Abstract
We report a transgenic zebrafish (Danio rerio) designed to respond to heavy metals using a metal-responsive promoter linked to a fluorescent reporter gene (DsRed2). The metallothionein MT-Ia1 promoter containing metal-responsive elements was derived from the Asian green mussel, Perna viridis. The promoter is known to be induced by a broad spectrum of heavy metals. The promoter-reporter cassette cloned into the Tol2 transposon vector was microinjected into zebrafish embryos that were then reared to maturity. A transgene integration rate of 28 % was observed. The confirmed transgenics were mated with wild-type counterparts, and pools of F1 embryos were exposed to sub-lethal doses of Cd2+, Cu2+, Hg2+, Pb2+ and Zn2+. The red fluorescence response of zebrafish embryos was observed 8 h post- exposure to these sub-lethal doses of heavy metals using a fluorescence microscope. Reporter expression estimated by real-time PCR revealed eightfold, sixfold and twofold increase on exposure to highest concentrations of Hg2+, Cd2+ and Cu2+, while Pb2+ and Zn2+ had no effect. This biosensor could be a first-level screening method for confirming aquatic heavy metal bio-toxicity to eukaryotes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic resources are the final recipients of terrestrial pollutants and effluent discharges from industrial and domestic sources, which exert toxic effects on aquatic organisms and species higher in the food chain. The threat of heavy metal pollution is well documented and remains a matter of concern (Jarup 2003; Verma and Singh 2005). Although often present in minute quantities, their non-biodegradable trait poses a risk to the environment and human health. Several techniques are available for accurate estimation of heavy metal concentrations in living systems (Breuil et al. 1998; Townsend et al. 1998; Tiemann et al. 1999). They require expensive equipment, considerable sample pre-treatment and cannot distinguish between bioavailable and non-available fractions. Therefore, it is essential to develop a reliable, efficient and cost-effective method which can monitor the presence of hazardous metals in the environment. Biosensors can act as an alternative to conventional analysis of heavy metals. Bio-available concentrations of contaminants can be directly measured by biosensors even when present at very low levels (Roberto et al. 2002). Researchers have developed metal-specific recombinant bacterial biosensors by fusing a metal regulatory protein gene promoter upstream of various reporter genes (Raja and Selvam 2011). However, being prokaryotic organisms, they may not indicate the toxic concentration ranges that trigger a response in eukaryotes. Therefore, attempts have been made to develop eukaryotic biosensors using yeasts, microalgae, ciliated protozoa and C. elegans, which facilitated the extrapolation of the results to higher eukaryotic organisms (Lagido et al. 2001; Gutierrez et al. 2015). In our study, we selected zebrafish, a common tropical eukaryotic multicellular vertebrate system, to develop a transgenic fish biosensor.
Zebrafish (Danio rerio) is widely used as a vertebrate model organism for research on toxicity (OECD 1992; Detrich et al. 1998), developmental biology (Driever et al. 1994), drug discovery, and number of other applications (Lele and Krone 1996), because zebrafish embryonic development is remarkably similar to that of humans. Zebrafish eggs are laid and fertilized externally, with large clutch size, rapid development and transparent embryos making it convenient to observe the movement and fate of individual cells during embryonic development (Kimmel et al. 1995). Carvan et al. (2000) gave a conceptual overview of the use of zebrafish for detecting detrimental effects of aquatic pollutants. Recently, transgenic zebrafish lines have been developed using stress promoters like heat shock protein (hsp) for detecting heavy metals (Blechinger et al. 2002; Seok et al. 2006, 2007; Wu et al. 2008; Lee et al. 2014).
Metallothioneins (MTs) are small, low molecular mass, cysteine-rich proteins that function as sensitive indicators of heavy metal contamination (Park et al. 2001). Metallothionein promoters containing metal-responsive elements (MREs) could be linked to fluorescent reporter genes to develop heavy metal-responsive biosensors. Although zebrafish has its own metallothionein gene, in the present study we used the metallothionein (MT-Ia1) promoter from green mussel, Perna viridis. This is mainly because being bivalve molluscs, green mussels have capability to accumulate high concentrations of heavy metals in their tissue as a result of filter feeding, and hence exhibit a high response towards heavy metals. Also, marine invertebrate metallothioneins are known to respond to a broad spectrum of heavy metals, Hg2+ > Cu2+ > Cd2+ > Zn2+ (Viarengo 1989). This property is particularly important, since a single biosensor potentially could be employed to detect several heavy metals simultaneously. Here, we report the use of MT-Ia1-DsRed2 transgene in a Tol2 transposon vector to develop a transgenic zebrafish heavy metal biosensor system.
