and (4) breeding recommendations to optimize mice matings.
Material and methods
Animal tissues
Tissue samples from 129S2/SvPasOrlRj, BALB/cJRj, C3H/HeNRj, C57BL/6JRj, DBA/1JRj and DBA/2JRj, as well as FVB/NRj and SJL/JRj mice, were purchased from Janvier Labs. SWR/J mice were purchased from Charles River Laboratories. SJL/JRj and SWR/J mice were housed in compliance with the National Institute of Health guidelines and with official Belgian guidelines. Same-sex, group-housed animals were housed in individually ventilated disposable cages (SealSafe Plus, Tecnilab-BMI, the Netherlands). Enrichment included nesting material (Carfil Cotton, Carfil Quality, Belgium), shelter (Carfil Dome Mini, Carfil Quality, Belgium) and Carfil ES-Brick (Carfil Quality, Belgium). Controlled laboratory conditions were: temperature (22 ± 2 °C), humidity (50 ± 10%) and a 12 h light–dark cycle, with ad libitum access to a commercial diet (Rats & Mice Maintenance, Carfil Quality, Belgium) and tap water. Animal health was assessed daily via clinical evaluation. Tissues from SJL/JRj and SWR/J mice were obtained via tail biopsy. The study was approved by the University of Liege Ethics Committee (approval number 19-2087).
Optimal marker set design simulation
We used simulations to obtain the distribution of the percentage of donor line genome in the “best” mice after a series of successive backcrosses. In this context, “best” refers to the mouse with the highest percentage of the donor line in its genome. The simulation program allowed us to specify the following:
-
The number of males (NBMALES) generated in each backcross (from which the “best” were chosen);
-
The number of backcrosses (NBBC);
-
The genetic map (MAPF) containing informative markers for the tested cross, as well as physical (in base pairs) and genetic (in recombination percentages) distances;
-
The number of simulations (NBSIMUL) to be carried out with these parameters, in order to obtain an idea of the measurement variability of the “best” individuals.
The program reported the percentage of donor genome for each individual in each backcross generation, and the “best” individual at the NBBC end. Obtaining this information from the NBSIMUL allowed obtaining a distribution of the “best” percentages. The program script is available upon request.
DNA extraction
DNA samples from 129/Sv, BALB/c, C3H, C57BL/6, FVB, DBA/1, DBA/2, SJL and SWR/J mouse tail biopsies were purified using the Nucleo Spin Tissue kit from Macherey–Nagel and quantified using a NanoDrop ND-1000.
Oligonucleotide primer design
Primers were modified from MGI or newly redesigned using Geneious 8.0.5 software to meet the following parameters: melting temperature (calculated as 4 °C*(# G/C nucleotides) + 2 °C*(# A/T nucleotides)) between 60 and 64 °C; length ranging from 20 to 23 bp; and amplicon length between 71 and 372 bp. Primers were purchased from IDT.
An exhaustive list of PCR primers is presented in Supplemental Table 1.
Microsatellite genotyping
Fluorescent PCRs were performed in a 10 μL volume with 1 × Green GoTaq®Flexi Buffer, 0.4 μM upstream and downstream primers, 200 μM of each dNTP, 1.625 mM MgCl2, 0,25 U GoTaq® Hot Start Polymerase (Promega) and 25 ng of genomic DNA. The following cycling conditions were applied: 5 min at 95 °C; 5 cycles of 30 s at 95 °C; 20 s at a temperature decreasing by 1 °C/cycle starting from 62 °C; 20 s at 72 °C; 23 cycles of 30 s at 95 °C; 20 s at 57 °C; 20 s at 72 °C; and a final extension of 10 min at 72 °C. Four to six PCR products were pooled and diluted 50 × in Hi-Di™ Formamide (Life Technologies) supplemented with 0.5 µl Red 500 DNA Size Standard (Nimagen), and then size separated using a 3730 DNA Analyzer (Applied Biosystems).
RFLP genotyping
PCRs were performed as for microsatellite genotyping using the following cycling conditions: 5 min at 95 °C; 5 cycles of 30 s at 95 °C; 20 s at a temperature decreasing by 1 °C/cycle starting from 62 °C; 20 s at 72 °C; 35 cycles of 30 s at 95 °C; 20 s at 57 °C; 20 s at 72 °C; and a final extension of 10 min at 72 °C. Restriction enzymes were purchased from New England Biolabs. Restriction digestions were performed in a total volume of 20 µl as follows: 10 µl PCR, 2 µl of CutSmart Buffer, and 1 U restriction enzyme. Digestions were performed overnight at the incubation temperature recommended by the manufacturer.
