Abstract
Greater than expected injury by western corn rootworm (WCR) (Diabrotica virgifera virgifera LeConte) to Cry3Bb1 expressing maize hybrids (Zea mays L.) has been reported in southwestern Nebraska. Affected areas of some fields are often associated with high pH calcareous soils where maize growth is poor and iron chlorosis is common. As part of a comprehensive study to understand potential causes of unexpected injury, experiments were conducted during 2013 and 2014 to ascertain whether the calcareous soil conditions and associated poor maize growth negatively affect the expression of Cry3Bb1. Quantitative determination of Cry3Bb1 protein expression levels in root tissues was carried out on plants at V5–V6 growth stage using the enzyme-linked immunosorbent assay. Cry3Bb1 and non-Bt near isoline maize hybrids were artificially infested with Cry3Bb1-susceptible WCR eggs to measure survival and efficacy of Cry3Bb1 maize in calcareous and non-calcareous soils. Results showed that there was not a significant difference in expression of Cry3Bb1 protein between plants from calcareous and non-calcareous soils (18.9–21.2 µg/g fresh weight). Western corn rootworm survival was about sevenfold greater from the non-Bt isoline than Cry3Bb1 maize indicating that Cry3Bb1 performed as expected when infested with a Cry3Bb1 susceptible rootworm population. When survival from calcareous and non-calcareous soils was compared, no significant differences were observed in each soil. A significant positive correlation between soil pH and expression of Cry3Bb1 protein in roots was detected from samples collected in 2014 but not in 2013. No such correlation was found between soil pH and survival of WCR. Results suggest that Cry3Bb1 expression levels were sufficient to provide adequate root protection against WCR regardless of soil environment, indicating that lowered Cry3Bb1 expression is not a contributing factor to the greater than expected WCR injury observed in some southwestern Nebraska maize fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The western corn rootworm (WCR), Diabrotica virgifera virgifera LeConte, is one of the most economically important insect pests of field maize (Zea mays L.) in North America (Rice 2004; Gray et al. 2009). Economic loss from the WCR is primarily caused by the larval stage which can cause substantial injury to maize roots. Larval injury can negatively impact maize photosynthetic rates and reduce plant vegetative biomass accumulation (Godfrey et al. 1993; Hou et al. 1997; Urías-López et al. 2000), cause plant instability (Levine and Oloumi-Sadeghi 1991; Spike and Tollefson 1991), and reduce grain yield (Urías-López and Meinke 2001; Gray and Steffey 1998; Dun et al. 2010; Tinsley et al. 2013). The invasive nature (Meinke et al. 2009) and adaptability of this species to selection pressure (Miller et al. 2009) has made management in field maize an ongoing challenge (Gray et al. 2009).
Genetically modified maize hybrids expressing insecticidal toxins from the soil bacterium, Bacillus thuringiensis Berliner (Bt) have been widely adopted by farmers as a primary tactic for management of corn rootworms (Andow et al. 2016). Bt-based transgenic maize provides an important alternative for farmers that has largely replaced the application of conventional soil insecticides at planting in continuous maize production. The rootworm-active protein Cry3Bb1 which was first registered in the United States as part of event MON863 and later event MON88017 has been a commercially available trait in field maize for corn rootworm control since 2003 (EPA 2003, 2012; Vaughn et al. 2005). Cry3Bb1 was initially marketed only in single rootworm-trait hybrids, but since 2009, the Cry3Bb1 × Cry34/35Ab1 pyramid has also been available to farmers in the U.S. (EPA 2009).
Greater than expected injury by WCR to single-trait Cry3Bb1 maize hybrids was initially observed in a number of locations between 2009 and 2011 (Gassmann et al. 2011, 2012, 2014; Potter and Ostlie 2011; Wright and Meinke 2011; Gray 2012, 2014; Wangila et al. 2015) and field evolved resistance to Cry3Bb1 has been confirmed in Iowa, Illinois, Minnesota, and Nebraska using laboratory bioassay techniques (Gassmann et al. 2011, 2012, 2014; Gray and Spencer 2015; Wangila et al. 2015; Zukoff et al. 2016). In recent years, to improve resistance management, single trait Cry3Bb1 maize hybrids have been phased out of the marketplace and replaced by maize hybrids expressing the Cry3Bb1 × Cry34/35Ab1 pyramid.
In southwestern Nebraska, the agro-ecosystem presents challenges to optimal maize production. Irrigated continuous maize is the predominant crop and often the most profitable; crop rotation options are limited. High soil pH (>7.0) is common in this agro-ecosystem and some fields contain patches of calcareous soil (pH often >8.0) in which maize can be chlorotic (i.e., iron chlorosis, Wortmann and Hergert 2013) and stunted, exhibit slower growth, or sustain stand reduction. Continuous maize often produces high annual densities of WCR that increases the risk of root injury and yield loss in many maize fields. Finally, there are increasing weed management problems (especially Palmer amaranth, Amaranthus palmeri), which are especially apparent in fields with patches of calcareous soils.