Materials and methods
Construction of biosensor plasmid
The metallothionein Ia1 (MT-Ia1) gene promoter (1190 bp) was amplified from genomic DNA of green mussel, P. viridis using Pvir-MTI-F (TCCTTCCTCAGCA TGAAAC) and Pvir-MTI-R (AAGTGGCTGTATGTCTCAGTTG) primers. A 25-µl PCR mix consisting of 1 µl of template (75 ng), 1 µl each of forward and reverse primers (10 pmol), 2 µl of dNTPs (2.5 mM each), 2.5 µl of 10 × Taq buffer (with 1.5 mM MgCl2) and 0.25 µl (1 U) of Taq DNA polymerase (Thermoscientific, USA) was prepared in nuclease-free water. PCR conditions included initial denaturation at 95 °C for 5 min; followed by 35 cycles of 94 °C for 30 s, 52 °C for 30 s and 72 °C for 1 min; and final extension at 72 °C for 8 min. The amplicon was T/A cloned and confirmed by sequencing. The promoter was then amplified from the clone using linker primers having BglII and EcoRV sites in the above-mentioned forward and reverse primers, respectively. This fragment was directionally cloned into the pTol2 vector (a gift from Dr. S.C. Ekker, Mayo Clinic, USA). The DsRed2 reporter gene (922 bp) was PCR-amplified from pFRM-DsRed2 plasmid (kind gift from Dr. S.C. Ekker, Mayo Clinic, USA) using linker primers (DsRed2-F: AAAGATATCAGTTCAGCCGGAATTCACC and DsRed2-R: AAAAAGCTTACAGA GTGAGCCGATCCGAG) with EcoRV and HindIII RE sites in the forward and reverse primers, respectively. PCR conditions were the same as above except that the annealing temperature was set at 55 °C. The amplicon was gel-purified, digested with EcoRV and HindIII restriction enzymes and cloned downstream to the MT-Ia1 promoter in the similarly digested pTol2-MT-Ia1 plasmid. The construct with the promoter-reporter cassette was transformed into E. coli DH5α cells. Positive clones were confirmed by colony PCR and sequencing (Sambrook and Russell 2001). The biosensor construct was named pTol2-MT-Ia1-DsRed2 (Fig. 1).
Schematic representation of the biosensor construct in mini Tol2 transposon vector. The construct comprises the metallothionein Ia1 (MT-Ia1) promoter from green mussel, P. viridis, the DsRed2 reporter gene, and the mini Tol2 terminal inverted repeat sequences required for transposition. (Color figure online)
Zebrafish maintenance
Wild-type zebrafish were maintained at 28 °C in aquaculture system (63 mg/l Red Sea Coral Pro Salt in RO-purified water) on a 14 h light and 10 h dark photoperiod cycle. Newly hatched fish larvae were fed Paramecium. Mature fish were fed thrice daily on freshly hatched brine shrimp. Adult zebrafish were bred in small tanks with 1:2 female-male ratio to obtain embryos (Westerfield 2000). The care and treatment of fish used in this study were in accordance with the guidelines of the CPCSEA [(Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment and Forests (Animal Welfare Division), Govt of India] on the care and use of animals in scientific research, and the work plan was approved by appropriate committees.
Microinjection of biosensor construct and screening for transgenics
Tol2 transposase mRNA was synthesized from the pDB600 plasmid (kind gift from Dr. S. C. Ekker, Mayo Clinic, USA) using the mMESSAGE mMACHINE Capped RNA In vitro Transcription kit (Ambion, USA) and stored at −80 °C. Zebrafish embryos at the one/two cell stage were microinjected at the blastoderm/yolk interface with 3 nL of solution containing 8.3 ng/µl of pTol2-MT-Ia1-DsRed2 plasmid and 100 ng/µl of Tol2 transposase mRNA. Injected embryos were reared till maturity in an aquaculture system with a few drops of methylene blue as an anti-fungal agent. After grow-out, the mature fish were mated with wild-type counterparts, and pools of 6 embryos were screened for the presence of the transgene by PCR amplification of the MT-Ia1 promoter and DsRed2 gene from genomic DNA. Transgenic parents were labelled and housed separately.