Data analysis
Genotyping results were analysed using Geneious 8.0.5 software (Dotmatics) or GeneMarker V2.7.4 software (Softgenetics). The mean number of alleles per locus, mean proportion of loci typed, mean expected heterozygosity and mean polymorphic information content (PIC) were calculated using Cervus 3.0.7 (Field Genetics Ltd) (Kalinowski et al. 2007).
Data availability statements
The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Results and discussion
Speed congenics strategy
In accordance with recommendations from the literature (Wakeland et al. 1997, Wong et al. 2002) and for practical reasons, we based our speed congenics approach on the genotyping of ten male carriers with a set of 96 markers. Indeed, it is relatively easy and fast to obtain forty offspring (of which ¼ are expected to be male and carrier) from a single male. The primers used were designed with compatible annealing temperatures; and all PCRs could conveniently be performed simultaneously in a 96-well plate.
To confirm our strategy’s validity, we performed theoretical computations to predict the percentage of recipient genome at each generation for the 18 crosses of interest, given our selected sets of markers. Results presented in Figs. 1 and 2 are based on 1000 permutations. Most importantly, we also calculated the theoretical number of backcross generations required to obtain individuals with > 99% of the receiver genome with a probability of > 99%. Hence, we confirmed that five generations of backcrossing between donor and receiver lines were sufficient to reach > 99% receiver genome (Fig. 2).
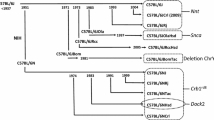
Schematic diagram illustrating the production of a congenic strain using the speed congenic method. Mean percentage recipient genome observed using a traditional congenic breeding strategy (TC) compared to percentage recipient genome of the best male carrier using a speed congenic breeding scheme (SC). For the SC, percentages were calculated based on 1000 permutations using the 129/Sv × BALB/c marker set. The red star represents the DNA region to be introgressed from the donor line to the receiver line. ♀ = female, ♂ = male
Marker development, analysis and pooling
The optimal number of markers per chromosome was chosen according to their size and varied from seven for chromosome 1 to three for chromosome 19. This resulted in a theoretical inter-marker interval ranging from 20.3 × 106 bp for chromosome 19 to 30 × 106 bp for chromosome 18. Microsatellites evenly distributed through the chromosomes were selected from MGI. Primers were either modified from MGI or newly redesigned to have similar melting temperatures, allowing for simultaneous amplification of any marker. For economic reasons, microsatellites were first PCR-amplified from the murine lines of interest with non-fluorescent primers. PCR products were then separated using a 4% agarose gel (Fig. 3) and/or the QIAxcel system (Qiagen). Microsatellites that appeared informative were subsequently amplified using a fluorescent primer and run on a 3730 DNA analyser to confirm the size difference between the lines (Table 1, Supplemental Table 2, Fig. 4). No markers were assigned to sex chromosomes, as these can be exchanged using a simple breeding strategy (Fig. 1).
In the few cases where no informative microsatellite could be found, we used restriction fragment length polymorphism (RFLP). Single nucleotide polymorphisms differing between mouse lines were retrieved from the Mouse Phenome Database at The Jackson Laboratory. Enzymes whose restriction site would be disrupted by the SNP were then uncovered using Geneious 8.0.5 software (Table 1, Supplemental Table 2).
Suggested informative marker sets for different introgressions are listed in Table 2. Markers (from Table 1 and Supplemental Table 2) were chosen to obtain the best possible coverage of each chromosome and to limit the use of RFLPs (which are more laborious to genotype). Size difference between microsatellite alleles was not taken into account, as we based our genotyping approach on using fluorescence-labelled primers. If genotyping costs were an issue, choosing a set of markers with ≥ 4 bp differences between alleles would allow genotyping on conventional agarose gels, although it would increase the hands-on time (Fig. 3).
Marker distribution uniformity across the genome varies depending on the considered cross. Hence, our marker sets, which allows introgression from 129/Sv towards BALB/c, or from C57BL/6 towards C3H, DBA/1, DBA/2 or SWR/J, comprises 96 markers covering the entire genome. In contrast, the marker set which allows introgression from FVB towards SWR/J comprises only 74 markers and leaves a few less-well-covered regions within the genome (i.e., chromosomes 3, 10, 11 and 14).
We evaluated the polymorphism of 255 microsatellites across our nine mouse lines of interest (129/Sv, BALB/c, C3H, C57BL/6, FVB, DBA/1, DBA/2, SJL and SWR/J). The successful amplification rate was 99.91%. The number of alleles per locus varied from two to eight, with a mean of 3.949. The mean expected heterozygosity was 0.6451, and the mean polymorphic information content (PIC) was 0.5582. Unexpectedly, microsatellite D14MIT257 had three alleles in FVB. This could be explained by a duplication of the region harbouring this microsatellite in this mouse line.