Unexpected WCR injury to single-trait Cry3Bb1 maize hybrids was initially observed in some southwestern Nebraska maize fields in 2011 (Wangila et al. 2015). This injury was especially apparent in the patches of calcareous soils (L. J. M, Personal communication). Previous research documented that WCR resistance to Cry3Bb1 had evolved in isolated parts of this region (Wangila et al. 2015) but the potential impact of other factors that could lead to rootworm injury in the calcareous high pH soil environment was unknown. The chlorotic appearance and reduced growth of single trait Cry3Bb1 maize in the calcareous soil raised the question as to whether Cry3Bb1 protein expression was compromised in calcareous soil maize possibly contributing to reduced efficacy.
Many studies have investigated factors affecting the expression of lepidopteran active Bt proteins (Nguyen and Jehle 2007; Luo et al. 2008; Badea et al. 2010; Székács et al. 2010a, b, 2012; Mejia and de Polania 2012), but only a few studies have focused on coleopteran active Bt proteins, especially Cry3Bb1 (Sidhu and Brown 2004; Nguyen and Jehle 2009; Marquardt et al. 2014). In particular, information on expression of Cry3Bb1 proteins in relation to soil factors/maize iron chlorosis is lacking. Therefore, as part of a larger project to develop sustainable maize rootworm management strategies in southwestern Nebraska, this study was conducted to investigate whether the high pH calcareous soil and associated poor maize growth leads to reduced expression of Cry3Bb1 protein which may contribute to unexpected rootworm injury. The null hypotheses tested included: (1) WCR survival is similar in calcareous soil and surrounding non-calcareous soil; and, (2) Cry3Bb1 protein expression is also similar in calcareous soil and surrounding non-calcareous soil.
Materials and methods
Experimental system and design
To address each hypothesis, irrigated first year maize-fields (300–500 ha) in Chase County Nebraska that had patches of calcareous soil (size range: 0.5–3 ha) were selected as study sites. Three fields were used for experiments in 2013 and two in 2014. Within each field, there were three (2013) and five (2014) replicates of calcareous soil and non-calcareous soil (called soil types throughout remainder of paper) arranged in a completely random design. Topsoil of calcareous soils at these sites was shallow, with weathered limestone bedrock fragments distributed throughout (up to 15% CaCO3, USDA 2016) which created a very high pH environment (often >7.8–8.0). Calcareous soil patches could be visually identified by color as they had a white chalky appearance and surrounding non-calcareous soil was darker brown to black. Within fields, soil sampling was conducted following standard sampling guidelines (USDA 2001; Ferguson et al. 2007) in the preselected replicates of each soil type to characterize soil texture and pH. Samples were collected with a 2.2 × 83.8 cm soil probe (Grainger, Inc. 100 Grainger Parkway Lake Forrest, IL) and analysis was conducted at AgSource Harris Laboratories (Lincoln, NE). A pH meter (Ben Meadows Company, Janesville, WI) was also used to record additional soil pH measurements as defined in Heggenstaller (2012). Soil texture classification in the calcareous patches was a Rosebud-Canyon loam (21.8% clay, 29.4% silt, 48.8% sand) and non-calcareous areas of fields were a combination of Canyon loam (22.0% clay, 37.9% silt, 40.1% sand) and either Kuma or Goshen silt loams (22.3–23.8% clay, 61.7–66.2% silt, 10.1–16% sand) (USDA 2016). The mean pH values of calcareous and non-calcareous soils obtained from the soil analyses were significantly different (i.e., 7.89 ± 0.1 and 7.35 ± 0.1, respectively; n = 19 in each soil; t test PROC GLIMMIX in SAS 2012, P < 0.05). Both soil types were alkaline soils, but 73% of the soil samples from the calcareous soils were >pH 7.8 which often leads to reduced availability of iron in maize (Shaver 2014). In this system, iron chlorosis was exhibited in maize grown in the calcareous soil but not the non-calcareous soil.
A maize hybrid (Stone 6021VT3) that expressed Cry3Bb1 protein and the near isoline maize hybrid (Stone 6021RR2) that does not express Cry3Bb1 (specified as isoline in remainder of paper) were used in this study. Seed pre-coated with neonicotinoid insecticide (Clothianidin, 0.25 mg AI per kernel) was provided by Monsanto Company (St. Louis, MO). When commercially planted maize reached V1 stage (Abendroth et al. 2011), seedlings were removed in each field from one 8 m row per replicate per soil type and replaced with the maize hybrids previously mentioned. Fifteen seeds of each maize hybrid were planted at a plant spacing of 25 cm in half of the row, respectively (4 m of each hybrid per soil type per rep) with hybrid position within rows randomly assigned. Lateral flow strips, an Enzyme Linked ImmunoSorbent Assay (ELISA)—based technique (Quantiplate TM kits; EnviroLogix, Portland, ME), were used to confirm the expression of Cry3Bb1 protein in maize leaf tissue before conducting experiments to address each hypothesis.
