Abstract
RNA interference (RNAi)-based host-induced gene silencing (HIGS) is emerging as a novel, efficient and target-specific tool to combat phytonematode infection in crop plants. Mi-msp-1, an effector gene expressed in the subventral pharyngeal gland cells of Meloidogyne incognita plays an important role in the parasitic process. Mi-msp-1 effector is conserved in few of the species of root-knot nematodes (RKNs) and does not share considerable homology with the other phytonematodes, thereby making it a suitable target for HIGS with minimal off-target effects. Six putative eggplant transformants harbouring a single copy RNAi transgene of Mi-msp-1 was generated. Stable expression of the transgene was detected in T1, T2 and T3 transgenic lines for which a detrimental effect on RKN penetration, development and reproduction was documented upon challenge infection with nematode juveniles. The post-parasitic nematode stages extracted from the transgenic plants showed long-term RNAi effect in terms of targeted downregulation of Mi-msp-1. These findings suggest that HIGS of Mi-msp-1 enhances nematode resistance in eggplant and protect the plant against RKN parasitism at very early stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant-parasitic nematodes (PPNs) impose a substantial economic burden on global agriculture and horticulture amounting to approximately $173 billion annual yield losses (Elling 2013). Additionally, changes in cultivation practices for efficient water utilization and global climate change result in the emergence of PPN problems in newer crops and geographical localities (Dutta et al. 2019). PPNs are among the most difficult crop pests to manage, considering that the most damaging ones such as root-knot (RKN: Meloidogyne spp.) and cyst (Heterodera and Globodera spp.) nematodes reside within the host plant for the majority of their life cycle (Palomares-Rius et al. 2017). Post hatching, second-stage juveniles (J2s) of Meloidogyne spp. invade the plant root and induce the formation of a hypermetabolic feeding cell (or giant cell, GC) in the vascular tissue for continuous nourishment of subsequent life stages until they attain the reproductive maturity (Mitchum et al. 2013). Cells are hypertrophied around the GCs to form galls that ultimately hinder the normal root growth and affect nutrient supply to distal plant tissues (Moens et al. 2009). The esophageal gland cells of RKN synthesize a repertoire of effector proteins that are secreted via the nematode stylet into the host cells. Effectors play essential roles in host penetration, GC establishment and maintenance, and suppression of host defence responses (Hewezi and Baum 2013).
The conventional PPN management practices heavily relied on the use of chemical nematicides, basic crop rotations and resistant crop cultivars (Dutta et al. 2019). However, many of the frontline nematicides are continually being withdrawn from market via governmental regulations owing to their non-specific and toxic effect on the environment. Considering that crop rotations are not effective to manage polyphagous RKNs, the landscape of global crop production is still under threat from PPNs due to the absence of effective, environmentally-friendly management tactics. Because of the limited availability of candidate resistance genes and sterility of certain crops impairing the progress of conventional breeding, a transgenic approach of nematode resistance appears to be a compelling alternative (Roderick et al. 2018). In view of this, a number of studies have shown that PPNs are susceptible to RNA interference (RNAi) and host-induced gene silencing (HIGS) strategy involving in planta generation of PPN gene-specific, double-stranded RNA (dsRNA) holds great promise (reviewed in Lilley et al. 2012; Dutta et al. 2015a). HIGS strategy has shown its utility by resisting the establishment and maintenance of PPN infection in different crop plants (Huang et al. 2006; Sindhu et al. 2009; Papolu et al. 2013; Dutta et al. 2015b; Shivakumara et al. 2017). The HIGS strategy potentially minimizes regulatory hurdles as it does not involve any novel protein/peptide expression thus reinforcing its native biosafety (Roderick et al. 2018).
The major concern in adopting HIGS strategy is the possibility of off-target effect of RNAi. Since the mechanism of RNAi occurs in a sequence-specific manner, endogenous transcripts having identity to the siRNAs (small interfering RNAs) produced from the introduced dsRNAs may cause silencing of non-target genes in non-targeted organisms (Rosso et al. 2009; Dutta et al. 2015a). To this end, PPN effectors serve as the candidate genes for HIGS strategy because effectors lack homology with the genes of organisms that belong to other taxa (Danchin et al. 2013; Dutta et al. 2015a). Earlier, a number of Meloidogyne incognita-specific effector genes were identified via bioinformatics mining of RKN esophageal gland cell microaspirated products (Huang et al. 2003, 2004). Next, using HIGS strategy, several of these genes including Mi-msp-9 (Xue et al. 2013), Mi-msp-12 (Xie et al. 2016), Mi-msp-16 (Huang et al. 2006; Yang et al. 2013; Dinh et al. 2014), Mi-msp-18, Mi-msp-20 (Shivakumara et al. 2017) and Mi-msp-40 (Niu et al. 2016), were shown to play decisive role in RKN parasitic success in different host plants.
The effector gene, Mi-msp-1 (synonymised with Mi-vap-1; Ding et al. 2000) belongs to venom allergen-like protein (VAP, homolog of plant and animal cysteine-rich secretory proteins) family which are highly transcribed during the parasitic process of PPNs (Gao et al. 2001; Wang et al. 2007; Lozano-Torres et al. 2014; Duarte et al. 2017; Luo et al. 2018). Recently, we have demonstrated the role of Mi-msp-1 in early parasitic stages of M. incognita specifically in pre- and post-parasitic J2s; Mi-msp-1 expression was localized to the subventral esophageal glands of pre-parasitic J2. In addition, using RNAi soaking experiments we showed that Mi-msp-1 is crucial for RKN infectivity and pathogenicity (Chaudhary et al. 2019). In the present study, we used Mi-msp-1 as a candidate in HIGS to manage RKN infectivity in eggplant.