We also calculated the percentage of heterozygote markers in each line. The mouse lines considered in our study are all long-established inbred lines and are traditionally expected to be homozygous at virtually all of their loci (Lutz et al. 2012). Interestingly, this was not the case, as the percentage of heterozygosity varied from 0.38% for C57BL/6 and SWR to 2.26% for BALB/c (Table 3). Possible sources of heterozygosity include contamination from outcrossing, incomplete inbreeding and mutation. Chebib et al. (2021) recently used whole-genome sequencing to assess the isogeneticity of four inbred mouse lines also used in our study (C3H/HeN, C57BL/6JRj, BALB/cAnNRj and FVB/NRj). They showed that individuals within strains are not isogenic; differences in genetic variation levels are explained by differences in the genetic distance from the colony nucleus. Indeed, the more generations separating a particular mouse ordered from a commercial breeder from the nucleus, the higher the probability that mutations may have accumulated.
To keep genotyping costs as low as possible, we ran six microsatellites per polyacrylamide capillary, thus making it possible to genotype six animals on a single 96-well plate. To enable this pooling, we designed PCRs with different lengths (ranging from 71 to 372 bp) and labelled them using the two least expensive available fluorophores (fluorescein (6-FAM) and hexachlorofluorescein (HEX)). Results obtained from 3 capillaries are shown in Fig. 4 as an example. Microsatellite pooling options for the 18 considered crosses are described in Table 2 and Supplemental Table 3.
Breeding recommendations
No markers were selected on the sex chromosomes, as they could be exchanged using a simple breeding strategy. The X chromosome could be exchanged by mating males from the donor line with females from the receiver line. Conversely, the Y chromosome could be exchanged by mating females from the donor line with males from the receiver line. As it is easier to obtain many offspring from males than from females, it is recommended to use females from the donor line at the F1 generation and males from the donor line at the F2 to F5 generations (Fig. 1). Indeed, all F1 individuals have a 50% donor–50% receiver genome; therefore, ten F2 carrier males are easily generated by crossing a sufficient number of F1 females with wild-type recipient males.
To rapidly obtain ten or more male carriers from an individual male progenitor, we recommend mating him with eight to ten females of the line of interest. This is best achieved by setting up two groups of four to five females and rotating the male between these groups every week. Females should be separated from the male and housed two by two as soon as they appear to be pregnant. Female offspring should be removed at weaning, whereas male offspring should be genotyped to verify which ones carry the gene of interest. Ten male carriers should then be genotyped for the set of informative markers covering the entire genome. The male with the highest proportion of receiver genome should be used to father the next generation. Notably, we recommend keeping the second and third best male carriers as backups in case the best male is unable to produce pups.
Scope of application
The use of speed congenics has decreased in recent years following the emergence of the clustered regularly interspaced short palindromic repeats/Cas9 (CRIPR/Cas9) system. This cutting-edge technology is used in many facets of life sciences, amongst others, to rapidly generate transgenic mice in virtually any genetic background. While speed congenics can be realized within 15–18 months, it takes only 8 months to generate a knock-in mice using Crispr/Cas9 (Liu et al. 2017). At present, it is often faster and cheaper to replicate a mutation in another mouse strain thanks to this new genome editing technique than to use speed congenics.
Nevertheless, while the use of the CRIPR/Cas9 system has transformed and eclipsed traditional transgenic technologies, restrictions remain. The main one is the limitation of the size of the DNA construct that can easily be introduced in the mouse genome (~ 5 kb) (Gurumurthy and Lloyd 2019; Qin and Wang 2020). Hence, traditional production of transgenic mice (by pronuclear injection or by injecting modified ES cells into receiver blastocysts) followed by introgression in the line of interest is still the gold standard for larger genes or when it is important to maintain intronic regions and regulatory elements.
Conclusions
We presented 255 microsatellites and 10 RFLPs which can be used in 18 marker panels to allow easy and fast introgression of genes of interest from three mouse lines commonly used for transgenesis (C57BL/6, 129/Sv and FVB) to six mouse lines relevant for biomedical research (BALB/c, C3H, DBA/1, DBA/2, SJL and SWR/J). In addition, our genotyping method was designed to allow simultaneous amplification of all markers to reduce required hands-on time and pooling different markers to reduce costs.
Analysis of our markers confirms a recently described lack of isogeny in well-established inbred mouse lines available from commercial breeders.