Western corn rootworm emergence and survival
Within each replicate of calcareous and non-calcareous soil, two plants each of Cry3Bb1 and isoline maize spaced 2-m apart were artificially infested with ca. 1300 WCR eggs per plant. The WCR population was susceptible to Cry3Bb1 (obtained from USDA-ARS North Central Agricultural Research Laboratory, Brookings, SD) which enabled measurement of adult survival in each soil type and hypothesis 1 to be addressed. The eggs were suspended in 1.25% agar-water solution, drawn in a 60 ml hypodermic syringe (Becton, Dickinson and company, Franklin Lakes, NJ) and mechanically infested in two holes, 6–10 cm deep (20 ml of egg-agar water per plant) on opposite sides of each plant. Eggs were infested when maize was V2–V3 stage (Abendroth et al. 2011) to synchronize the appropriate larval rootworm-maize phenological interaction commonly observed in southwestern Nebraska. In early July, all infested plants were caged using single plant emergence cages, 76 cm long × 38 cm wide, a modified design of Fisher (1980). Each cage was partially buried in the soil and secured around the plant stalk to prevent adult WCR escape but allowed normal plant growth throughout the season. Adults that emerged were trapped in cylindrical glass collection jars (PL2 0090, Solo Cup Company, Lake Forest, IL) placed on the cages. The jars were replaced on a weekly basis during the emergence period (July-late September) and beetles collected were taken to the laboratory and counted. This experiment was conducted in 2013 and consisted of four total cages per soil type per replicate, and three replicates per soil type in each of three fields (total n = 72).
Plant tissue collection
The expression of Cry3Bb1 protein in maize root tissues from calcareous and non-calcareous soil treatments (hypothesis 2) was conducted in 3 fields during 2013 and 2 fields during 2014. A subset of plants (not caged in 2013) as described above was used to quantify the expression of Cry3Bb1 proteins in root tissues at V5–V6 growth stage (Abendroth et al. 2011), a plant growth stage that matches the maize growth phenology at the study location when WCR eggs typically hatch and larvae actively colonize maize roots and an optimal maize growth stage that favors WCR larval development (Hibbard et al. 2008). Three plants expressing Cry3Bb1 protein were collected per soil type per replicate in each field during 2013 (total n = 54) and 2014 (total n = 60). Soil from each plant root was gently washed three times under running water and then dried using paper towels. Due to the fact that Bt toxin is most concentrated in root tips and young root tissue (Meissle et al. 2009), a randomly chosen young root tip (composite sample about 4 cm long) was cut using a clean scissor and placed inside a small plastic vial (5 × 5 ml graduated plastic vials), snap frozen in liquid nitrogen and then stored at −80 °C until used for Cry3Bb1 expression using quantitative ELISA (Stave 2002; Grathaus et al. 2006).
Protein extraction and quantitation
Proteins were extracted from maize roots prepared as previously described by Marquardt et al. (2014) by grinding 100 mg of maize root tissue, in an ice-cold mortar with 1 ml of PBST buffer (pH 7.40). Protein extract was cleared by centrifugation at 12,000g for 10 min at 4 °C and the supernatant containing Cry3Bb1toxin was used for ELISA assays. Protein concentration was measured by the Bradford (1976) procedure using bovine serum albumin (BSA) as standard. The established standard (R2 = 0.9992; Online Resource 1) was saved for quantification of total protein expressed in root samples prior to the ELISA assays. Three replicates per sample were tested.
Quantification of expressed Cry3Bb1 toxin using ELISA
Cry3Bb1 protein was quantified by ELISA assay following the protocol described by Marquardt et al. (2014). Briefly, protein samples were diluted from mg l−1 to μg l−1 prior performing ELISA assays. A standard curve was generated using 97% pure and trypsin-activated Cry3Bb1 protein obtained from Monsanto Company (St. Louis, MO), using eight different concentrations of pure Cry3Bb1 toxin that were prepared with PBST-buffer (pH 7.40). Standards and maize root samples were added to a 96-well ELISA plate and the color development was measured using a 96-well microplate reader (BioTek instruments, INC, Highland Park, Winooski, VT) at 650 nm. The standard curve was established (R2 = 0.9929; Online Resource 2) and saved for use in quantification of Cry3Bb1 proteins. Positive and negative controls were used in all ELISA assays. Each sample was evaluated by triplicate.