LC50 studies
To obtain 24-h LC50 values for various heavy metals, dechorionated 48-h old wild-type zebrafish embryos were exposed to 0, 0.1, 0.2, 0.3, 0.4 or 0.5 ppm (parts per million) of Hg2+and Cu2+; 0, 5, 6, 7, 8, 9 or 10 ppm of Cd2+; 0, 5, 7, 9, 11, 13 or 15 ppm of Pb2+ ions or 0, 10, 12, 14, 16, 18 or 20 ppm of Zn2+ ions at 28.5 °C for 24 h. These concentrations were determined from the results of earlier dose-ranging studies for each metal ion (data not shown). Unexposed controls were maintained alongside. Triplicates were run with 10 embryos in each Petri plate. Surviving individuals were counted 24-h post-treatment. The LC50 values obtained were used to decide the concentration range to be used for induction studies.
Induction of transgenics with heavy metals and fluorescence microscopy
Confirmed transgenics were bred with wild counterparts to obtain F1 progeny, which were dechorionated and exposed to different heavy metals at 48 h post-fertilization (hpf) to confirm and quantify reporter expression. The embryos were exposed to 0.05, 0.1, 0.3 or 0.5 ppm of Hg2+, Cd2+, Cu2+ or Pb2+ ions; or 5, 10, 15 or 20 ppm of Zn2+ ions for 8 h. Unexposed transgenic embryos and unexposed wild-type embryos constituted the two negative control sets. Duplicates were run with ten embryos in each treatment group. 8 h post-exposure, DsRed2 expression was observed under a Zeiss Axioscope 2 compound microscope with a rhodamine filter set (excitation and emission wavelengths of 546 ± 10 and 570 ± 10 nm, respectively). A Zeiss Axiocam digital camera was used for imaging the fluorescent reporter expression in the transgenic zebrafish. Constant exposure settings were used throughout the imaging of experimental larvae. The fluorescence images were used to quantify the red fluorescence in the control as well as experimental groups by ImageJ software. The relative fluorescence intensity in the larvae after metal exposure was calculated by normalizing to the background fluorescence in the un-induced controls.
Real-time PCR
Quantitative RT-PCR (qRT-PCR) was performed to confirm expression of the reporter gene in zebrafish larvae. Ten embryos from each treatment group (as described above) were pooled and stored in RNAlater solution (Qiagen, NL) at −20 °C until total RNA was isolated using RNeasy mini kit (Qiagen, NL). First-strand cDNA was synthesized from 1 µg of total RNA and oligo(dT) primer using First Strand cDNA Synthesis kit (Thermoscientific, USA). Real-time PCR was performed on ABI 7500 Real-time PCR machine using Quantifast SYBR Green PCR mix (Qiagen, NL). Primers qDsR-F (CTACCTGGTGGAGTTCAAGTCC) and qDsR-R (CGCTACAGGAACAGGTGGTG) were designed to amplify a 165-bp fragment of the DsRed2 gene, while qGapdh-F (GTGGAGTCTACTGGTGTCTTC) and qGapdh-R primers (GTGCAGGAGGCA TTGCTTACA) were designed to amplify a 173-bp fragment of the GAPDH gene that served as reference for baseline-level gene expression. Gene Runner software v. 3.0 was the primer design software used. A 15-µl PCR mix comprising 7.5 µl of 2 × SYBR Green master mix (Thermoscientific, USA), 1 µl of cDNA, 1 µl each of forward and reverse primers (0.3 µM) and 4.5 µl of nuclease-free water was prepared. The PCR program included an initial denaturation at 95 °C for 5 min; with 40 cycles of 95 °C for 15 s and 60 °C for 30 s. Experimental duplicates were run. The comparative Ct method was used to estimate the relative expression of DsRed2 mRNA. Fold-change in DsRed2 expression was calculated by the \(2^{{ - {\Delta \Delta }C_{\text{t}} }}\) method (Schmittgen and Livak 2008).
Southern blotting
Southern blot hybridization was carried out to confirm the genomic integration of the biosensor cassette following standard protocol (Sambrook and Russell 2001). A 165-bp region of the DsRed2 gene sequence within the biosensor cassette was used as the probe. Probe labelling was done using the PCR DIG probe synthesis kit (Roche Applied Science, Switzerland) following the manufacturer’s instructions. Briefly, genomic DNA was isolated from F1 embryos (offspring of transgenic × wild-type), and the presence of the transgene was confirmed by PCR as mentioned above. The genomic DNA was partially digested with HindIII which had a single site at one end of the promoter-reporter cassette. The restriction enzyme-digested fragments were separated on 1 % agarose gels and transferred to a positively charged nylon membrane (Pall Life Sciences, USA) using alkaline upward capillary transfer. The membrane was probed with DIG-labelled probe, and the hybridization was detected using the DIG-Nucleic Acid detection kit (Roche Applied Science, Switzerland) following the manufacturer’s instructions. The results were documented using a Syngene Gel documentation system.
