Abstract
Transgenic cotton expressing the toxin Cry1Ac from Bacillus thuringiensis L. (Bt) is widely cultivated in Pakistan after its formal approval in 2010. The exposure of the local target pests to the Cry1Ac endotoxin for this duration might have changed the baseline susceptibility. To probe the status of resistance in one of the main target pests, Helicoverpa armigera, field-collected larvae were reared in the lab for conducting leaf fed bioassays. Twenty-six cotton accessions collected from farmers, including 25 Bt-cotton and one non-Bt, were tested to quantify the level of Cry1Ac, an insecticidal crystalline protein (ICP), in leaves of lower, middle and upper canopies of plants. The concentration of ICP was tested through Enzyme-linked Immunosorbent Assay and found significantly variable (P < 0.01) between and within accessions. The highest mean expression was observed in Accession-2 and Accession-4, while the lowest in Accession-21 and Accession-19. Among fresh leaf tissues from different parts of the plant, the highest mean expression was recorded at 60 days after sowing in upper canopy leaves of cotton accessions, which decreased in lower parts of the plant with the lowest mean expression in lower canopy leaves. Laboratory bioassays, to calculate lethal dose, for H. armigera showed that LD50 and LD95 were 0.62 μg/g and 1.59 μg/g of fresh tissue weight, respectively. A strong positive correlation also exists between the levels of Cry1Ac protein and insect mortality (r = 0.84). These findings suggested the future risk of cultivation of Bt cotton, carrying single Cry1Ac gene, in Pakistan, as resistance surging in H. armigera against Cry protein. These results may also have significant implications for the resistance management in Bt crops, especially cotton, in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transgenic cotton, carrying Cry endotoxin protein, has revolutionized the pests’ control with little or no harmful effect to humans and other living organisms around the globe (Alvi et al. 2012). It has brought many direct and indirect benefits to farmers including less use of pesticides and eco-friendly attitude, beneficial insects’ preservation, high yield with low production cost, and ultimately higher farmer profits (Abedullah and Qaim 2015). Moreover, it also helped to get more production, with high sustainability (Godfray et al. 2010). But, the continued success of transgenic crops has been adversely affected by the evolution of pest resistance to Bt proteins produced by Bt crops (Tabashnik and Carriere 2017). The expression of Cry proteins during sporulation phase may cause little difference in their effectiveness as compared to other proteins i.e. Vips (Chakroun et al. 2016).
Nevertheless, to get the sustainable high production from cotton, viable pest control is also important and for this the plant should produce toxin at a lethal level, above which target arthropod pest should not survive. The ability of producing such toxic material, by the host plant, should be equally present in the whole plant parts especially during the period of pest attack. Many studies have shown that the Bt protein production is highly variable, not only among the different genotypes (Cheema et al. 2015), but also within plant parts and tissues (Khan et al. 2018), while it decreases constantly with the age of plant (Zaman et al. 2015). It is empirical that transgene must be expressed consistently for the effective control of target pests (Naik et al. 2018). But unfortunately, according to various reports published in last few years, the benefits of Bt cotton with Cry1Ac are under serious threat because of the development of resistance in bollworm, which is mainly due to extensive use of chemicals (Sayyed and Wright 2006), cultivation of unapproved Bt cotton varieties and inappropriate high expression of Cry1Ac (Alvi et al. 2012). The cases of practical resistance to the different Bt toxins in trans-genic crops has raised from 3 in 2005 to 16 in 2016 and the insect resistance reported from different geographies has considered to be a serious threat to the efficacy of Cry1Ac proteins (Tabashnik and Carriere 2017). Previous studies from India (Naik et al. 2018), USA (Tabashnik et al. 2013), South Africa (Van Rensburg 2007), Argentina (Grimi et al. 2015) and Brazil (Yano et al. 2016) have reported the practical resistance in seven major insect species, excluding H. armigera, against different Cry toxins including Cry1Ac in cotton and corn. While, in H. armigera, early warning of resistance has been reported from China (Jin et al. 2015) and Pakistan (Alvi et al. 2012), and no decrease in susceptibility of Cry1Ac has found from Australia (Downes and Bird 2015). Strikingly, H. armigera has gained more resistance to Bollgard-I cotton since 2012 (Tabashnik and Carriere 2017). Despite of different reports on effectiveness of Bt cotton from China, several studies have reported a low but significantly increasing trend of resistance to Cry1Ac in field populations, providing an early warning of potentially more serious problems (Wei et al. 2017). Additionally, monitoring data from different regions of the world have also provided an early warning of field-evolved resistance to Cry1Ac in H.armigera (Tabashnik et al. 2013).
However, to check the resistance status of H. armigera against Cry1Ac the baselines susceptibility studies have been conducted and lethal dose (LD50) of Cry1Ac for China, Pakistan, India, Spain and Australia have been found but variable with an array from 0.09 to 9.07 μg/ml, 335.7 μg/ml of diet, 1.90 μg/g, 2.04 μg/g and 1.62 μg/g of fresh tissue weight, respectively (Heckel 1994; Ibargutxi et al. 2006; Jalali et al. 2004; Kranthi et al. 2005; Wu et al. 2006; Alvi et al. 2012). Furthermore, only one study has been conducted on fresh cotton leaf tissues in Pakistan since the adaption of Bt cotton. According to that work the critical value (LD95) for the H. armigera is 770 ± 25 ng/g, below which the pest can survive but it’s hard to survive above the given value (Ullah et al. 2014). But, this study provides limited information about the practical resistance status of H. armigera in Pakistan. Inspite of many reports that have been reported the lethal level (LD50), which is increasing with the passage of time, and the status of resistance insurgency in H. armigera against Bt cotton, farmers are still growing it on large scale. Both, the increasing trend in pest resistance and cultivation of Bollgard-I cotton with inconsistant expression of endotoxin are posing threat for increased pest attack which will eventually affect the cotton production in coming few years in Pakistan. Meanwhile, low and highly variable expression of Cry1Ac is not affordable and has also been a serious threat to different cotton producing countries that may lead to the induction of resistance (Tabashnik and Carriere 2017). Many developing countries including Pakistan are at higher risk, where unapproved Bt varieties are grown commercially on large scale and have inadequate baseline study for their cultivation.