Data analysis
The WCR emergence data per cage was transformed with the function log (n + 1) and analyzed as a multilocation 3 × 2 × 2 generalized mixed model ANOVA (PROC GLIMMIX) in SAS (SAS Institute 2012). The fixed factors in the model included, three fields, two soil types (calcareous and non-calcareous soil) and two maize hybrids (Cry3Bb1 and near isoline) and their interaction. The random factor in the model was the field (SAS Institute 2012). Non-transformed data are reported in this paper.
Data on expression of Cry3Bb1 proteins in root tissues was analyzed as a two-way mixed model ANOVA, using PROC GLIMMIX in SAS (SAS Institute 2012). Data for each year (2013 and 2014) were analyzed separately. Each ANOVA included the fixed factors field and soil type. In both analyses, the Kenward–Roger adjustment was used to adjust for the standard error means for fixed effects (Littell et al. 2006).
Correlation analyses were used to measure the strength of association between soil pH and WCR emergence from Cry3Bb1 and isoline hybrids. Also, correlation was measured between soil pH and concentration of Cry3Bb1 protein in maize root tissues. The significance of correlation was determined against the null hypothesis of P = 0 using the Pearson correlation coefficient (PROC CORR in SAS). For all analyses, the treatment differences were determined by the LSMEANS test at P = 0.05 level of significance (SAS Institute 2012).
Results
Survival of WCR in 2013 field studies
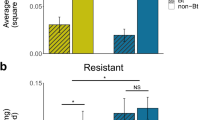
Western corn rootworm emergence was not significantly impacted by the three-way or any of the two way interactions (Table 1). Also, no main effects were statistically significant except maize hybrid (Table 1; Fig. 1). The number of WCR adults recovered in emergence cages of non-Bt maize was greater (12.33 ± 1.92) than that recovered on Cry3Bb1 maize (1.81 ± 0.52; Fig. 1). The overall means for WCR emergence in each soil type were 6.78 ± 1.95 and 7.36 ± 1.84 rootworms per cage in calcareous and non-calcareous soil, respectively. There was not a significant correlation between soil pH and WCR emergence in either Cry3Bb1 (r = 0.0911; df = 33; P = 0.4105; Fig. 2a) or isoline maize (r = 0.2128; df = 34; P = 0.2125; Fig. 2b).
Western corn rootworm (WCR) adult emergence from Cry3Bb1and non-Bt isoline maize artificially infested with 1300 eggs per plant of a Cry3Bb1 susceptible WCR population. The design included a total of four cages per soil type per replicate, and three replicates per soil type in each of three fields, n = 72, conducted during 2013
Expression of Cry3Bb1 protein in maize root tissues at V5–V6 growth stage
The interaction between field and soil type did not significantly affect the mean quantity of Cry3Bb1 protein in maize root tissues of plants in either 2013 or 2014 (Table 2). Similarly, there were no statistically significant main-effects during either year (Table 2). Mean Cry3Bb1 expression levels are presented for each soil type within years in Fig. 3. In 2013, there was not a significant correlation between soil pH and concentration of Cry3Bb1 protein (r = 0.0374; df = 81; P = 0.7364; Fig. 4a); however, in 2014, a significant positive correlation was detected (r = 0.4411; df = 49; P = 0.0012; Fig. 4b).
Discussion
Similar levels of Cry3Bb1 protein expression obtained from maize plants growing in calcareous and non-calcareous soil in 2013 and 2014 indicate that the high pH calcareous soils within the associated environment of this study did not compromise Cry3Bb1 expression (Table 2; Fig. 3). These results are similar to those reported by Badea et al. (2010), who reported no difference between expression level of Cry1Ab proteins in maize growing in three soil types under greenhouse conditions. The significant but weak correlation between soil pH and plant expression of Cry3Bb1 protein in 2014 (Fig. 4b) suggests that a small increase in expression with higher pH can occur within the pH range evaluated. This increase in plant expression at highest pH was somewhat variable and mainly observed in calcareous soil but was not large enough to cause significant differences in expression when soil types were compared. Therefore, the increase in Cry3Bb1 expression is probably not biologically relevant in the southwestern Nebraska system. It would be interesting to investigate if pH values lower than measured in this study would significantly contribute to lower expression.
A number of agronomic and environmental factors have been shown to affect the expression level of Bt proteins in plants (Trarore et al. 2000; Bruns and Abel 2003; Chen et al. 2005; Clark et al. 2005; Icoz and Stotzky 2007, 2008; Luo et al. 2008; Marquardt et al. 2014; Trtikova et al. 2015). In maize, Marquardt et al. (2014) found a positive linear relationship between nitrogen applied in the soil and Cry3Bb1 expression under greenhouse conditions. A similar positive correlation between the concentrations of Bt expressed and applied nitrogen rates was reported from experiments with MON-810 (Cry1Ab) and DBT 418 (Cry1Ac) Bt maize hybrids (Bruns and Abel 2003). Variable Cry1Ab expression levels in maize have also been associated with different moisture and temperature stress conditions (Trtikova et al. 2015). In this study, a uniform rate of nitrogen was applied within each field across soil types. If differences in plant nitrogen uptake or available nitrogen per soil type were present, they were not large enough to significantly affect Cry3Bb1 expression. A key factor in our study system was irrigation. Maize in each field was grown under center pivot irrigation which may have mediated some potential differential effects of agronomic or environmental factors on plant expression of Cry3Bb1 in each soil type.