Statistical analysis
Real-time PCR and relative fluorescent intensity data were analyzed by one-way ANOVA, and Tukey’s test was done to determine statistical significance of changes observed in reporter expression among different treatment groups.
Results
Microinjection of biosensor construct and screening for transgenics
The biosensor construct pTol2-MT-Ia1-DsRed2 was microinjected into one/two cell stage zebrafish embryos along with in vitro transcribed Tol2 transposase mRNA. In total, 1117 embryos were injected during the course of this study, and about 60 % survival was obtained at 72 h post-injection. However, only 87 fish (8 %) survived to maturity, of which 32 produced offspring (F1) when mated with wild-type mates. Genomic DNA was isolated from pools of 48 h-old F1 embryos, and the transgenics were confirmed by PCR amplification of MT-Ia1 and DsRed2 regions of the transgene cassette. Four males and five females of the 32 microinjected broodstock were confirmed as transgenics, and the integration rate was calculated to be 28 %. The transgenic parents were mated with their wild counterparts to obtain F1 embryos for further studies.
LC50 of heavy metals for zebrafish embryos
All the heavy metals caused a dose-dependent increase in mortality of zebrafish embryos. The LC50 at 24 h was estimated as 0.4 ± 0.01, 0.5, 8.5 ± 0.21, 8.75 ± 0.35, 17 ± 0.28 ppm for Hg2+, Cu2+, Cd2+, Pb2+ and Zn2+ ions, respectively. The sub-lethal concentrations used for the subsequent induction studies were decided based on these values.
Reporter detection in transgenic larvae by fluorescence microscopy
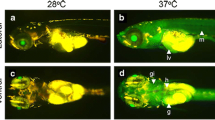
Heavy metal-induced DsRed2 reporter expression recorded by fluorescence microscopy could be seen in almost 90 % of the transgenic embryos in a mosaic pattern detected largely in dorsal and ventral retina, gills and the skin epithelium (Fig. 2). The relative fluorescence intensity in the experimental groups as quantified by ImageJ software revealed highest RFP expression in Hg2+-induced larvae (Fig. 3). The fluorescence intensity also increased with increasing metal concentration. Low levels of red fluorescence were observed in egg yolk and eyes of some unexposed transgenic larvae, and those exposed to Zn2+ and Pb2+. This indicates some leakage from the promoter because no fluorescence was recorded in the wild-type controls. However, this leaky expression was never observed in body parts other than eyes and yolk sac, which could be because of the transparency of these body parts. The larvae were phenotypically normal at all metal concentrations used for induction, except at 0.5 ppm for Hg2+, where no animals survived. No other mortality was recorded during the experiment.
Fluorescence imaging of F1 transgenic zebrafish (D. rerio) larvae (48 h post-fertilization) exposed for 8 h to Hg2+, Cd2+, Cu2+, Pb2+ or Zn2+ at doses that gave maximum fluorescence. Fluorescence expression is observed only in the yolk sac of un-induced transgenic control, while it is present in other body parts in the induced larvae. BL bright field, FL fluorescence. Scale bar 200 µm
Quantification of DsRed2 fluorescence in F1 transgenic zebrafish larvae exposed to Hg2+, Cd2+, Cu2+, Pb2+ or Zn2+ (a–e) at different doses. The mean fluorescence intensity values normalized to background fluorescence from un-induced controls for each experimental group were plotted. Data represents mean ± SEM. (ns not significant; **p < 0.01; ***p < 0.001)
Reporter detection in transgenic larvae by real-time PCR
Heavy metal-induced larvae from each plate were pooled, and expression of the DsRed2 reporter gene in all the treatments and controls was confirmed by real-time PCR (Fig. 4). While the DsRed2 transcript was absent in the wild-type controls, it was detected in the unexposed transgenic controls, showing leaky expression from the promoter. The difference in mean fold-change in reporter expression calculated with respect to the unexposed transgenic controls was significant in larvae induced with Hg2+, Cd2+ and Cu2+ ions, and the fold-change difference increased with the concentration of inducing ions. Eightfold increase was observed on exposure to 0.3 ppm Hg2+ (p < 0.001), while no larvae survived the 0.5 ppm exposure. In case of Cd2+ (p < 0.001) and Cu2+ (p < 0.01) ions, sixfold and twofold significant up-regulation, respectively, was noted at 0.5 ppm. No increase in reporter expression was observed on induction with lead and zinc. The heavy metals induced promoter function in the order Hg2+ > Cd2+ > Cu2+ at the highest sub-lethal concentration tested.