Materials and methods
Bioinformatics
To interrogate the MSP-1 homologs in related species, BLASTp and tBLASTn algorithms were performed against non-redundant protein sequence database in NCBI and nematode genomic and transcriptomic sequence databases at http://www6.inra.fr/meloidogyne_incognita/ and http://parasite.wormbase.org/Meloidogyne_hapla_prjna29083/ using the Mi-msp-1 protein sequence as the query with expect value threshold of 100. The derived protein sequences were aligned using Clustal Omega tool (http://www.ebi.ac.uk/Tools/msa/clustalo) with default parameters. Signal peptides for secretion were predicted using SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/). Transmembrane domains were predicted using Phobius server (http://phobius.sbc.su.se/). Conserved domains within the MSP-18 homologs were investigated in InterProScan (https://www.ebi.ac.uk/interpro/) and SMART (http://smart.embl-heidelberg.de/). Motif database algorithm (http://molbiol-tools.ca/Motifs.htm) was used to predict common motifs. Primers to amplify the targeted dsRNA of corresponding Mi-msp-1 gene were designed using OligoAnalyzer tool (https://eu.idtdna.com/). Potential off-target sites in the targeted dsRNA were investigated at http://dsCheck.RNAi.jp/. RT-qPCR (reverse transcription quantitative PCR) primers to detect transgene expression were designed using OligoAnalyzer tool.
Nematode culturing
A pure culture of M. incognita race 1 was multiplied on eggplant (Solanum melongena cv. Pusa Purple Long) in a greenhouse. Egg masses were extracted from the galled roots of eight-week-old plant using sterilized tweezers and were kept for hatching in a modified Baermann assembly (Southey 1986). Freshly hatched J2s were used for further experiments. For RT-qPCR studies, different post-parasitic life stages of RKN were carefully separated from the infected root using sterilized tweezers under the microscope.
Cloning of Mi-msp-1 from M. incognita
Total RNA (400 ng) was extracted from pre-parasitic J2s and reverse-transcribed to cDNA using random primers (SuperScript VILO, Invitrogen) as depicted previously (Shivakumara et al. 2016). The target sequence of Mi-msp-1 was PCR amplified from the cDNA using high-fidelity Platinum Taq DNA polymerase enzyme (Thermo Fisher Scientific) by following the manufacturer’s instructions. Amplified fragment was cloned into pGEM-T vector (Promega) and the identity of the insert was ascertained by Sanger sequencing.
Development of Mi-msp-1 RNAi construct for HIGS
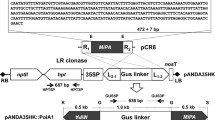
The RNAi Gateway ready pK7GWIWG2(I) binary vector was obtained from VIB-UGent Center for Plant Systems Biology, Ghent University, Ghent, Belgium. The 515 bp sequence of Mi-msp-1 flanked by attB1 and attB2 recombination sites were PCR amplified from the pGEM-T clone and sub-cloned into an entry vector, pDONR221 (Invitrogen) prior to cloning in the destination vector, pK7GWIWG2(I) in sense and antisense orientation connected by an intron (Shivakumara et al. 2017). Primer details are provided in Supplementary Table 1. The recombinant pK7GWIWG2(I) containing the hairpin RNA of Mi-msp-1 (Supplementary Figure 1) was transformed into competent Agrobacterium tumefaciens (LBA4404) cells by electroporation and positive clones were selected via screening against antibiotic (kanamycin) resistance by following the protocol described previously (Papolu et al. 2013; Dutta et al. 2015b).
Generation of transgenic plants
Surface-sterilized (by 70% ethanol and 1% NaOCl) eggplant (cv. Pusa Purple Long) seeds were germinated on Murashige and Skoog (MS) agar (HiMedia), pH 5.8. 1 cm2 cut leaf explants of 15-day-old seedling were infected with A. tumefaciens (LBA4404) transformants harboring the Mi-msp-1 hairpin RNA. Pre-cultivation and co-cultivation of explants were performed as described earlier (Papolu et al. 2013; Dutta et al. 2015b; Supplementary Figure 2). Rooting was induced in the explants by supplementing the MS agar with 0.1 mg/L NAA (Sigma-Aldrich). Plants with hardened roots were transferred to the greenhouse facility at the host institute till the production of seeds of primary events (T0). Explants co-cultured with A. tumefaciens harboring the empty construct (that underwent no antibiotic selection) were used as the control.
Detection of Mi-msp-1 transgene via PCR and Southern hybridization
Genomic DNA was extracted from the harvested leaves of T0 events using a Nucleospin plant II DNA isolation kit (Macherey–Nagel) by following the manufacturer’s protocol. Putative transformants were confirmed initially via PCR using Taq DNA polymerase (Thermo Fisher Scientific) with specific primer pairs (enlisted in Supplementary Table 1) followed by electrophoresis of the amplified products in 1% agarose gel. In order to investigate the integration pattern and copy number of Mi-msp-1 transgene in PCR-positive T0 events, Southern blot hybridization was performed. Genomic DNA (10 µg) of each event was digested with SacI (New England Biolabs; cuts once in the T-DNA), resolved on 0.8% agarose gel followed by transblotting to a nitrocellulose membrane (Bio-Rad). The 515 bp PCR product of Mi-msp-1 was used as the probe. Probe labelling, hybridization and blot development was performed as previously described (Papolu et al. 2013; Dutta et al. 2015b).