The significantly greater WCR survival (ca. sevenfold) from Cry3Bb1 versus non-Bt isoline maize in this study (Table 1) indicates that Cry3Bb1 was performing as expected when infested with a Cry3Bb1 susceptible rootworm population. The lack of a significant difference in rootworm survival between soil types suggests that potential differences in soil physical properties between soil types did not significantly influence rootworm population dynamics in this study. Soil factors that could vary by soil type that have been shown to affect WCR egg survival, larval establishment, or larval survival and movement in the soil (i.e., texture, porosity, moisture, compaction, etc., Turpin et al. 1972; MacDonald and Ellis 1990; Beckler et al. 2004; Meinke et al. 2009), did not appear to significantly impact the results of this study in an irrigated system.
In this study, Cry3Bb1 protein expression was quantified from only one maize growth stage (V5–V6). Previous studies have documented that expression of Cry3Bb1 can be variable among maize root tissues (Meissle et al. 2009, 2011) and often declines in root tissue as plants age (Vaughn et al. 2005; Nguyen and Jehle 2009). This suggests that declines in Cry3Bb1 expression may have been found in this study as well if sampling would have been extended over multiple growth stages. However, the key point to consider with any Bt technology is whether or not variability in expression adversely impacts efficacy. The decline in Cry3Bb1 expression reported by Vaughn et al. (2005) was not associated with a decline in efficacy in greenhouse or field trials. If a decline in efficacy did occur over time in our study, the adult survival data suggest that efficacy was not significantly affected. Efficacy may be preserved partly because a large proportion of mortality associated with dietary exposure to Cry3Bb1 on vegetative stage plants occurs during the first instar (Becker 2006) when Cry3Bb1 expression levels are the greatest (Vaughn et al. 2005; Nguyen and Jehle 2009).
Results of this study suggest that greater than expected WCR injury to Cry3Bb1 expressing maize hybrids in calcareous soils of southwestern Nebraska might be due to factors other than reduced Cry3Bb1 expression. The stunted growth of maize in patches of calcareous soil can lead to later pollination than found in non-calcareous soils. Also, A. palmeri weed growth can dominate patches of calcareous soil and bloom late in the growing season. Because the WCR is highly attracted to maize or weed pollen and younger plant tissues (Darnell et al. 2000; Campbell and Meinke 2006), the phenological contrast between maize and weeds in calcareous soils and surrounding maize can act as a sink for immigrating beetles causing densities to rise in calcareous soil patches later in the season (Wangila 2016). Trap crop situations can lead to greater oviposition and higher larval densities the following season (Hill and Mayo 1974). Therefore, larval densities and associated injury potential in some patches of calcareous soil may be greater than densities found in the remainder of the field. Secondly, the smaller root size of stunted plants in calcareous soil compared to the root mass of plants in non-calcareous soil may cause these plants to be more susceptible to larval injury and lodging per WCR density present. A third factor that can be layered across both soil types is the evolution of resistance to Cry3Bb1 as a result of trait overuse (Wangila et al. 2015). In fields where western corn rootworm populations exhibit low levels of resistance to Cry3Bb1, (i.e., early stages of resistance evolution), an interaction of rootworm density, stunted plant size, and resistance may lead to visibly greater than expected injury in calcareous soils before it occurs in non-calcareous soils. An example of this may have been a Chase Co. NE field in 2011 where greater than expected injury was observed in patches of calcareous soil but only a low resistance level was recorded when single-plant bioassays were conducted (field PA, Wangila et al. 2015).
In summary, the Cry3Bb1 protein expression data and WCR survival data presented in this paper collectively support the conclusion that Cry3Bb1 expression levels in the southwestern Nebraska irrigated agroecosystem are sufficient to provide adequate root protection against WCR regardless of soil type. Therefore we accept the null hypotheses addressed in this paper. This study increases our understanding of Cry3Bb1 protein expression in maize plants especially in a high soil pH environment and also helps narrow down possible causes of greater than expected injury to Cry3Bb1 observed in the southwestern Nebraska agro-ecosystem.