Although non-Bt host crops, especially okra, are serving as a natural refuge for H. armigera and playing a key role in delaying Cry1Ac resistance in field populations of target pest (Jin et al. 2015), but the cultivation of transgenic cotton expressing double Cry protein such as Cry1Ac + Cry2Ab, or three-toxin pyramided cotton such as Cry1Ac + Cry2Ab + Vip3Aa have also been reported as an alternative tactics for alleviating Bt resistance in H. armigera (Liu et al. 2017). Therefore, the present study was aimed to assess the expression and efficacy of Cry1Ac protein, required for controlling H. armigera, in locally bred Bt-cotton accessions and also to know about the practical resistance status of H. armigera in Punjab, Pakistan. The findings are of utmost importance in a scenario, where government is going to introduce double/triple gene Bt cotton.
Materials and methods
The seed of cotton accessions collected from 560 farmers was grown in randomized complete block design in the cotton field at University of Agriculture, Faisalabad (UAF: 31°25′N, 73°09′E). Then 26 accessions, including 25 Bt-cotton containing the Cry1Ac gene from Bt (event MON531) and one non-Bt accession (Accession-1) as a check, were randomly selected from the total cultivated accessions for insect bioassays and to check the expression of Cry1Ac (Table 1).
Leaf sampling and sample extraction
All the fresh leaves were collected from upper, middle and lower canopies of the three different randomly selected plants of every single accession at 60DAS. Two leaf punch samples (approximately 10 mg each) were taken by snapping the cap of disposable Eppendorf tube down on the leaf. The actual weight of the leaf samples was measured by analytical balance (Model No. AP310-0, Ohaus Corporation Florham Park. N.J.07932, Switzerland). The tissue was ground by rotating the pestle against the sides of the tube with twisting motions. This process was continued until the leaf tissues were ground well and 500 μl of 1 × extraction buffer was added to the tube. Grinding step was repeated to mix tissues with 1 × extraction buffer. This protocol was repeated for each sample to be tested and a new tube and pestle was used for each. The samples were centrifuged at 13,500 rpm for 10 min and supernatant was taken.
Quantification
For the quantification of Cry1Ac protein, the required solutions i.e., wash buffer and 1 × extraction buffer were prepared from the supplied wash buffer salt and 5 × extraction buffer in the commercial quantification kit (QuantiPlate™ Kit, EnviroLogix, Inc., USA), respectively. The amount of Cry1Ac in each sample was quantified and the supernatants were diluted prior to assay according to the manual provided with a commercial quantification kit. Whereas, for 1:11 dilution, the extraction buffer at the rate of 500 μl 1 × and 50 μl sample extract were added to dilution tubes labeled for each sample and then mixed thoroughly. After that, ELISA was performed for the quantification of Cry1Ac following the same protocol written in the kit’s manual. The amount of Cry1Ac was calculated as parts per billion (ppb), which corresponds to nanogram per gram (ng/g) fresh weight of leaf but the calculated amount was then converted in parts per million, which corresponds to microgram per gram (μg/g).
Insect bioassays
Insect rearing
The larvae and adults of H. armigera were collected from cotton and okra fields of Faisalabad and reared on artificial diet up to pupation. The diet recipe used consisted of 150 g chickpea seed-flour, 24 g yeast, 1.5 g methyl-4-hydroxy benzoate, 0.75 g sorbic acid, 2.35 g ascorbic acid, 6 ml linseed oil, 0.75 g streptomycine, 9 g agar–agar, 0.02 g vitamin mixture and 700 ml ddH2O. After emergence of adults from pupae, moths were kept in glass cages (30 × 30 × 30 cm) at 25 °C ± 2 °C and 70 ± 5% R.H. Adults were provided with water based 10% solution on cotton swab for feeding and a layer of liner strip was hung inside the box and placed on the inner surface of the box for oviposition. The liners were removed daily and eggs laid on liner strips were kept in transparent polythene bags with some moisture for hatching. After hatching the 1st instars larvae were shifted on artificial diet in petri plates. There, the larvae were kept feeding until developed to 2nd instar. Then the 2nd instar larvae were used for further feeding on Bt cotton leaves.
Bioassay methodology
For each accession the plants were randomly selected to take leaf samples for bioassays. Three leaves, each from the upper, middle and the lower (at height 1/3 from top, 1/2 height of plant and 1/3 from plant base respectively) portion of the plant, were detached from the plants of the transgenic and the non-transgenic accessions in triplicate form. The leaf area and weight were also recorded to find out the correlation between weight of the leaf and the amount of the Cry1Ac protein present inside the leaf. The petiole of each leaf was wrapped in wet tissue after taking punches for ELISA to determine toxin level. Leaves were placed in glass petri plates (6 inches diameter) and after that petri plates were wrapped by para-film sheet. Five 2nd instar larvae were weighed and placed in the petri plates containing leaves for bioassay. The day, leaves were placed along with larvae was marked as zero (0) day of bioassay (0DOB) and the data of mortality and weight gain or loss by insects were recorded on 3DOB.