Detection of Mi-msp-1 transgene expression using RT-qPCR
In order to increase homozygosity in subsequent events, PCR-confirmed T0 plants were self-pollinated and T1 seeds were obtained. Surface-sterilized T1 seeds were germinated in MS agar supplemented with kanamycin (100 mg/L) and transferred to pots containing autoclaved soil. To analyze the Mi-msp-1 transcript abundance in T1 plants, RT-qPCR was performed with three independent biological and three technical replicates. Total RNA was extracted from harvested leaves of PCR-confirmed T1 plants using a Nucleospin plant II RNA isolation kit (Macherey–Nagel) followed by reverse-transcription to cDNA using a cDNA sysnthesis kit (Superscript VILO, Invitrogen). RT-qPCR experiment was conducted in Realplex2 thermal cycler (Eppendorf) with 10 µl reaction mixture containing 1.5 ng cDNA, 750 nM of each primer and 5 µl SYBR Green PCR master-mixes (Eurogentec). The amplification reaction conditions and melt curve program were followed as previously described (Shivakumara et al. 2016). Ct (cycle threshold) values were imported from Realplex2 software (Eppendorf). Mi-msp-1 expression in different transgenic lines were calculated as average Δct values—the difference between the ct mean of transgene and normalizer gene, 18S rRNA of eggplant (Papolu et al. 2016; Shivakumara et al. 2017). Primer details are documented in Supplementary Table 1.
Detection of Mi-msp-1 integration loci in transgenic eggplant
In order to analyse the T-DNA integration sites in the transformed plants, Mi-msp-1 integration and flanking sequences in different T2 events (obtained via selfing of homozygous T1 plants) were identified via genome walking (Universal genome walker 2.0, Clontech laboratories). Two µg genomic DNA (extracted from harvested leaves of T2 plants) was used as the template in PCR reaction (catalysed by Platinum Taq DNA polymerase, Thermo Fisher Scientific) with T-DNA-specific primers (listed in Supplementary Table 1). PCR products were cloned and sequence verified as explained above. Obtained sequences were analysed in NCBI-BLAST and Sol Genomics Network (http://www.solgenomics.net/). Further, event-specific PCR was carried out to validate the specific integration sites in progeny plants. Primer details are listed in Supplementary Table 1.
Resistance evaluation of transgenic eggplant against M. incognita
Homozygous T1, T2 and T3 lines (obtained via selfing of homozygous T2 plants) harbouring the HIGS construct of Mi-msp-1 were screened for resistance against RKN. The roots of a 30-day-old plant growing in 250 ml pots (containing equal proportion of soil and soilrite; Keltech Energies Ltd.) were inoculated with approximately 500 RKN J2s near the root zone. Infected plants were grown in a growth chamber at 27 ± 2 °C, 60% relative humidity and 16:8 h light:dark photoperiod. At 30 dpi (days post inoculation), plants were harvested, roots were washed carefully and different agronomic characters such as root and shoot weight were recorded along with the control plants. Nematode parasitic success was documented in terms of number of galls, egg masses, eggs/egg mass and multiplication factor (MF) as explained in Papolu et al. (2013, 2016) and Dutta et al. (2015b). Each of the transgenic lines contained at least 12 plants and the experiment was repeated twice.
Additionally, at 2 and 7 dpi, representative plants from each line were harvested and roots were stained with acid fuchsin (Shivakumara et al. 2017) to analyse the RKN early infection ability on transformed plants.
Transcription analysis of Mi-msp-1 in RKNs extracted from transgenic plants
Total RNA was extracted from post-parasitic J2 (at 4 dpi), J3/J4 (15 dpi) and females (25 dpi) feeding on the selective T3 plants and reverse-transcribed to cDNA as described above. RT-qPCR was performed and fold change in expression of Mi-msp-1 gene was calculated by augmented comparative ct method and log10-transformed (Shivakumara et al. 2017). 18S rRNA of M. incognita was used as the normalizer gene. Primer details are given in Supplementary Table 1.
Statistical analysis
Data were checked for normality and compared using one-way ANOVA followed by Tukey’s HSD test in SAS statistical package. Statistical comparisons were made among different treatments or compared individually to controls, as stated in the corresponding figure legends.
Results
Mi-msp-1 target identification for generating HIGS constructs
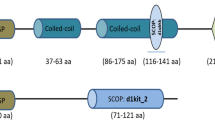
The protein homology search of Mi-msp-1 (Genbank Accession No. AAD01511) in the NCBI non-redundant database using BLASTp revealed 16 (M. hispanica), 31 (Bursaphelenchus mucronatus), 32 (Heterodera glycines, Globodera rostochiensis), 33 (Ditylenchus destructor) and 38 (H. avenae) percent identity with the corresponding proteins of different PPNs (query coverage ranged from 87 to 94%). Mi-msp-1 showed 34% identity with the SCP (secretory cysteine-rich protein)-like extracellular protein of Caenorhabditis elegans (93% query coverage). Intriguingly, when interrogated via BLAST-based homology in Meloidogyne genomic resources (http://www6.inra.fr/meloidogyne_incognita/) and M. hapla genome in Wormbase Parasite (http://parasite.wormbase.org/Meloidogyne_hapla_prjna29083/) Mi-msp-1 exhibited 66, 95 and 97% similarity to M. hapla (MhA1_Contig481), M. arenaria (Mare1s19144g093341) and M. javanica (Mjav1s01723g017848), respectively. This indicates that Mi-msp-1 is highly conserved among some of the RKN species. Mi-msp-1 contains the SCP-like conserved domain at position 27–190 aa (amino acids) within which allergen V5 domain is predicted at position 155–165 aa (Fig. 1a). Mi-msp-1 contains the predicted N-myristoylation (at position 52–57, 130–135, 193–198, 209–214 aa), N-glycosylation (85–88, 110–113 aa) and tyrosine kinase phosphorylation (56–58, 94–101 aa) motifs.