References
Abendroth LJ, Elmore RW, Boyer MJ, Marlay SK (2011) Corn growth and development. PM1009. Iowa State University Extension and Outreach, Ames
Andow DA, Pueppke SG, Schaafsma AW, Gassmann AJ, Sappington TW, Meinke LJ, Mitchell PD, Hurley TM, Hellmich RL, Porter RP (2016) Early detection and mitigation of resistance to Bt maize by western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 109:1–12
Badea ME, Chelu F, Lăcătuşu A (2010) Results regarding the levels of Cry1Ab protein in transgenic corn tissue (MON810) and the fate of Bt protein in three soil types. Roman Biotechnol Lett 15:55–62
Becker SC (2006) Stage-specific development and mortality of western and Northern corn rootworm reared on transgenic event MON 863 and on a non-transgenic isoline field corn hybrid. Masters thesis, University of Nebraska
Beckler AA, French B, Chandler LD (2004) Characterization of western corn rootworm (Coleoptera: Chrysomelidae) population dynamics in relation to landscape attributes. Agric For Entomol 6:129–139
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruns HA, Abel CA (2003) Nitrogen fertility effects on Bt delta-endotoxin and nitrogen concentrations of maize during-early growth. Agron J 95:207–211
Campbell LA, Meinke LJ (2006) Seasonality and adult habitat use by four Diabrotica species at prairie-corn interfaces. Environ Entomol 35:922–936
Chen D, Ye G, Yang C, Chen Y, Wu Y (2005) The effect of high temperature on the insecticidal properties of Bt cotton. Environ Exp Bot 53:333–340
Clark BW, Phillips TA, Coats JR (2005) Environmental fate and effects of Bacillus thuringiensis (Bt) proteins from transgenic crops: a review. J Agric Food Chem 53:4643–4653
Darnell SJ, Meinke LJ, Young LJ (2000) Influence of corn phenology on adult western corn rootworm (Coleoptera: Chrysomelidae) distribution. Environ Entomol 29:587–595
Dun Z, Mitchell PD, Agosti M (2010) Estimating Diabrotica virgifera virgifera damage functions with field data: applying an unbalanced nested error component model. J Appl Entomol 134:409–419
Environmental Protection Agency [EPA] (2003) Bacillus thuringiensis Cry3Bb1 protein and the genetic material necessary for its production (vector zmir13L) in event MON863 corn (006484) fact sheet. http://www.epa.gov/oppbppd1/biopesticides/ingredients/
Environmental Protection Agency [EPA] (2009) Pesticide fact sheet. MON 9034_TC1507_MON 88017_DAS- 59122-7. http://www.epa.gov/oppbppd1/biopesticides/pips/smartstax-factsheet.pdf
Environmental Protection Agency [EPA] (2012) Current and previously registered section 3 PIP registrations. http://www.Epa.gov/pesticides/ biopesticides/pips/pip_list.htm
Ferguson RB, Hergert GW, Shapiro CA, Wortmann CS (2007) Guidelines for Soil Sampling. G1740. http://www.ianrpubs.unl.edu/live/g1740/build/g1740.pdf
Fisher JR (1980) A modified emergence trap for quantitative adult corn rootworm studies (Coleoptera: Chrysomelidae). J Kansas Entomol Soc 53:363–366
Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW (2011) Field-evolved resistance to Bt Maize by Western Corn Rootworm. PLoS ONE. doi:10.1371/journal.pone.0022629
Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW (2012) Western corn rootworm and Bt maize: challenges of pest resistance in the field. GMCrops Food 3:235–244
Gassmann AJ, Petzold-Maxwell JL, Clifton EH, Dunbar MW, Hoffmann AM, Ingber DA, Keweshan RS (2014) Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc Natl Acad Sci 111:5141–5146
Godfrey LD, Meinke LJ, Wright RJ (1993) Field corn vegetative and reproductive biomass accumulation: response to western corn rootworm (Coleoptera: Chrysomelidae) root injury. J Econ Entomol 86:1557–1573
Grathaus DG, Bandla M, Currier T, Giroux R, Jenkins RG, Lipp M, Shan G, Stave JW, Pantella V (2006) Immunoassay as an analytical tool in agricultural biotechnology. J AOAC Int 89:913–928
Gray ME (2012) Continuing evolution confirmed of field resistance to Cry3Bb1 in some llinois fields by western corn rootworm. Integ Pest Manag Bul Issue 20:2. http://www.bulletin.ipm.illinois.edu/article.php?id=1704. Accessed 28 October 2015
Gray ME (2014) Field evolved western corn rootworm resistance to Bt (Cry3Bb1) confirmed in three additional Illinois counties. Integ Pest Manag Bul. http://www.bulletin.ipm.illinois.edu/?p=1913. Accessed 28 Oct 2015
Gray ME, Spencer JL (2015) Western corn rootworm: Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) resistance to Bt maize and crop rotation: management challenges and opportunities. Bull R Entomol Soc 39:100–101