Real-time PCR analysis of DsRed2 mRNA expression in F1 transgenic zebrafish larvae exposed to Hg2+, Cd2+, Cu2+, Pb2+ or Zn2+ (a–e) at different doses. Data represents mean ± SEM. ns expression not significantly different from control; **expression significantly higher than control (p < 0.01); ***expression highly significant above the control (p < 0.001)
Confirmation of transgene integration
The transgenic fish identified by PCR screening were mated with wild-type fish, and 15-day old F1 embryos were pooled, with 10 embryos in each pool. Assuming one integration event per transgenic individual, on mating with wild-type counterparts, 50 % of the embryos would be expected to carry the transgene. It was expected that each integration event would be visible as one hybridized band since there was no other restriction site for HindIII in the transgene. If the transgene construct is extra-chromosomal, then a single band of ~4.7 kb is expected. However, six bands of sizes 6, 3.8, 2.8, 2.5, 2.4 and 1.0 kb were detected in pooled F1 embryos from a single cross (transgenic × wild), indicating at least six integration events in the transgenic parent (Fig. 5). Since the DNA was from a pool of embryos and not from an individual sample, the results could not be used for copy number estimation.
Discussion
Use of zebrafish, a small tropical fish, has revolutionized the field of developmental biology and soon became the favourite vertebrate model. Owing to its transparent body and easy maintenance, it has been used extensively for developing transgenic models expressing reporter proteins for environmental toxicity testing. Several researchers worldwide have developed transgenic zebrafish lines expressing fluorescent proteins under the control of promoter elements such as estrogen response elements (ERE; Chen et al. 2010, Lee et al. 2012a, b), aryl hydrocarbon response elements (AHREs; Mattingly et al. 2001), heat-shock protein (hsp) promoters (Blechinger et al. 2002; Seok et al. 2007; Wu et al. 2008), glycoprotein hormone promoters (Ji et al. 2012; Cheng et al. 2014), DNA-damage inducible promoters (Gireesh-Babu et al. 2012) and tissue-specific promoters (Wan et al. 2006; Sun et al. 2010; Almeida et al. 2014) for aquatic pollution monitoring. So far, most of the transgenic zebrafish biosensors developed for heavy metal detection are based on hsp promoter elements that are triggered by several other stressors (Blechinger et al. 2002). In most cell types even prior to stress, HSPs constitute 1–2 % of total protein, suggesting a strong constitutive basal expression from the hsp promoter (Stephanou and Latchman 2011). In this scenario, it would be difficult to quantify the heavy metal levels using reporter expression, as it indicates a cumulative effect rather than a heavy metal-specific effect. MTs are reported to be specifically induced in response to heavy metal treatment, whereas the synthesis of hsp70 appears to be a general stress response (Misra et al. 1989). Cioci et al. (2000) compared the responsiveness of transgenic Caenorhabditis elegans containing hsp-regulated versus MTl-regulated transgenes, and reported that the MTl reporter system is a more sensitive monitor for metal exposure. Also, metallothionein gene promoters contain metal-response elements (MREs) that are responsive to heavy metals, and hence are a better choice for developing biosensors for heavy metals. Recently, Liu et al. (2016) have generated a mt:egfp transgenic zebrafish as a biosensor with a zebrafish MT promoter that is sensitive to environmental concentrations of zinc and cadmium.