Mi-msp-1 target identification for generating dsRNA constructs for HIGS. a Multiple sequence alignment of MSP1 orthologs from four Meloidogyne species. Asterisk and colon signs indicate conserved and similar amino acids, respectively. The blue box indicates predicted signal peptides (SPs, 1-21 aa) except M. hapla for which no SP was predicted. The green box indicates extracellular cysteine-rich secretory protein (SCP, 27-190 aa) domain. Underlined (with arrow) regions indicate venom allergen 5 domain (155–165 aa). (B) Illustration of Mi-msp-1 (Accession No. AF013289) open reading frame (49–900 bp) and the conserved regions for which the dsRNA (indicated as two-headed arrows) was generated in this study have homology. Numbers indicate the position of nucleotides. Green and blue boxes indicate position of SP and SCP-like domain respectively. Box with diagonal pattern indicates allergen V5 domain. (Color figure online)
A 515 bp sequence (spanning between 128 and 642 bp of Mi-msp-1, Accession No. AF013289) containing the conserved SCP-like domain was identified as the target sequence for HIGS construct development (Fig. 1b). The target dsRNA sequence was queried in dsCheck database (http://dsCheck.RNAi.jp/; Naito et al. 2005) to identify the potential off-target sites. No exact match for processed siRNAs (19 nucleotides) of the target dsRNA in the existing database of C. elegans, Drosophila melanogaster, Rattus norvegicus, Oryza sativa and Arabidopsis thaliana could be found. In addition, no nucleotide match for Mi-msp-1 dsRNA was found in eggplant genome server (http://www.solgenomics.net/).
Molecular characterization of transgenic eggplant harbouring HIGS construct of Mi-msp-1
Using leaf-disc co-culture method, A. tumefaciens (LBA4404) containing Mi-msp-1 RNAi construct was transferred to eggplant (Supplementary Figure 2). The regenerated 17 events (T0) that survived against kanamycin selection were genotyped via PCR. The presence of Mi-msp-1, its orientation within the T-DNA (sense or antisense), and nptII (Neomycin phosphotransferase, corresponds to kanamycin resistance) marker fragment was detected in 14 events (Supplementary Figure 3). No significant difference in the root and shoot weight of control and transgenic plants could be found (Supplementary Figure 4), indicating that neither the antibiotic resistance gene nor the RNAi construct introgression affected the growth of transgenic plants.
The PCR-confirmed 14 T0 lines were subjected to Southern hybridization to analyze the integration pattern of Mi-msp-1 transgene. Single copy insertion of Mi-msp-1 was documented in line numbers 2, 3, 4, 6, 13 and 14, while double or multiple copy insertion was observed in line numbers 8, 9, 10, 11 and 12. No or very faint hybridization signal was detected in line numbers 1, 5 and 7 (Fig. 2a). Only the single copy events harboring the RNAi construct were used for further study.
Southern blot for a T0 and b T2 eggplant transformants harboring the HIGS construct of Mi-msp-1. Genomic DNA extracted from untransformed/empty vector control (UC) plants did not show any hybridization signal. Positive control (PC) indicates the probe used for hybridization was specific to Mi-msp-1 gene. M: Lambda HindIII digest marker. Lanes 1–14: DNA samples from different T0 events
T1 progeny plants were generated by selfing the selected T0 lines in the greenhouse. T1 lines were genotyped through PCR. At least five plants per event were tested. Gene-specific, sense, antisense fragments, as well as nptII, were detected in all the events (Supplementary Figure 5). Homozygous T1 lines were selfed to generate T2 plants. Accordingly, T3 plants were generated by selfing of homozygous T2 plants. PCR-based genotyping of T2 and T3 lines indicated the amplification of expected fragments in all the events tested (data not shown). Using Southern hybridization single copy integration of Mi-msp-1 was confirmed in progeny plants of selected T2 lines, namely 2.1, 3.3, 4.2, 6.2, 6.3, 13.1 and 14.2 (Fig. 2b). Hence, Mi-msp-1 RNAi transgene was stably integrated and inherited in the progeny plants.
RT-qPCR assay indicated the overexpression of Mi-msp-1 transcripts in all the selected T1 lines. On the contrary, no Mi-msp-1 expression was detected in the RNA isolated from control plants. Therefore, Mi-msp-1 expression data are simply presented as Δct relative to the ct of 18S rRNA. As ct value is inversely proportional to quantitative expression value, significantly (P < 0.05) greater expression of Mi-msp-1 was recorded in line numbers 6.2, 13.1 and 14.2, compared to line numbers 2.1, 3.3 and 4.2 (Fig. 3). Similar results were obtained with T2 and T3 lines as well (data not shown).
Detection of Mi-msp-1 expression in T1 eggplant transformants by RT-qPCR. Relative transcript levels of Mi-msp-1 are expressed as Δct values which denote the difference in ct mean of transgene and the reference gene (18S rRNA of eggplant). Higher Δct values indicate the lower expression of transgene in the corresponding event. Each bar represents mean ± SEM derived from three independent biological and three technical replicates. Bars with different letters are statistically different at P < 0.05, Tukey’s HSD test
Assessment of Mi-msp-1 integration loci in transgenic eggplant
In order to characterize the T-DNA insertion sites in selective T2 events, event numbers 6.2 and 14.2 (these lines exhibited greatest expression of Mi-msp-1 transgene RT-qPCR assays) were evaluated via genome walking. Fragments flanking the T-DNA were detected in the progeny plants of events 6.2 and 14.2 using event-specific primers. The absence of these fragments in other selected events suggested the independent integration of T-DNA in events 6.2 and 14.2 (Supplementary Figure 6). Sequences flanking the T-DNA region showed homology with the genome sequence of eggplant (http://www.solgenomics.net/). The sequence of the T-DNA flanking PCR product corresponding to events 6.2 and 14.2 showed 100% (Sequence ID: Sme2.5_00538.1) and 98.34% (Sequence ID: Sme2.5_04649.1) identity with the S. melongena draft genome sequence, respectively.