References
Andrews K, Hunter S, Torrevillas B, Céspedes N, Garrison M, Strickland J, Wagers D, Hansten G, New D, Fagnan M, Luckhart S (2021) A new mouse SNP genotyping assay for speed congenics: combining flexibility, affordability, and power. BMC Genom 22:378
Auerbach AB, Norinsky R, Ho W, Losos K, Guo Q, Chatterjee S, Joyner AL (2003) Strain-dependent differences in the efficiency of transgenic mouse production. Transgenic Res 12:59–69
Chebib J, Jackson B, López-Cortegano E, Tautz D, Keightley P (2021) Inbred lab mice are not isogenic: genetic variation within inbred strains used to infer the mutation rate per nucleotide site. Heredity (edinb) 126:107–116
Doetschman T (2009) Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol 530:423–433
Grove E, Eckardt S, McLaughlin KJ (2016) High-speed mouse backcrossing through the female germ line. PLoS ONE 11(12):e0166822
Grover A, Sharma PC (2016) Development and use of molecular markers: past and present. Crit Rev Biotechnol 36:290–302
Gurumurthy CB, Lloyd KCK (2019) Generating mouse models for biomedical research: technological advances. Dis Models Mech 12(1):dmm029462
Heiman-Patterson TD, Blankenhorn EP, Sher RB, Jiang J, Welsh P, Dixon MC, Jeffrey JI, Wong P, Cox GA, Alexander GM (2015) Genetic background effects on disease onset and lifespan of the mutant dynactin p150Glued mouse model of motor neuron disease. PLoS ONE 10:e0117848
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106. https://doi.org/10.1111/j.1365-294X.2007.03089.x
Li J, Ortiz LA, Hoyle GW (2002) Lung pathology in platelet-derived growth factor transgenic mice: effects of genetic background and fibrogenic agents. Exp Lung Res 28:507–522
Liu ET, Bolcun-Filas E, Grass DS, Lutz C, Murray S, Shultz L, Rosenthal N (2017) Of mice and CRISPR: the post-CRISPR future of the mouse as a model system for the human condition. EMBO Rep 18(2):187–193
Lutz C, Linder CC, Davisson MT (2012) Strains, stocks, and mutant mice. In: Hedrich HJ (ed) The laboratory mouse, 2nd edn. Elsevier Science, London, UK, pp 37–50
Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, Moore KJ (1997) Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet 17(3):280–284
Montagutelli X (2000) Effect of the genetic background on the phenotype of mouse mutations. J Am Soc Nephrol Suppl 16:S101-105
Ogonuki N, Inoue K, Hirose M, Miura I, Mochida K, Sato T, Mise N, Mekada K, Yoshiki A, Abe K, Kurihara H, Wakana S, Ogura A (2009) A high-speed congenic strategy using first-wave male germ cells. PLoS ONE 4:e4943
Qin W, Wang A (2020) Generating mouse models using CRISPR/Cas9. Addgene. https://blog.addgene.org/generating-mouse-models-using-crispr/cas9 Accessed 28 July 2023
Schuster-Gossler K, Lee AW, Lerner CP, Parker HJ, Dyer VW, Scott VE, Gossler A, Conover JC (2001) Use of coisogenic host blastocysts for efficient establishment of germline chimeras with C57BL/6J ES cell lines. Biotechniques 31:1022–1026
Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, Overbeek PA (1991) FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A 88:2065–2069
Wakeland E, Morel L, Achey K, Yui M, and Longmate J. (1997) Speed congenics: A classic technique in the fast lane (relatively speaking). Immunol Today 18:472–477
Wong GT (2002) Speed congenics: applications for transgenic and knock-out mouse strains. Neuropeptides 36:230–236
Acknowledgements
The authors wish to thank the GIGA Genomics Plateform (ULiege) for technical assistance, and Dr. Fabien Ectors for valuable discussions.
Funding
This research was funded by Laboratoires Prevor, Moulin de Verville.
Author information
Authors and Affiliations
Contributions
AV and DD initiated the project and designed the study; AV, AT and LD performed the laboratory work; FF wrote the script for the optimal marker set design- simulation; AV and AT analysed the results; AV wrote the original draft; DD and JB reviewed the draft, JB acquired the fundings. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The study was approved by the University of Liege Ethics Committee (Approval Number 19-2087).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Laere, AS., Tromme, A., Delaval, L. et al. A timely, user-friendly, and flexible marker-assisted speed congenics method. Transgenic Res 32, 451–461 (2023). https://doi.org/10.1007/s11248-023-00365-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-023-00365-7