Statistical analysis
Repeated measure analysis of variance (ANOVA) was performed for Bt protein concentration, leaf damage percentage and lepidopteron pest (H. armigera) population using accessions as treatment and means were separated by tukey’s (HSD) test. Lethal doses i.e., LD50, LD90 and LD95 were calculated through probit analyses. Whereas, the correlation test was done to check the statistical relationship between insect mortality and the concentration of Cry1Ac protein. All of these statistical analyses were performed on computer software GenStat (10th Edition) and Statistix 8.1.
Results
Expression of Cry1Ac endotoxin protein
The range of Bt toxin in the 234 fresh leaf samples were, 0–0.5 μg/g in 49.15% samples, 0.51–1.00 μg/g in 19.66% samples, 1.01–1.50 μg/g in 11.11% samples, 1.51–2.00 μg/g in 10.26% samples, 2.01–2.50 μg/g in 6.84% samples and 2.51–3.00 μg/g in 2.99% samples. The fresh leaf samples having the expression of Cry1Ac endotoxin in the range of 0.00–0.50 μg/g were maximum (49.15%) while the samples of fresh leaves having the Cry1Ac endotoxin protein expression in the range of 2.51–3.00 μg/g of the fresh tissue weight were minimum (2.99%). While, the total numbers of larvae used in all conducted bioassays were 936 and, 49.15% of the total used larvae were naturally fed on the fresh leaf tissues having the Cry1Ac protein concentration range from Zero (0.00) μg/g to 0.50 μg/g. Dead and alive larvae ratio under lowest expression of Cry1Ac was 99:361. However, not a single alive larva was detected under the highest expression of Cry1Ac. Alive and dead larvae ratio under the highest expression of Cry1Ac was 0:28 (see Online Resource Table 1 and Online Resource Fig. 1).
Quantification of Cry1Ac in upper, middle and lower canopy leaves
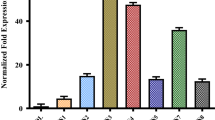
Among the tested three leaf canopies, upper, middle and lower of the cotton plants, the highest expression of Cry1Ac was recorded in upper canopy leaves of the cotton plant in all accessions (Fig. 1). The highest expression (2.73 μg/g) of Cry1Ac in upper canopy leaves was observed in Accession-2 followed by Accession-4, Accession-5, Accession-6, Accession-15, Accession-16, Accession-17, Accession-25, Accession-26, Accession-14, Accession-24, Accession-3, Accession-8, Accession-11, Accession-10, Accession-12, Accession-13, Accession-23, Accession-20, Accession-18, Accession-22, Accession-19 and Accession-21. While the accession Accession-21 shown the minimum expression (0.97 μg/g) of Cry1Ac among all the tested transgenic cotton accessions. However, the middle canopy leaves showed an intermediate expression of Cry1Ac than upper and lower canopy leaves. The expression was significantly variable among the middle canopy leaves of the transgenic cotton accessions. The highest expression (0.89 μg/g) of Cry1Ac was noted in Accession-2 followed by Accession-4, Accession-5 and Accession-6 and the minimum expression (0.23 μg/g) of Cry1Ac was noted in Accession-21 followed by Accession-19, Accession-22 and Accession-18. Conversely, lower canopy leaves expressed lowest expression of Cry1Ac than any other position leaves i.e., upper canopy leaves and middle canopy leaves. The expression was highly variable among the lower canopy leaves of the transgenic cotton accessions, being maximum (0.21 μg/g) in Accession-2 and minimum (0.002 μg/g) in Accession-21. Whereas, the maximum expression levels of the leaves of the middle and the lower canopies were less than the minimum expression levels of upper and middle canopy leaves, respectively.
Mean comparison of expression variability of Cry1Ac in various leaf canopy layers. Lower canopy leaves expression of Cry1Ac in lower canopy leaves of randomly selected three plants from a single accession. Middle canopy leaves expression of Cry1Ac in lower canopy leaves of randomly selected three plants from a single accession. Upper canopy leaves expression of Cry1Ac in lower canopy leaves of three randomly selected plants from a single accession
Cry1Ac expression variability between accessions
The ELISA results of three different plants of a single transgenic cotton accession have shown that the expression variation of Cry1Ac was present within an accession but it was not highly variable. Conversely, Cry1Ac expression variation was highly significant between the tested locally bred cotton accessions. All the transgenic cotton accessions i.e., Accession-2, Accession-4, Accession-5, Accession-6, Accession-15, Accession-16, Accession-17, Accession-25, Accession-26, Accession-14, Accession-24, Accession-3, Accession-8, Accession-11, Accession-10, Accession-12, Accession-13, Accession-23, Accession-20, Accession-18, Accession-22, Accession-19 and Accession-21 differed significantly on the basis of Cry1Ac concentration from check which was a non-transgenic accession (Accession-1) (Table 2). Whereas, maximum (1.28 μg/g) average expression of Cry1Ac was noted in Accession-2 followed by Accession-4 (1.21 μg/g) and Accession-5 (1.16 μg/g). The minimum (0.00 μg/g) average expression of Cry1Ac among all accessions was recorded in non-Bt Accession-1, and likewise among all transgenic accessions the minimum expression of target protein was recorded in Accession-21 (0.40 μg/g) followed by Accession-19 (0.43 μg/g) and Accession-22 (0.47 μg/g) (Fig. 2).
Leaf biotoxicity assay
Mortality results
Laboratory insect bioassays were carried out with the 2nd instar larvae of H. armigera to check the efficacy of Bt cotton accessions. The 2nd instar larvae of H. armigera were fed on the detached leaves of Bt cotton. The number of dead and alive larvae was counted from each petri plate (Fig. 3). Transgenic cotton accessions exhibited a variable survival percentage of H. armigera larvae that ranged between 22.22 and 75%. The variation in expression levels of ICP had shown a varying survival rate of H. armigera larvae (Fig. 2). For all that, this survival is also indicating an alarming behavior of insect resistance.