Evaluation of eggplant transformants for resistance against M. incognita
Six independent T1 events (2.1, 3.3, 4.2, 6.2, 13.1 and 14.2) harbouring the Mi-msp-1 RNAi construct were tested for resistance against RKN to assess the HIGS effect of the target gene. At 30 dpi, the average numbers of galls per plant was significantly (P < 0.01) reduced by 33.78–46.92% in transgenic lines compared to control plants harbouring the empty vector. Accordingly, the number of egg masses was reduced by 41.05–51.16% in all RNAi lines compared to control plants (Fig. 4; Supplementary Table 2). As adult RKN females produces its progeny in a single egg mass, the number of egg masses implies the similar number of reproducing females. Therefore, HIGS of Mi-msp-1 resulted in the reduced galling of roots presumably because attenuated development of RKN J2 to females in transgenic lines. In corroboration, RKN fecundity in terms of number of eggs per egg mass was markedly (P < 0.01) decreased by 36.08–41.20% in RNAi lines than control. Consequently, the calculated multiplication factor (MF, indicative of nematode reproductive fitness and parasitic success on a given host plant) of RKN was substantially (P < 0.01) reduced by 62.37–70.62% in transgenic lines in comparison to the control plants (Fig. 4; Supplementary Table 2). Within the T1 events no significant (P > 0.01) difference in RKN parasitism was documented. However, within the T2 and T3 lines, events 14.2 and 13.1 showed significantly (P < 0.01) greatest and least reduction in MF, respectively (Supplementary Figure 7). Overall, the level of resistance in T1 lines was comparable in both T2 and T3 lines (Supplementary Table 2; Supplementary Figure 7). This suggests the no resistance breakdown in progeny plants of Mi-msp-1 RNAi lines.
Effect of HIGS of Mi-msp-1 on development and reproduction of M. incognita in eggplant a Absolute numbers of galls induced, egg masses, eggs per egg mass and the respective MF (multiplication factor) of M. incognita in different T1 events (2.1, 3.3, 4.2, 6.2, 13.1 and 14.2) and control plants harboring empty vector are shown at 30 dpi. Each bar represents the mean ± SE of n = 12, and bars with different letters (within each parameter) indicate a significant difference at P < 0.01, Tukey’s HSD test. b Photograph shows that intensity of galling was higher in control plants than T1 event number 14.2 which had comparatively higher root mass. Scale bar = 5 cm
In addition, perturbed infectivity of RKN due to HIGS effect of Mi-msp-1 in early parasitic stages of nematodes was documented. Significantly (P < 0.01) less penetrating J2 (at 2 dpi) and spike-tail J3/J4 stages (at 7 dpi) were observed in different T1 lines (2.1, 3.3, 4.2, 6.2, 13.1 and 14.2) compared to control plants (Fig. 5). This documented the HIGS effect of Mi-msp-1 in early parasitic stages of RKN.
Penetration and development of M. incognita in eggplant T1 lines (2.1, 3.3, 4.2, 6.2, 13.1 and 14.2) harboring Mi-msp-1 HIGS construct and control plants containing empty construct. Nematodes were stained with acid fuchsin at 2 (as post-parasitic J2) and 7 (as spike-tail stages) dpi. Each bar represents the mean ± SE of nematode numbers in each plant (n = 12), bars with different letters indicate a significant difference at P < 0.01, Tukey’s HSD test. Lower panel shows the stained nematodes in control and transgenic lines. Scale bar = 500 µm
Expression analysis of Mi-msp-1 in nematodes extracted from transgenic eggplant
In order to analyse the long-term effect of HIGS on suppressing the target transcripts in feeding worms, RT-qPCR was performed in post-parasitic J2, J3/J4 and young females of M. incognita extracted from the roots of different T3 lines (2.1, 3.3, 4.2, 6.2, 13.1 and 14.2). The mRNA levels of Mi-msp-1 in post-parasitic J2s were significantly (P < 0.01) downregulated by 2.5 ± 0.35 to 5.5 ± 0.5 fold in specimens exposed to transgenic lines, compared to worms extracted from non-transgenic control plants. Similarly, Mi-msp-1 transcripts were significantly (P < 0.01) attenuated by 1.1 ± 0.3 to 3.5 ± 0.7 fold in J3/J4 stages in transgenic lines compared to control. Consequently, a significant (P < 0.01) suppression of Mi-msp-1 by 1 ± 0.35 to 2.5 ± 0.55 fold was recorded in T3 lines compared to control (Fig. 6). Our results suggest that dsRNA/siRNA molecules corresponding to Mi-msp-1 gene were ingested by M. incognita during the early parasitic process.