Here, we report the use of a broad-spectrum metallothionein (MT-Ia1) promoter from a marine invertebrate, P. viridis, for developing a zebrafish biosensor system. Viarengo (1989) reported that marine invertebrate metallothioneins are responsive to heavy metals in the order of Hg2+ > Cu2+ > Cd2+ > Zn2+. Khoo and Patel (1999) reported the promoter sequence of the P. viridis MT-Ia1 gene. The green mussel from which it is derived is itself used as a bioindicator of heavy metals (Putri et al. 2012). The MT-Ia1 promoter consists of three active protein-binding (AP1) sites and two metal-responsive elements (MRE) that regulate its expression in response to heavy metals (Khoo and Patel 1999). The DsRed2 fluorescent reporter used here is a variant of the original red fluorescent protein isolated from a coral, Discosoma sp. Unlike GFP, it has higher signal-to-noise ratio and is resistant to photobleaching (Baird et al. 2000, Clay and Ramakrishnan 2005). However, the wild-type protein has disadvantages of slow maturation and insolubility. Through a combination of random and site-directed mutagenesis, Bevis and Glick (2002) developed a variant called DsRed2 with improved solubility, enhanced fluorescence and speed of maturation. An asparagine-to-glutamine substitution at position 42 resulted in 10–15 times faster maturation of DsRed, but also increased the level of green emission. This green emission was further suppressed by additional amino acid substitutions, and a reduction of net charge near the N terminus of the protein resulted in enhanced solubility.
Use of the Tol2 transposon vector in this study resulted in a germ-line integration rate of 28 %, which is higher than the 1.8 % obtained by Wu et al. (2008) and Lee et al. (2011). In these studies, transgenesis was performed by microinjecting a linearized naked plasmid vector that integrates into the host genome less efficiently. The Tol2 transposon vector has been used effectively before and is known to integrate randomly into the vertebrate genome (Kawakami et al. 1998; Balciunas et al. 2006; Zou et al. 2006). The high germ-line integration rates (60–70 %) reported earlier for the Tol2 transposon vector could be due to the use of a constitutive promoter that helped in screening the embryos for GFP fluorescence before being reared to maturity (Urasaki et al. 2006). Since an inducible promoter was used here, all embryos were reared, and this could have resulted in the lower integration rate of 28 %. Although Tol2 has been observed to create single-copy insertions most often (Kawakami 2007), at least six integration events were detected in one transgenic individual in this study. Using a similar vector, Urasaki et al. (2006) found the total number of insertions transmitted by a single founder fish varies from 1 to 15.
The mosaic pattern of reporter expression observed after induction with heavy metals could be due to pigmentation obscuring the fluorescence, integration of head-to-tail concatemers, position effects at the integration site or variation in copy number, as was observed for GFP expression in the F1 embryos of transgenic Nile tilapia (Fujimura and Kocher 2011). Although no red fluorescence was detected in wild-type controls, some leaky expression of DsRed2 was seen in un-induced transgenic embryos by real-time PCR. This leaky expression could be due to the basal expression of this promoter in the absence of inducing heavy metals. Background GFP fluorescence was observed in transgenic C. elegans containing the MT2 promoter fused to the GFP gene, which was 10-fold to 15-fold lower than the post-induction values (Ma et al. 2009). MTs are closely associated with homeostasis of essential metal ions and hence some level of constitutive expression is expected. MT expression is also known to be controlled by developmentally regulated pathways (Chen et al. 2004). Since, in the present study zebrafish larvae (48 hpf) were used for heavy metal exposure assay, there is a possibility of basal expression from the MT-Ia1 promoter. However, under the fluorescence microscope, this expression was visible as fluorescence in the eyes and yolk sac which was easily distinguishable from metal-induced fluorescence in other body parts. This pattern of background fluorescence in the control was found to be consistent across different larvae and experimental groups. Hence, this fluorescence is not expected to interfere in any way with the heavy metal detection assay.
Kusik et al. (2008) used an electrophile-responsive element (EPRE) and luciferase-green fluorescence protein (LUC-GFP) fusion reporter to detect Hg2+ ions ranging from 0.1 to 1 µM. Human and zebrafish hsp70 promoters have been employed by a number of workers to detect Cd2+ (0.2–125 µM), CuSO4 (1–1.5 µM) and As3+ (10–300 µM), and reporter response was found to be dose-dependent (Blechinger et al. 2002; Seok et al. 2006, 2007). Wu et al. (2008) developed a heat shock-inducible gfp transgenic zebrafish line by cloning the zebrafish hsp27 promoter in a pEGFP-1 vector. The transgenic line has been maintained for six generations. Induction was carried out using cadmium (1.35–135 µM) and sodium arsenate (64–640 µM). It was selectively inducible by arsenic in a dose-dependent manner. In the present work too, the transgenic larvae showed a dose dependent induction pattern for the heavy metals studied. According to the Environmental (Protection) Rules of India (1986), the water quality standards for effluent discharge of mercury, cadmium and copper are 0.01, 1–2, and 3 ppm, respectively. The minimum detection limit by the zebrafish biosensor developed in the present study is 0.1 ppm for mercury and 0.5 ppm for cadmium and copper as evident by real-time PCR. However, it is to be noted that the transgenic larvae used here are mosaic for the transgene. Hence, the exact detection range of the heavy metals by this biosensor may be determined only once a true transgenic line is established. At that point, these transgenic zebrafish might be used to detect the presence of heavy metals in freshwater samples. For this, the adult transgenic zebrafish biosensors could be exposed to effluent water samples in the laboratory and the expression of red fluorescence protein be studied by fluorescence microscopy. However, it is even possible to visualize the red fluorescence in daylight or under ultra-violet light (385 nm) provided it expresses in large quantities, alleviating the need for fluorescence microscopy. If this becomes a reality, the day will come where these zebrafish can be introduced into cages near effluent treatment plants to test effluent water samples prior to their discharge in receiving water bodies.