Mortality analysis of the ELISA samples shown that 99 out of 460 larvae died in the range from 0 to 0.5 μg/g in 115 bioassays, 117 out of 184 larvae died in the range from 0.51 to 1.00 μg/g in 46 bioassays, 81 out of 104 larvae died in the range from 1.00 to 1.50 μg/g in 26 bioassays, 90 out of 96 insects were dead in the range from 1.51 to 2.00 μg/g in 24 bioassays, 63 out of 64 insects were dead in the range from 2.01 to 2.50 μg/g in 16 bioassays and 28 out of 28 insects were dead in the range from 2.51 to 3.00 μg/g in 7 bioassays (see Online Resource Table 2). Similarly, the per cent survival decrease with higher levels of Cry1Ac doses in leaf samples (Fig. 2). Interestingly, maximum larvae mortality with 100% casualties was observed on the concentration of Cry1Ac endotoxin protein ranged from 2.51 μg/g to 3.00 μg/g and it was followed by the concentration ranged from 2.01 to 2.50 μg/g with 98.44% mortality. Minimum larvae mortality with 21.52% casualties was recorded on the concentration of Cry1Ac endotoxin protein that ranged from 0.00 to 0.50 μg/g and it was followed by the concentration range from 0.51 to 1.00 μg/g with 63.59% mortality (Table 2). However, the graph in Fig. 2 representing the tendency of lethality for a particular accession to impose death/mortality upon larvae when fed on its leaves, containing toxin. Only 33.33% insects were killed when fed on leaves of Aceesion-21 carrying Bt toxin @ 0.40 μg/g. Whereas, insect mortality and the concentration of toxin in leaves were shown a correlation which indicated that the insect mortality was increased with increase in toxin levels. So, the value (0.84) of correlation between mortality and dose was calculated and it indicates that a strong positive correlation exists between mortality and Cry 1Ac concentration (Fig. 4). Hence, the phenomenon was clearly witnessed, with an increase in the concentration of Cry1Ac level in Bt cotton leaves, the insect mortality was also increased.
Lethal dose of Cry1Ac for H. armigera
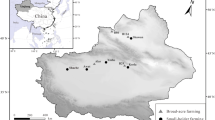
Lethal dose of Cry1Ac for H. armigera was estimated through Probit Analysis (A best analysis used in toxicology and to analyse different dose–response and their relationships.) using statistical software GenStat 10th Edition. The estimates of LD50, LD90, LD95 and LD99 for local population of H. armigera are 0.62 μg/g, 1.38 μg/g, 1.59 μg/g and 2.00 μg/g respectively (Table 3) at 95% Confidence Interval (CI). Based on these findings the status of insect resistance, to different farmers collected Bt accessions, in different districts of Punjab can be seen in Fig. 5.
Discussion
Transgenic cotton, harboring Cry1Ac has significantly decreased the application of pesticides against the target pests but extensive cultivation of Bt cotton at different geographies has resulted in the evolution of field-evolved resistance in selected lepidopteran pests, that is destabilizing the pest control and the technology (Banerjee et al. 2017; Tabashnik and Carriere 2017). Therefore, one of the objectives of current study was to determine the expression of ICP produced by the gene Cry1Ac in Pakistani transgenic cotton accessions. In this regard, the molecular approaches have revealed that the gene remained stable in subsequent transgenic plants’ leaves, while expression level of the targeted gene varied along with the different leaf canopies (Fig. 1). The variability among the accessions due to the age of the plants reported in previous studies (Spielman et al. 2017; Cheema et al. 2015) has been validated along with the expression variation in different leaf canopies, as leaves get older the expression level of Cry1Ac decreases, thus there was instability in the expression of an insecticidal gene over the crop growth period and the leaves of Bt cotton. The leaves have the maximum expression of Cry1Ac gene with more toxin concentration as compared to certain fruiting parts of cotton plant (Adamczyk et al. 2001). Upper canopy leaves had shown maximum expression compared with middle and lower canopy leaves, and the reasons behind this variation have been reported as; (1) low cell division rate, and (2) low efficiency of the promoter or gene in the older leaf tissues as compared with the fresh or upper canopy leaves (Bakhsh et al. 2012). The reduced levels of Cry1Ac transcripts are result of failure of 35S promoter which is also linked with lowering the transcript level at post squaring in another transgene as well (Olsen et al. 2005). The toxin in different fruiting parts in older age crop cannot provide full protection against target insect pest (Kranthi et al. 2005). Therefore, toxic level in plant is required to be maintained at a minimum level at all growth stages and specifically during the period of higher pest attack. In this study, we found a gradual decline in endotoxin expression along the leaves position from top to bottom. Due to low concentration and higher susceptibility, lower leaves of crop have been found an easy target by bollworms.