Gray ME, Steffey KL (1998) Corn rootworm (Coleoptera: Chrysomelidae) larval injury and root compensation of 12 hybrids: an assessment of the economic injury index. J Econ Entomol 91:723–740
Gray ME, Sappington TW, Miller NJ, Moeser J, Bohn MO (2009) Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annu Rev Entomol 54:303–321
Heggenstaller A (2012) Managing soil pH for crop production. Crop Insights 22:1–4
Hibbard BE, Schweikert YM, Higdon ML, Ellersieck MR (2008) Maize phenology affects establishment, damage, and development of the western corn rootworm (Coleoptera: Chrysomelidae). Environ Entomol 37:1558–1564
Hill RE, Mayo ZB (1974) Trap corn to control corn rootworms. J Econ Entomol 67:748–750
Hou X, Meinke LJ, Arkebauer TJ (1997) Soil moisture and larval western corn rootworm injury: influence on gas exchange parameters in corn. Agron J 89:709–717
Icoz I, Stotzky G (2007) Cry3Bb1 protein from Bacillus thuringiensis in root exudates and biomass of transgenic corn does not persist in soil. Transgenic Res 17:609–620
Icoz I, Stotzky G (2008) Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biol Biochem 40:559–586
Institute SAS (2012) SAS/STAT user’s, 3rd edn. SAS Institute Inc, Cary
Levine E, Oloumi-Sadeghi H (1991) Management of diabroticite rootworms in corn. Ann Rev Entomol 36:229–255
Littell RC, Milliken GA, Stroup WA, Schabenberger RD (2006) SAS for mixed models, 2nd edn. SAS Institute Inc., Cary
Luo Z, Dong H, Li W, Ming Z, Zhu Y (2008) Individual and combined effects of salinity and waterlogging on Cry1Ac expression and insecticidal efficacy of Bt cotton. Crop Protect 27:1485–1490
MacDonald PJ, Ellis CR (1990) Survival time of unfed, first instar western corn rootworm (Coleoptera: Chrysomelidae) and the effects of soil type, moisture, and compaction on their mobility in soil. Environ Entomol 19:666–671
Marquardt PT, Krupke CH, Camberato JJ, Johnson WG (2014) The effect of nitrogen rate on transgenic corn Cry3Bb1 protein expression. Pest Manag Sci. doi:10.1002/ps.3611
Meinke LJ, Sappington TW, Onstad DW, Guillemaud T, Miller NJ, Judith K, Nora L, Furlan L, Jozsef K, Ferenc T (2009) Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric For Entomol 11:29–46
Meissle M, Christina P, Romeis J (2009) Susceptibility of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) to the entomopathogenic fungus Metarhizium anisopliae when feeding on Bacillus thuringiensis Cry3Bb1-expressing maize. Appl Environ Microbiol 75:3937–3943
Meissle M, Hellmich RL, Romeis J (2011) Impact of Cry3BB1-expressing Bt maize on adults of the western corn rootworm Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Pest Manag Sci 67:807–814
Mejia RA, de Polania IZ (2012) Expression of the Cry1Ab toxin in transgenic corn Yieldgard® in the eastern plains of Colombia. Southwest Entomol 37:209–223
Miller N, Guillemaud T, Giordano R, Siegfried BD, Gray ME, Meinke LJ, Sappington TW (2009) Genes, gene flow and adaptation of Diabrotica virgifera virgifera. Agric Forst Entomol 11:47–60
Nguyen HT, Jehle JA (2007) Quantitative analysis of the seasonal and tissue-specific expression of Cry1Ab in transgenic maize Mon810. J Plant Dis Prot 114:82–87
Nguyen HT, Jehle JA (2009) Expression of Cry3Bb1 in transgenic corn MON88017. J Agric Food Chem 57:9990–9996
Potter B, Ostlie K (2011) Performance problems with Bt- rootworm corn: trait resistance in Minnesota and corn rootworm management. Cropping systems team. http://www.nwroc.umn.edu/prod/groups/cfans/@pub/@cfans/@swroc/documents/asset/cfans_asset_404474.pdf
Rice ME (2004) Transgenic rootworm corn: assessing potential agronomic, economic, and environmental benefits. Plant Health Program. doi:10.1094/PHP-2004-0301-01-RV
Shaver TM (ed.) (2014) Nutrient management for agronomic crops in Nebraska. Extension Circular EC155. Univ. Nebraska-Lincoln Extension, Lincoln. http://extensionpublications.unl.edu/assets/pdf/ec155.pdf
Sidhu RS, Brown S (2004) Petition for the determination of nonregulated status for MON 88017 corn. Monsanto Company 04-CR-108U
Spike BP, Tollefson JJ (1991) Yield response of corn subjected to western corn rootworm (Coleoptera: Chrysomelidae) infestation and lodging. J Econ Entomol 84:1585–1590
Stave JW (2002) Protein immunoassay methods for detection of biotech crops: application, limitations, and practical considerations. J AOAC Int 85:780–786
Székács A, Lauber E, Juracsek J, Darvas B (2010a) Cry1Ab toxin production of MON 810 transgenic maize. Environ Toxicol Chem 29:182–190