Effect of HIGS on the transcript abundance of Mi-msp-1 in M. incognita post-parasitic stages extracted from the roots of eggplant T3 events (2.1, 3.3, 4.2, 6.2, 13.1 and 14.2). Using 18S rRNA gene of M. incognita as reference, expression of Mi-msp-1 was quantified via augmentative comparative ct method and fold change values were log10-transformed. Bars represent mean expression level ± SE of three biological and three technical replicates. Asterisks indicate significant differential expression (P < 0.01) in comparison with the worms extracted from non-transgenic control plants. In lower panel photomicrographs show different nematode stages, i.e. post-parasitic J2 (post J2), J3/J4 and young females (youngF) extracted at 4, 15 and 25 dpi, respectively. Scale bar = 500 µm
Discussion
RNAi is a nematode management approach that can replace the highly toxic and less specific chemical-based PPN management strategies (Roderick et al. 2018). The present work identifies the potential of a RKN-specific parasitism gene, Mi-msp-1, in conferring nematode resistance in eggplant using HIGS approach. Nevertheless, a possibility of off-target effect on a wide range of organisms in the rhizosphere due to the higher degree of sequence conservation among the target transcripts cannot be avoided while adopting this technique if the design of dsRNA molecule is improper. In view of this, a 515 bp sequence of Mi-msp-1 (containing the SCP-like domain that codes for VAP proteins) was identified as the dsRNA sequence for HIGS construct development in our study. Earlier, using this sequence M. incognita parasitic ability was considerably suppressed in tomato and adzuki bean via in vitro and in vivo RNAi approach (Chaudhary et al. 2019). Incidentally, Mi-msp-1 protein exhibited higher degree of sequence homology to corresponding proteins of M. hapla (66%), M. arenaria (95%) and M. javanica (97%), but showed negligible sequence conservation in other PPNs and C. elegans. This exemplifies its potential as RNAi target to manage RKNs. It is to be noted that the effector genes are the excellent candidate for HIGS in view of the biosafety concerns since effectors lack significant homology to nematodes other than PPNs (Danchin et al. 2013; Dutta et al. 2015a). In addition, the transformed dsRNA sequences had no 19 nucleotide matches in the genomes of Arabidopsis thaliana, Oryza sativa, Drosophila melanogaster, Rattus norvegicus and C. elegans that represent five different organisms in dsCheck server (Naito et al. 2005) which employs off-target search algorithm to analyse the identical nucleotide matches for siRNAs processed from the target dsRNAs. Mi-msp-1 dsRNA sequences also had no nucleotide matches in the published genome of eggplant, which was selected as the host for this study. This indicates the rational design of dsRNA molecule in order to reduce the likely risk.
Using Agrobacterium-mediated transformation transgenic eggplants that express Mi-msp-1 dsRNA were generated. Since the efficacy of HIGS is compromised by copy number effects of the RNAi transgene (multi-copy T-DNA integration at the loci near to hypermethylated region of the host genome may lead to transcriptional gene silencing of RNAi cassettes; Kerschen et al. 2004; Majumdar et al. 2017), only the single-copy events harbouring the Mi-msp-1 dsRNA (confirmed via Southern hybridization) were used for subsequent studies. Selected six homozygous T0 lines were selfed to generate T1 lines and by continuous selfing of subsequent homozygous lines T3 plants were generated. The stable integration and inheritance of the RNAi transgene was ascertained in the majority of these transformants. However, the expression of the RNAi transgene (quantified via RT-qPCR) was variable among the different T1/T2/T3 events, suggesting that the Mi-msp-1 transgene was randomly integrated at various transcriptionally active sites in the eggplant genome. In order to validate this hypothesis, T-DNA integration sites were characterized in selective T2 events, 6.2 and 14.2, via genome walking. As the event-specific PCR failed to detect the expected fragments (containing eggplant sequence adjacent to T-DNA) in events other than 6.2 and 14.2, the independent introgression of T-DNA (containing Mi-msp-1 dsRNA cassette) in different T2 events was confirmed.
Resistance screening studies indicated a pronounced decline in M. incognita development and reproduction in all the T1 events compared to non-transgenic control plants with reductions of 33.78–46.92, 41.05–51.16, 36.08–41.20 and 62.37–70.62% in terms of gall number, egg masses, eggs per egg mass and multiplication factor, respectively. Further, the consistent HIGS effect was observed in T2 and T3 events suggesting no resistance breakdown in progeny plants. The partial nematode resistance documented in our study is in agreement with the HIGS studies of other effector genes of PPNs (Huang et al. 2006; Dutta et al. 2015a; Niu et al. 2016; Xie et al. 2016; Shivakumara et al. 2017). In order to achieve superior nematode resistance, a combinatorial HIGS was attempted using fusion constructs of cysteine and serine protease gene of M. incognita in tomato (Antonino de Souza Júnior et al. 2013). Although, the nematode resistance level could not be increased in terms of efficacy of dual construct compared to single gene cassette in that study. This may be because the higher level of dsRNA delivery saturates the dsRNA processing (by RNaseIII/Dicer enzyme) ability of both hosts and nematodes. The failure to amplify the silencing signal by RdRp (RNA-dependent RNA polymerase that generates secondary siRNAs from primary ones; Pak et al. 2012) due to higher supply of exogenous RNAs can also be another reason in this case. Conversely, crossing of two Arabidopsis RNAi lines expressing housekeeping genes enhanced HIGS efficacy against M. incognita (Charlton et al. 2010). Given the transient nature of RNAi effect (Rosso et al. 2009) it is practically impossible to achieve complete nematode resistance. As RKNs complete three generations in an annual crop, a 60% reduction in RKN multiplication per generation is sufficient to minimize nematode population below the economic threshold (Fuller et al. 2008; Shivakumara et al. 2017). Notably, T2 and T3 events conferred 46.68–73.22 and 44.47–72.92% M. incognita resistance, respectively, in terms of reduced multiplication factor in our study. Additionally, RKN penetration and development (to J3/J4) was retarded in transgenic eggplant compared to control plants, suggesting that HIGS of Mi-msp-1 had negatively altered the early-stage parasitic behavior of M. incognita.
In order to evaluate the long-term HIGS effect of Mi-msp-1 on RKN development, different post-parasitic nematode stages were extracted from T3 eggplant and subjected to RT-qPCR analysis. A significant reduction in Mi-msp-1 expression was documented in post-parasitic J2, J3/J4 and young females extracted from all the T3 transformants compared to nematodes isolated from non-transgenic control plants. It is assumed that the Mi-msp-1 HIGS effect in M. incognita is systemic and is propagated to the entire nematode body upon uptake of dsRNA/siRNA molecule via the stylet orifice. Moreover, RNAi effect was perpetuated across the developmental stages of nematodes till maturity and speculatively M. incognita offspring with defunct Mi-msp-1 would be produced. The heritable nature of RNAi is well studied in C. elegans (Grishok et al. 2000), M. javanica (Gleason et al. 2008) and M. chitwoodi (Dinh et al. 2014). It remains unclear in the present study whether RKNs have ingested dsRNAs directly and processed them into siRNAs (via Dicer) or they ingested the host-processed siRNAs directly. Either or both is possible as RKNs can efficiently ingest large biomolecules via stylet orifice (Li et al. 2007; Zhang et al. 2012).