The expression of Cry1Ac was significantly different in the accessions and it was found that the transgenic cotton accessions such as Accession-2, Accession-4, Accession-5, Accession-6, Accession-15, Accession-16, Accession-17, Accession-25, Accession-26, Accession-14 and Accession-24 had shown the maximum expression of targeted gene as compared to other tested Bt-cotton accessions (Fig. 2). The reason of high expression in these accessions than any other accession can be their effective back cross breeding programme and heat tolerance property. Possibly, due to the high level of acclimatization of the accessions in current environment, which was favorable for the crop and ultimately for the expression of Cry1Ac protein than any other accession, these accessions have showed highest expression of Cry1Ac. Low expression of Cry1Ac protein in other accessions can be attributed to the non-promoting genetic background, the poor acclimatization of the accessions and unfavorable environment for expression of Cry protein. Generally, the new varieties, particularly in cotton, produce maximum yield during first couple of years and then there is a decline in the yield potential, attributable to many factors including the nature of the crop (often cross pollination), and seed mixing during the production and supply chains that ultimately affect the expression of ICP that has also been recorded in present study. However, this expression variability can also be due to the internal and external environmental factors including temperature, nucleotide sequence, insertion point, transgene copy number and the promoter site of the gene that vary the expression of Cry1Ac and avoid showing a consistent expression (Carrière et al. 2018). Bt concentration is altered by a number of known environmental factors, however, still some of the factors requires further probe (Dong and Li 2007). The expression of Cry1Ac δ-endotoxins is highly variable in the commercial cultivars of transgenic cotton due to dissimilar genetic background and their varying level of mRNA transcripts through qPCR (Olsen et al. 2005). In this regard, the variation in Cry1Ac expression observed by Khan et al. (2018) and in present study is suggesting that genetic background has been a contributing factor towards this variation. Therefore, by keeping a check on the expression level of the varieties at the seed distribution channels and the farmers, will provide an opportunity to grow latest varieties that will have better performance for biological control of targeted insect pests.
Insect resistance is highly associated with the benefits of planting Bt crops including lessen use of insecticides and significant decline in overall target pest populations (Shi et al. 2013). However, sustainable use of the Bt crops is under serious threat due to an evolution of resistance in target pest populations (Carrière et al. 2016). Insect resistance to Bt crops has been categorized into three different groups; (1) practical resistance, (2) no decrease in susceptibility, and (3) early warning of resistance. The criteria for grouping are very simple and can be found from Online Source Table 2. However, this study on H. armigera has shown the emergence of resistance against different cultivated accessions in Pakistan. The phenomenon is clear from the comparison between the present and the earlier studies (see Online Source Fig. 2). Earlier the toxin dosage (0.77 μg/g) of Cry1Ac in Bt cotton with Mon531 event, was enough to kill 95% population of H. armigera which is not good in current situation. However, to kill the same percentage of population of same insect, plants need to produce toxin minimum at the rate of 1.59 μg/g. Thus, this rise in LD level clearly indicates the surge in insect resistance to transgenic cotton. So, to confirm the expression results and the insect resistance to the Bt cotton further in field conditions, leaf biotoxicity assays were conducted to determine the mortality percentage of 2nd instar larvae of H. armigera at different leaf canopies of the plants at the 60DAS of crop age. Low and variable expression of Cry1Ac in different cotton accessions under study affected the mortality and the efficacy of ICP against targeted insect pests. Less mortality of 2nd instar H. armigera has been recorded at lower canopy and middle canopy leaves, due to the low expression of Cry1Ac, as compared with upper canopy leaves subjected to bioassays. The reasons behind the low mortality rate of targeted insect pest at middle and lower canopy leaves of transgenic cotton as compared to upper canopy leaves was the hardness of the leaves and the low expression level of the Cry1Ac protein. The disliking of the larvae to eat lower canopies leaves, which were relatively harder, cause weight loss due to starvation and did not cause mortality. Strikingly, during this phase cannibalism was observed (Fig. 6) which can be studied in more details in future. However, larvae loved to eat the soft leaf tissues and it became the reason of high mortality of larvae at upper canopy leaves. When a larvae chews on an upper canopy leaf, which is soft (eat more) and has a high concentration of Cry1Ac protein, it is likely to cause high mortality. The reason of high mortality at high concentration has been correlated with more binding of toxin with more receptors in the midgut of the larvae and vice versa (Soberón et al. 2018). Additionally, as the results are expressing the evolution of resistance hence it has also become the reason for the survival of H. armigera on lower canopy leaves (see Online Resource Fig. 1 and Online Resource Table 1). It is reported that the decreasing expression of ICP increase the rate of survival of targeted insect pests and the varying mortality rates also indicate a gradual decline in insecticidal proteins levels (Bakhsh et al. 2012). Hereafter, for the effectiveness in the control of lepidopteran pests, especially in case of bollworms, the sustainability in the expression of Cry1Ac in Bt-cotton is very crucial.
The percentage of leaf damage by the larvae of H. armigera was found highly associated with the expression of Cry1Ac in leaves. Leaves with higher toxin level in upper canopy leaves, were less damaged by the larvae and vice versa. On the other hand, though the expression was low in middle and lower canopies’ leaves but the damage percentage was not so high, which might be the outcome of harder scaffold of leaves. The study makes us able to correlate the mortality of H. armigera with the expression level of Cry1Ac protein in Bt-cotton leaf tissues, thus finding a critical expression level (LD95 = 1.59 μg/g), which is almost double than the previously reported critical level by Ullah et al. (2014). Farmers’ complaints are quite addressed regarding survival of American bollworms on Bt-cotton plants. The different survival percentage on different plant canopies clearly explains the differential rate of H. armigera survival on lower leaves in Bt-cotton fields of Pakistan (Fig. 1). However, one of the reasons of high value of the LD95 shown by our results was the survival of more larvae on low concentration of Cry1Ac that is parallel to the pest resistance, as the pest get older, they become more resistant to ICP. Finally, in comparison with the previous findings from Pakistan, it was concluded that the insects are surge in resistance and also 2nd instar larvae require more toxin to die as compared to 1st instar larvae. Hence, the level of resistance in H. armigera against the ICP produced by the Bollgard-I transgenic cotton is increasing with the passage of time.