Székács A, Lauber E, Takács E, Darvas B (2010b) Detection of Cry1Ab toxin in the leaves of MON 810 transgenic maize. Anal Bioanal Chem 396:2203–2211
Székács A, Weiss G, Quist D, Takács E, Darvas B, Meier M, Swain T, Hilbeck A (2012) Inter-laboratory comparison of Cry1Ab toxin quantification in MON 810 maize by enzyme-immunoassay. Food Agric Immunol 23:99–121
Tinsley NA, Estes RE, Gray ME (2013) Validation of a nested error component model to estimate damage caused by corn rootworm larvae. J Appl Entomol 137:161–169
Trarore SB, Carlson RE, Pilcher CD, Rice ME (2000) Bt and non-Bt maize growth and development as affected by temperature and drought stress. Agron J 92:1027–1035
Trtikova M, Wikmark OG, Zemp N, Widmer A, Hilbeck A (2015) Transgene expression and Bt protein content in transgenic Bt maize (MON810) under optimal and stressful environmental conditions. PLoS ONE 10(4):e0123011
Turpin FT, Dumenil LC, Peters DC (1972) Edaphic and agronomic characters that affect potential for rootworm damage to corn in Iowa. J Econ Entomol 65:1615–1619
United States Department of Agriculture [USDA] (2001) Soil quality test kit guide. http://www.nrcs.usda.gov/Internet/FSE_documents/nrcs142p2_050956.pdf
United States Department of Agriculture [USDA] (2016) Web soil survey. https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx
Urías-López MA, Meinke LJ (2001) Influence of western corn rootworm (Coleoptera: Chrysomelidae) larval injury on yield of different types of maize. J Econ Entomol 94:106–111
Urías-López MA, Meinke LJ, Higley LG, Haile FJ (2000) Influence of western corn rootworm (Coleoptera: Chrysomelidae) larval injury on photosynthetic rate and vegetative growth of different types of maize. Environ Entomol 29:861–867
Vaughn T, Cavato T, Brar G, Coombe T, DeGooyer T, Ford S, Groth M, Howe A, Johnson S, Kolacz K, Pilcher C, Purcell J, Romano C, English L, Pershing J (2005) A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci 45:931–938
Wangila DS (2016). Resistance management of western corn rootworm, Diabrotica virgifera virgifera LeConte, to corn traits in Nebraska. Dissertation, University of Nebraska
Wangila DS, Gassmann AJ, Petzold-Maxwell JL, French BW, Meinke LJ (2015) Susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to Bt corn events. J Econ Entomol 108:742–751
Wortmann, CS, Ferguson RB, Hergert, GW, Shapiro CA, Shaver TM. 2013. Micronutrient management in Nebraska. NebGuide G1830. Univ. Nebraska-Lincoln Extension, Lincoln. http://extensionpublications.unl.edu/assets/pdf/g1830.pdf
Wright B, Meinke L (2011) Corn rootworm management update: Ensure diversity when selecting seed for 2012. http://cropwatch.unl.edu/corn-rootworm-management-ensure-diversity
Zukoff SN, Ostlie KR, Potter B, Meihls LN, Zukoff AL, French L, Ellersieck MR, French BW, Hibbard BE (2016) Multiple assays indicate varying levels of cross resistance in Cry3B1-selected field populations of the western corn rootworm to mCry3A, eCry3.1Ab, and Cry34/35Ab1. J Econ Entomol 109:1387–1398
Acknowledgements
The authors thank the farmers that allowed us to conduct studies in their fields. We thank Larry Appel, a crop consultant in Grant, NE. for helping us identify fields in southwestern Nebraska where we conducted our studies. We thank James Brown for helping with setting and changing emergence cages in the field. Thanks to AgSource Harris laboratories, Lincoln, NE. for analyzing soil samples. We also thank Monsanto Company, St. Louis, MO. for providing purified Cry3Bb1 protein and seed. This study was funded by the University of Nebraska Foundation-Elliott Research Fund Grant 01014960.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2017_14_MOESM1_ESM.docx
Bradford’s Assay standard curve using Bovine Serum Albumin (BSA) to quantify the total protein concentration in maize root samples at V5–V6 growth stage sampled from fields during 2013 and 2014 (DOCX 39 kb)
11248_2017_14_MOESM2_ESM.docx
The Cry3Bb1 standard curve used to quantify the concentration of Cry3Bb1 proteins in maize roots at V5–V6 growth stage sampled from the field during 2013 and 2014 (DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Wangila, D.S., Valencia J, A., Wang, H. et al. Influence of calcareous soil on Cry3Bb1 expression and efficacy in the field. Transgenic Res 26, 419–428 (2017). https://doi.org/10.1007/s11248-017-0014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-017-0014-5