HIGS is emerging as a powerful strategy for enhancing disease resistance trait in plants to various pests and pathogens (Majumdar et al. 2017). Of late, a few of the RNAi-based genetically modified crops are on course for commercialization, e.g. Pioneer’s PlenishR high oleic acid soybean by DuPont and SmartStax Pro to control western corn earworm by Monsanto. This strategy is more advantageous than classical transgenic approaches as HIGS is not dependent on host’s requirement to produce foreign proteins (Atkinson et al. 2012) that eventually may become allergenic/toxic. HIGS also ensures continuous delivery of dsRNA/siRNA molecules generated by the host plants for uptake by biotrophic pathogens including PPNs. However, compared to other pathogens the status of RNAi-based control of PPNs is still in its infancy (Roderick et al. 2018). In this context, HIGS of Mi-msp-1 holds great potential for management of M. incognita (this parthenogenetic species has broadest host range and is more devastating compared to its sexual relatives).
References
Atkinson HJ, Lilley CJ, Urwin PE (2012) Strategies for transgenic nematode control in developed and developing world crops. Curr Opin Biotechnol 23:251–256
Charlton WL, Harel HYM, Bakhetia M, Hibbard JK, Atkinson HJ, McPherson MJ (2010) Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. Int J Parasitol 40:855–864
Chaudhary S, Dutta TK, Shivakumara TN, Rao U (2019) RNAi of an esophageal gland specific Mi-msp-1 gene alters the early stage infection behaviour of root-knot nematode, Meloidogyne Incognita. J Gen Plant Pathol. https://doi.org/10.1007/s10327-019-00837-x
Danchin EGJ, Arguel MJ, Campan-Fournier A, Perfus-Barbeoch L, Magliano M, Rosso M-N et al (2013) Identification of novel target genes for safer and more specific control of root-knot nematodes from a pan-genome mining. Plos Pathogen 9:e1003745
de Souza Júnior JDA, Coelho RR, Lourenço IT, da Rocha Fragoso R, Viana AAB, de Macedo LLP et al (2013) Knocking-down Meloidogyne incognita proteases by plant-delivered dsRNA has negative pleiotropic effect on nematode vigor. PLoS One 8:e85364
Ding X, Shields J, Allen R, Hussey R (2000) Molecular cloning and characterisation of a venom allergen AG5-like cDNA from Meloidogyne incognita. Int J Parasitol 30:77–81
Dinh PT, Zhang L, Brown CR, Elling AA (2014) Plant-mediated RNA interference of effector gene Mc16D10L confers resistance against Meloidogyne chitwoodi in diverse genetic backgrounds of potato and reduces pathogenicity of nematode offspring. Nematology 16:669–682
Duarte A, Maleita C, Egas C, Abrantes I, Curtis R (2017) Significant effects of RNAi silencing of the venom allergen-like protein (Mhi-vap-1) of the root-knot nematode Meloidogyne hispanica in the early events of infection. Plant Pathol 66:1329–1337
Dutta TK, Banakar P, Rao U (2015a) The status of RNAi-based transgenic research in plant nematology. Front Microbiol 5:760
Dutta TK, Papolu PK, Banakar P, Choudhary D, Sirohi A, Rao U (2015b) Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root-knot nematodes. Front Microbiol 6:260
Dutta TK, Khan MR, Phani V (2019) Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: current status and future prospects. Curr Plant Biol. https://doi.org/10.1016/j.cpb.2019.02.001
Elling AA (2013) Major emerging problems with minor Meloidogyne species. Phytopathol 103:1092–1102
Fuller VL, Lilley CJ, Urwin PE (2008) Nematode resistance. New Phytol 180:27–44
Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS (2001) Molecular characterisation and expression of two venom allergen-like protein genes in Heterodera glycines. Int J Parasitol 31:1617–1625
Gleason CA, Liu QL, Williamson VM (2008) Silencing a candidate nematode effector gene corresponding to the tomato resistance gene Mi-1 leads to acquisition of virulence. Mol Plant-Microbe Interact 21:576–585
Grishok A, Tabara H, Mello CC (2000) Genetic requirements for inheritance of RNAi in C. elegans. Science 287:2494–2497
Hewezi T, Baum TJ (2013) Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant-Microbe Interact 26:9–16
Huang G, Gao B, Maier T, Allen R, Davis EL, Baum TJ et al (2003) A profile of putative parasitism genes expressed in the esophageal gland cells of the root-knot nematode Meloidogyne incognita. Mol Plant-Microbe Interact 16:376–381
Huang G, Dong R, Maier T, Allen R, Davis EL, Baum TJ et al (2004) Use of solid-phase subtractive hybridization for the identification of parasitism gene candidates from the root-knot nematode Meloidogyne incognita. Mol Plant Pathol 5:217–222
Huang G, Allen R, Davis EL, Baum TJ, Hussey RS (2006) Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci USA 103:14302–14306
Kerschen A, Napoli CA, Jorgensen RA, Müller AE (2004) Effectiveness of RNA interference in transgenic plants. FEBS Lett 566:223–228
Li XQ, Wei JZ, Tan A, Aroian RV (2007) Resistance to root-knot nematode in tomato roots expressing a nematicidal Bacillus thuringiensis crystal protein. Plant Biotechnol J 5:455–464
Lilley CJ, Davies LJ, Urwin PE (2012) RNA interference in plant parasitic nematodes: a summary of the current status. Parasitology 139:630–640