The commercially grown Bollgard-I cotton cultivars differ in the expressed quantity of Cry1Ac protein. Our results are in line with the findings of many researchers who think that there is a correlation between the “decline in efficacy” to “increased survival rates of H. armigera” (Khan et al. 2018; Olsen et al. 2005; Wan et al. 2005). A strong positive correlation has been found among mortality of larvae and the concentration of Cry1Ac. Instead, high rate of larvae survival has been observed at low concentration of Cry1Ac which shows that there was an inverse relation in the survival rate of larvae and the concentration of Cry1Ac (see Online Resource Table 1). Therefore, to find out the LD of Cry1Ac against H. armigera, which was another objective of the study, the second instar larvae of H. armigera were fed on the leaves collected from the Bt cotton plants at the age of 60DAS from field to estimate the LD of Cry1Ac endotoxin protein. Although the attack of 1st instar larvae can be more on cotton crop and the 1st instar stage might cause significant damage to plant but one of the reasons for the selection of 2nd instar larvae of H. armigera was that the larvae at 1st instar were delicate and minute, in consequence difficult to handle. The larvae were also dying while shifting so, it was preferred to use 2nd instar larvae. Another reason was the most dangerous and damage causing stage of larvae, after 1st instar, is 2nd instar. Additionally, a study on 1st instar has already been published from Pakistan (Ullah et al. 2014). Meanwhile, several reports of using 2nd and 3rd instar larvae for bioassays have been published and supporting this selection (Alvi et al. 2012; Wu et al. 2006; Kranthi et al. 2001). Interestingly, the reason to choose the leaves from 60DAS crop for bioassays to test the efficacy of Cry1Ac against H. armigera was that there were reports of having the highest concentration at the age of 60DAS in leaves followed by 90DAS and 120DAS (Khan et al. 2018; Ullah et al. 2014). However, regarding LD, many laboratory experiments have been conducted in USA, Australia, China and India that have shown that the H. armigera has ability to develop resistance against Cry1Ac that expresses in all parts of the cotton plant throughout the growth period. Therefore, the baselines susceptibility values of Cry1Ac for H. armigera in China (0.091 to 9.073 μg/ml and 0.01 to 0.71 μg/ml of diet), India (1.9 μg/g), Spain (2.04 μg/g) and Australia (1.62 μg/g) have been found variable (Heckel 1994; Ibargutxi et al. 2006; Jalali et al. 2004; Kranthi et al. 2005; Wu et al. 1999). Thus, from this study we also found that the lethal level of Cry1Ac expression required to kill 50% population of the larvae of H. armigera was 0.62 μg/g. All the lethal doses i.e., LD50 (0.62 μg/g), LD90 (1.38 μg/g), LD95 (1.59 μg/g) and LD99 (2.00 μg/g) are required to kill their respective percentage of indigenous population of larvae of H. armigera (Table 3).
Keeping in view the LD95 (1.59 μg/g), from the current study, the breeders should develop such Bt cotton varieties which must have at least 1.59 μg/g of fresh weight concentration of Cry1Ac with stable expression in leaves and reproductive parts of cotton plants to control the maximum attack of H. armigera. Because of the high variation in the expression of Cry1Ac endotoxin crystalline protein, the given LD95 is recommended only for 2 to 3 years. It is also suggested that the bioassays should be conducted regularly on yearly basis because the climatic conditions are changing rapidly and the larvae have ability to get the resistance against Cry1Ac. In addition, according to our results of Principle Component Analysis the best performing accessions of our study with high concentration of Cry1Ac and maximum larvae mortality were Accession-2, and Accession-16 (see Online Resource Fig. 3). Finally, the decline in the effectiveness of insecticidal genes in different leaf positions, the present study actually prompted the research to find possible new promoters and insect pest management strategies such as a struggle has been made in confinement of the expression of Cry1Ac to insect biting sites. Expression of insecticidal genes through the plant and growth period of the cotton plant was quite sustainable (Khabbazi et al. 2018). Similarly, as experiment has performed to make consistent gene expression by the use of promoter (RbcS) that would control the insecticidal gene expression during the whole life span of a cotton plant. In regards of biosafety concerns the use of this promoter is also safe and useful as the activities of this promoter are limited to the green plant parts, hence gene will not be expressed in the roots and the cotton seeds, in the end no menace to the soil organisms and safety of the products and by products of cotton would also be guaranteed (Bakhsh et al. 2012). Alternatively, the improvement of the toxicity of insecticidal gene cry2Ah1 also conferred high resistance to Cry1Ac-resistant and susceptible cotton bollworm (Li et al. 2018). So, for the sustainable solution to these problems of inconsistent effectiveness and evolution of resistance against GM cotton, the different conventional breeding methods, gene-pyramiding and the selection procedure are the ways that must be adopted to place the other promoters of the gene that can produce more substantial expression of transgenes through plant and the cotton growth season.
Conclusion
Sixteen transgenic Bt cotton accessions including Accession-2, 3, 4, 5, 6, 7, 8, 9, 11, 14, 15, 16, 17, 24, 25 and 26 are resistant to bollworm at 60DAS stage in bioassays, though there are differences in resistant level and Bt toxin content in these transgenic cottons.These accessions are better to grow under field conditions because they have shown the concentration higher than the value (0.62 μg/g) of LD50 and this level of Cry1Ac concentration is still effective in controlling American bollworm in Pakistan due to the presence of alternate non-Bt hosts. Inversely, due to the presence of high variability in ICP expression, there is a need to develop such mechanisms that could maintain and control the variable expression of transgene in cotton plant. In future, the resistant accessions should be exploited directly or indirectly in conventional breeding to improve cotton germplasm at desirable level. There is also the need to understand the molecular mechanisms to impede the evolution of pest resistance to Bt crops.