Conclusion
The present transgenic zebrafish biosensor for sensing heavy metal toxicity employs the P. viridis MT-Ia1 metallothionein promoter fragment known to respond to a broad spectrum of heavy metals. These fish are expected to report presence of heavy metals in the bio-available fraction at levels toxic to eukaryotes and can be used as a primary screening method for water samples from areas likely to be polluted by the heavy metals. Quantification can be thus limited to positive samples to minimize the cost of expensive analytical techniques.
References
Almeida DV, Vaz B, Figueiredo MA, Junior ASV, Marins LF (2014) Fluorescent transgenic zebrafish as a biosensor for growth-related effects of methyl parathion. Aquat Toxicol 152:147151
Baird GS, Zacharias DA, Tsien RY (2000) Biochemistry, mutagenesis, and oligomerization of DsRED, a red fluorescent protein from coral. Proc Natl Acad Sci USA 97:11984–11989
Balciunas D, Wangensteen KJ, Wilber A, Bell J, Geurts A, Sivasubbu S, Wang X, Hackett PB, Largaespada DA, Mclvor RS, Ekker SC (2006) Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet 2(11):e169. doi:10.1371/journal.pgen.0020169
Bevis BJ, Glick BS (2002) Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat Biotechnol 20(1):83–87
Blechinger SR, Warren JT Jr, Kuwada JY, Krone PH (2002) Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ Health Perspect 110(10):1041–1046
Breuil P, Di Benedetto D, Poyet JP (1998) On-line analysis of copper and zinc in industrial effluents by UV-visible spectrometry (in French). Analusis 26(8):63–66
Carvan MJ, Dalton TP, Stuart GW, Nebert DW (2000) Transgenic zebrafish as sentinels for aquatic pollution. Ann N Y Acad Sci 919(1):133–147
Chen WY, John JA, Lin CH, Lin HF, Wu SC, Lin CH, Chang CY (2004) Expression of metallothionein gene during embryonic and early larval development in zebrafish. Aquat Toxicol 69(3):215–227
Chen H, Hu J, Yang J, Wang Y, Xu H, Jiang Q, Gong Y, Gu Y, Song H (2010) Generation of a fluorescent transgenic zebrafish for detection of environmental estrogens. Aquat Toxicol 96(1):53–61
Cheng X, Chen X, Jin X, He J, Yin Z (2014) Generation and characterization of gsuα: EGFP transgenic zebrafish for evaluating endocrine-disrupting effects. Toxicol Appl Pharmacol 278(1):78–84
Cioci LK, Qiu L, Freedman JH (2000) Transgenic strains of the nematode Caenorhabditis elegans as biomonitors of metal contamination. Environ Toxicol Chem 19(8):2122–2129
Clay H, Ramakrishnan L (2005) Multiplex fluorescent in situ hybridization in zebrafish embryos using tyramide signal amplification. Zebrafish 2(2):105–111
Detrich HW, Westerfield M, Zon LI (1998) Overview of the zebrafish system. Method Cell Biol 59:3–10
Driever W, Stemple D, Schier A, Solnica-Krezel L (1994) Zebrafish: genetic tools for studying vertebrate development. Trends Genet 10(5):152–159
Fujimura K, Kocher TD (2011) Tol2-mediated transgenesis in tilapia (Oreochromis niloticus). Aquaculture 319(3):342–346
Gireesh-Babu P, Pawar N, Krishnan P, Kumar AP, Zaidi SGS, Bhartiya D, Kumar N, Sivasubbu S, Rajendran KV, Chaudhari A (2012) Functional characterization of the zebrafish gadd45αb gene promoter and its application as a biosensor. Curr Sci 103(4):388–394
Gutierrez JC, Amaro F, Martín-Gonzalez A (2015) Heavy metal whole-cell biosensors using eukaryotic microorganisms: an updated critical review. Front Microbiol 6:1–8
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182