Lozano-Torres JL, Wilbers RHP, Warmerdam S, Finkers-Tomczak A, Diaz-Granados A, van Schaik CC et al (2014) Apoplastic venom allergen-like proteins of cyst nematodes modulate the activation of basal plant innate immunity by cell surface receptors. Plos Pathogen 10:e1004569
Luo S, Liu S, Kong L, Peng H, Huang W, Jian H et al (2018) Two venom allergen-like proteins, HaVAP 1 and HaVAP 2, are involved in the parasitism of Heterodera avenae. Mol Plant Pathol. https://doi.org/10.1111/mpp.12768
Majumdar R, Rajasekaran K, Cary JW (2017) RNA interference (RNAi) as a potential tool for control of mycotoxin contamination in crop plants: concepts and considerations. Front Plant Sci 8:200
Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL (2013) Nematode effector proteins: an emerging paradigm of parasitism. New Phytol 199:879–894
Moens M, Perry RN, Starr JL (2009) Meloidogyne species—a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes, UK. CAB International Publishers, Wallingford, pp 1–17
Naito Y, Yamada T, Matsumiya T, Ui-Tei K, Saigo K, Morishita S (2005) dsCheck: highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res 33:W589–W591
Niu J, Liu P, Liu Q, Chen C, Guo Q, Yin J et al (2016) Msp40 effector of root-knot nematode manipulates plant immunity to facilitate parasitism. Sci Rep 6:19443
Pak J, Maniar JM, Mello CC, Fire A (2012) Protection from feed-forward amplification in an amplified RNAi mechanism. Cell 151:885–899
Palomares-Rius JE, Escobar C, Cabrera J, Vovlas A, Castillo P (2017) Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front Plant Sci 8:1987
Papolu PK, Gantasala NP, Kamaraju D, Banakar P, Sreevathsa R, Rao U (2013) Utility of host delivered RNAi of two FMRF amide like peptides, flp-14 and flp-18, for the management of root knot nematode, Meloidogyne incognita. PLoS One 8:e80603
Papolu PK, Dutta TK, Tyagi N, Urwin PE, Lilley CJ, Rao U (2016) Expression of a cystatin transgene in eggplant provides resistance to root-knot nematode, Meloidogyne incognita. Front Plant Sci 7:1122
Roderick H, Urwin PE, Atkinson HJ (2018) Rational design of biosafe crop resistance to a range of nematodes using RNA interference. Plant Biotechnol J 16:520–529
Rosso M-N, Jones JT, Abad P (2009) RNAi and functional genomics in plant parasitic nematodes. Ann Rev Phytopathol 47:207–232
Shivakumara TN, Papolu PK, Dutta TK, Kamaraju D, Chaudhary S, Rao U (2016) RNAi-induced silencing of an effector confers transcriptional oscillation in another group of effectors in the root-knot nematode, Meloidogyne incognita. Nematology 18:857–870
Shivakumara TN, Chaudhary S, Kamaraju D, Dutta TK, Papolu PK, Banakar P et al (2017) Host-induced silencing of two pharyngeal gland genes conferred transcriptional alteration of cell wall-modifying enzymes of Meloidogyne incognita vis-à-vis perturbed nematode infectivity in eggplant. Front Plant Sci 8:473
Sindhu AS, Maier TR, Mitchum MG, Hussey RS, Davis EL, Baum T (2009) Effective and specific in planta RNAi in cyst nematodes: expression interference of four parasitism genes reduces parasitic success. J Exp Bot 60:315–324
Southey JF (1986) Laboratory methods for work with plant and soil nematodes. Ministry of Agriculture, Fisheries, and Food Reference Book, London, p 402
Wang X, Li H, Hu Y, Fu P, Xu J (2007) Molecular cloning and analysis of a new venom allergen-like protein gene from the root-knot nematode Meloidogyne incognita. Exp Parasitol 117:133–140
Xie J, Li S, Mo C, Wang G, Xiao X, Xiao Y (2016) A novel Meloidogyne incognita effector Misp12 suppresses plant defense response at latter stages of nematode parasitism. Front Plant Sci 7:964
Xue B, Hamamouch N, Li C, Huang G, Hussey RS, Baum TJ, Davis EL (2013) The 8D05 parasitism gene of Meloidogyne incognita is required for successful infection of host roots. Phytopathol 103:175–181
Yang Y, Jittayasothorn Y, Chronis D, Wang X, Cousins P, Zhong G-Y (2013) Molecular characteristics and efficacy of 16D10 siRNAs in inhibiting root-knot nematode infection in transgenic grape hairy roots. PLoS One 8:e69463
Zhang F, Peng D, Ye X, Yu Z, Hu Z, Ruan L et al (2012) In vitro uptake of 140 kDa Bacillus thuringiensis nematicidal crystal proteins by the second stage juvenile of Meloidogyne hapla. PLoS One 7:e38534
Acknowledgements
Ph.D. Student SC acknowledges her co-guide Dr. Vishakha Raina, School of Biotechnology, KIIT, Bhubaneswar, India. Current investigation was funded by Department of Biotechnology, Government of India (Grant No. BT/PR5908/AGR/36/727/2012).
Author information
Authors and Affiliations
Contributions
SC performed all the experiments. TKD wrote the MS and analysed of the data. NT, TNS, PKP and KAC helped in performing experiments. UR conceived the experiment and edited the MS. All the authors read and approved the final MS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaudhary, S., Dutta, T.K., Tyagi, N. et al. Host-induced silencing of Mi-msp-1 confers resistance to root-knot nematode Meloidogyne incognita in eggplant. Transgenic Res 28, 327–340 (2019). https://doi.org/10.1007/s11248-019-00126-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-019-00126-5