Abbreviations
- Bt :
-
Bacillus thuringiensis
- Cry1Ac:
-
Crystal protein produced by the bacterium Bacillus thuringiensis during sporulation
- DAS:
-
Days after sowing
- DOB:
-
Days of bioassay
- ELISA:
-
Enzyme-linked Immunosorbent Assay
- GM:
-
Genetically modified
- ICP:
-
Insecticidal crystalline protein
- LD:
-
Lethal dose
- R.H.:
-
Relative humidity
References
Abedullah Kouser S, Qaim M (2015) Bt cotton, pesticide use and environmental efficiency in Pakistan. J Agric Econ 66:66–86. https://doi.org/10.1111/1477-9552.12072
Adamczyk JJ, Hardee DD, Adams LC, Sumerford DV (2001) Correlating differences in larval survival and development of bollworm (Lepidoptera: Noctuidae) and fall armyworm (Lepidoptera: Noctuidae) to differential expression of Cry1Ac δ-endotoxin in various plant parts among commercial cultivars of transgenic Bacillus thuringiensis cotton. J Econ Entomol 94:284–290. https://doi.org/10.1603/0022-0493-94.1.284
Alvi AH, Sayyed AH, Naeem M, Ali M (2012) Field evolved resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis toxin Cry1Ac in Pakistan. PLoS ONE 7:e47309. https://doi.org/10.1371/journal.pone.0047309
Bakhsh A, Rao AQ, Shahid AA, Husnain T (2012) Spatio temporal expression pattern of an insecticidal gene (cry2A) in transgenic cotton lines. Not Sci Biol 4:115. https://doi.org/10.15835/nsb448217
Banerjee R, Hasler J, Meagher R, Nagoshi R, Hietala L, Huang F, Narva K, Jurat-Fuentes JL (2017) Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci Rep 7:10877. https://doi.org/10.1038/s41598-017-09866-y
Carrière Y, Fabrick JA, Tabashnik BE (2016) Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol 34:291–302. https://doi.org/10.1016/j.tibtech.2015.12.011
Carrière Y, Degain BA, Unnithan GC, Harpold VS, Heuberger S, Li X, Tabashnik BE (2018) Effects of seasonal changes in cotton plants on the evolution of resistance to pyramided cotton producing the Bt toxins Cry1Ac and Cry1F in Helicoverpa zea. Pest Manag Sci 74:627–637. https://doi.org/10.1002/ps.4746
Chakroun M, Banyuls N, Bel Y, Escriche B, Ferré J (2016) Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol Mol Biol Rev 80:329–350. https://doi.org/10.1128/MMBR.00060-15
Cheema HMN, Khan AA, Khan MI, Aslam U, Rana IA, Khan IA (2015) Assessment of Bt cotton genotypes for the Cry1Ac transgene and its expression. J Agric Sci 154:109–117. https://doi.org/10.1017/s0021859615000325
Dong H, Li W (2007) Variability of endotoxin expression in Bt transgenic cotton. J Agron Crop Sci 193:21–29. https://doi.org/10.1111/j.1439-037X.2006.00240.x
Downes S, Bird L (2015) 2014–15 End of season resistance monitoring report. https://www.cottoninfo.com.au/sites/default/files/documents/2015-16%20End%20of%20Season%20Resistance%20Monitoring%20Report.pdf
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818. https://doi.org/10.1126/science.1185383
Grimi D, Ocampo F, Martinelli S, Head G (2015) Detection and characterization of Diatraea saccharalis resistant to Cry1A. 105 protein in a population of northeast San Luis province in Argentina. In: Congreso Argentino de Entomología
Heckel DG (1994) The complex genetic basis of resistance to Bacillus thuringiensis toxin in insects. Biocontrol Sci Technol 4:405–417. https://doi.org/10.1080/09583159409355351
Ibargutxi MA, Estela A, Ferré J, Caballero P (2006) Use of Bacillus thuringiensis toxins for control of the cotton pest Earias insulana (Boisd.)(Lepidoptera: Noctuidae). Appl Environ Microbiol 72:437–442. https://doi.org/10.1128/AEM.72.1.437-442.2006
Jalali S, Mohan K, Singh S, Manjunath T, Lalitha Y (2004) Baseline-susceptibility of the old-world bollworm, Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) populations from India to Bacillus thuringiensis Cry1Ac insecticidal protein. Crop Prot 23:53–59. https://doi.org/10.1016/S0261-2194(03)00170-4
Jin L, Zhang H, Lu Y, Yang Y, Wu K, Tabashnik BE, Wu Y (2015) Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat Biotechnol 33:169–174. https://doi.org/10.1038/nbt.3100
Khabbazi SD, Khabbazi AD, Özcan SF, Bakhsh A, Başalma D, Özcan S (2018) Expression of GNA and biting site-restricted cry1Ac in cotton; an efficient attribution to insect pest management strategies. Plant Biotechnol Rep 12:273–282. https://doi.org/10.1007/s11816-018-0493-8
Khan MI, Khan AA, Cheema HMN, Khan RSA (2018) Spatio-temporal and intra-plant expression variability of insecticidal gene (Cry1Ac) in upland cotton. Int J Agric Biol 20:715–722. https://doi.org/10.17957/IJAB/15.0546
Kranthi K, Kranthi S, Wanjari R (2001) Baseline toxicity of Cry1A toxins to Helicoverpa armigera (Hubner)(Lepidoptera: Noctuidae) in India. Int J Pest Manage 47:141–145. https://doi.org/10.1080/09670870151130651
Kranthi KR, Naidu S, Dhawad C, Tatwawadi A, Mate K, Patil E, Bharose A, Behere G, Wadaskar R, Kranthi S (2005) Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Hübner)(Noctuidae: Lepidoptera). Curr Sci 89:291–298
Li S, Wang Z, Zhou Y, Li C, Wang G, Wang H, Zhang J, Liang G, Lang Z (2018) Expression of cry2Ah1 and two domain II mutants in transgenic tobacco confers high resistance to susceptible and Cry1Ac-resistant cotton bollworm. Sci Rep 8:508. https://doi.org/10.1038/s41598-017-19064-5
Liu L, Gao M, Yang S, Liu S, Wu Y, Carrière Y, Yang Y (2017) Resistance to Bacillus thuringiensis toxin Cry2Ab and survival on single-toxin and pyramided cotton in cotton bollworm from China. Evol Appl 10:170–179. https://doi.org/10.1111/eva.12438
Naik VCB, Kumbhare S, Kranthi S, Satija U, Kranthi KR (2018) Field evolved-resistance of Pink bollworm, Pectinophora gossypiella (Saunders)(Lepidoptera: Gelechiidae) to transgenic Bt-cotton expressing Cry1Ac and Cry2Ab in India. Pest Manag Sci 74:2544–2554. https://doi.org/10.1002/ps.5038
Olsen K, Daly J, Holt H, Finnegan E (2005) Season-long variation in expression of Cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae). J Econ Entomol 98:1007–1017. https://doi.org/10.1603/0022-0493-98.3.1007
Sayyed AH, Wright DJ (2006) Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera: Plutellidae). Pest Manag Sci 62:1045–1051. https://doi.org/10.1002/ps.1270
Shi G, Chavas J-P, Lauer J (2013) Commercialized transgenic traits, maize productivity and yield risk. Nat Biotechnol 31:111–114. https://doi.org/10.1038/nbt.2496
Soberón M, Monnerat R, Bravo A (2018) Mode of action of Cry toxins from Bacillus thuringiensis and resistance mechanisms. In: Gopalakrishnakone P, Stiles B, Alape-Girón A, Dubreuil J, Mandal M (eds) Microbial toxins, toxinology. Springer, Dordrecht, pp 15–27. https://doi.org/10.1007/978-94-007-6449-1_28
Spielman DJ, Zaidi F, Zambrano P, Khan AA, Ali S, Cheema HMN, Nazli H, Khan RSA, Iqbal A, Zia MA, Ali GM (2017) What are farmers really planting? Measuring the presence and effectiveness of Bt cotton in Pakistan. PLoS ONE 12:e0176592. https://doi.org/10.1371/journal.pone.0176592
Tabashnik BE, Carriere Y (2017) Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol 35:926–935. https://doi.org/10.1038/nbt.3974
Tabashnik BE, Brévault T, Carrière Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521. https://doi.org/10.1038/nbt.2597
Ullah I, Asif M, Arslan M, Ashfaq M (2014) Temporal expression of Cry1Ab/c protein in Bt-cotton varieties, their efficacy against Helicoverpa armigera (Lepidoptera: Noctuidae) and population dynamics of sucking arthropods on them. Int J Agric Biol 16:579–585
Van Rensburg J (2007) First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S Afr J Plant Soil 24:147–151. https://doi.org/10.1080/02571862.2007.10634798
Wan P, Zhang Y, Wu K, Huang M (2005) Seasonal expression profiles of insecticidal protein and control efficacy against Helicoverpa armigera for Bt cotton in the Yangtze River valley of China. J Econ Entomol 98:195–201. https://doi.org/10.1093/jee/98.1.195
Wei Y, Wu S, Yang Y, Wu Y (2017) Baseline susceptibility of field populations of Helicoverpa armigera to Bacillus thuringiensis Vip3Aa toxin and lack of cross-resistance between Vip3Aa and cry toxins. Toxins 9:127. https://doi.org/10.3390/toxins9040127
Wu K, Guo Y, Lv N (1999) Geographic variation in susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis insecticidal protein in China. J Econ Entomol 92:273–278. https://doi.org/10.1093/jee/92.2.273
Wu K, Guo Y, Head G (2006) Resistance monitoring of Helicoverpa armigera (Lepidoptera: Noctuidae) to Bt insecticidal protein during 2001–2004 in China. J Econ Entomol 99:893–898. https://doi.org/10.1603/0022-0493-99.3.893
Yano SA, Specht A, Moscardi F, Carvalho RA, Dourado PM, Martinelli S, Head GP, Sosa-Gómez DR (2016) High susceptibility and low resistance allele frequency of Chrysodeixis includens (Lepidoptera: Noctuidae) field populations to Cry1Ac in Brazil. Pest Manag Sci 72:1578–1584. https://doi.org/10.1002/ps.4191
Zaman M, Mirza MS, Irem S, Zafar Y (2015) A temporal expression of Cry1Ac protein in cotton plant and its impact on soil health. Int J Agric Biol 17:280–288
Acknowledgements
This study was conducted with partial support from the Pakistan Strategy Support Program of the International Food Policy Research Institute, with funding from the United States Agency for International Development (USAID). The authors acknowledge the assistance and insights from David J. Spielman, Hina Nazli, Patricia Zambrano, Fatima Zaidi, and Shaukat Ali.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: HMNC, AAK, SAK. Performed the experiments: SA, HMNC, JNA. Analyzed the data: SA, AAK. Contributed materials/reagents/analysis tools: HMNC, AAK, JNA. Wrote the paper: SA, HMNC, AAK. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmad, S., Cheema, H.M.N., Khan, A.A. et al. Resistance status of Helicoverpa armigera against Bt cotton in Pakistan. Transgenic Res 28, 199–212 (2019). https://doi.org/10.1007/s11248-019-00114-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-019-00114-9