Ji C, Jin X, He J, Yin Z (2012) Use of TSHβ: EGFP transgenic zebrafish as a rapid in vivo model for assessing thyroid-disrupting chemicals. Toxicol Appl Pharmacol 262(2):149–155
Kawakami K (2007) Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. doi:10.1186/gb-2007-8-S1-S7
Kawakami K, Koga A, Hori H, Shima A (1998) Excision of the Tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene 225(1):17–22
Khoo HW, Patel KH (1999) Metallothionein cDNA, promoter, and genomic sequences of the tropical green mussel, Perna viridis. J Exp Zool 284(4):445–453
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310
Kusik BW, Carvan MJ III, Udvadia AJ (2008) Detection of mercury in aquatic environments using EPRE reporter zebrafish. Mar Biotechnol 10(6):750–757
Lagido C, Pettitt J, Porter AJR, Paton GI, Glover LA (2001) Development and application of bioluminescent Caenorhabditis elegans as multicellular eukaryotic biosensors. FEBS Lett 493(1):36–39
Lee HC, Chen YJ, Liu YW, Lin KY, Chen SW, Lin CY, Lu YC, Hsu PC, Lee SC, Tsai HJ (2011) Transgenic zebrafish model to study translational control mediated by upstream open reading frame of human chop gene. Nucleic Acids Res 39(20):e139. doi:10.1093/nar/gkr645
Lee O, Takesono A, Tada M, Tyler CR, Kudoh T (2012a) Biosensor zebrafish provide new insights into potential health effects of environmental estrogens. Environ Health Perspect 120(7):990–996
Lee O, Tyler CR, Kudoh T (2012b) Development of a transient expression assay for detecting environmental oestrogens in zebrafish and medaka embryos. BMC Biotechnol 12(1):32. doi:10.1186/1472-6750-12-32
Lee HC, Lu PN, Huang HL, Chu C, Li HP, Tsai HJ (2014) Zebrafish Transgenic line huORFZ is an effective living bioindicator for detecting environmental toxicants. PLoS ONE 9(3):e90160. doi:10.1371/journal.pone.0090160
Lele Z, Krone P (1996) The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol Adv 14(1):57–72
Liu L, Yan Y, Wang J, Wu W, Xu L (2016) Generation of mt:egfp transgenic zebrafish biosensor for the detection of aquatic zinc and cadmium. Environ Toxicol Chem. doi:10.1002/etc.3362
Ma H, Glenn TC, Jagoe CH, Jones KL, Williams PL (2009) A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ Toxicol Chem 28(6):1311–1318
Mattingly CJ, McLachlan JA, Toscano WA (2001) Green fluorescent protein (GFP) as a marker of aryl hydrocarbon receptor (AhR) function in developing zebrafish (Danio rerio). Environ Health Perspect 109(8):845–849
Misra S, Zafarullah M, Price-Haughey J, Gedamu L (1989) Analysis of stress-induced gene expression in fish cell lines exposed to heavy metals and heat shock. BBA-Gene Struct Expr 1007(3):325–333
OECD (1992) OECD Guidelines for the Testing of Chemicals. Test No. 203: Fish, Acute Toxicity Test, Organisation for Economic Co-operation and Development, Paris
Park JD, Liu Y, Klaassen CD (2001) Protective effect of metallothionein against the toxicity of cadmium and other metals. Toxicology 163(2):93–100
Putri LSE, Prasetyo AD, Arifin Z (2012) Green mussel (Perna viridis L.) as bioindicator of heavy metals pollution at Kamal estuary, Jakarta Bay, Indonesia. J Environ Res Dev 6(3):389–396
Raja CE, Selvam GS (2011) Construction of green fluorescent protein based bacterial biosensor for heavy metal remediation. Int J Environ Sci Technol 8(4):793–798
Roberto FF, Barnes JM, Bruhn DF (2002) Evaluation of a GFP reporter gene construct for environmental arsenic detection. Talanta 58(1):181–188
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schmittgen TD, Livak KJ (2008) Analyzing real time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Seok SH, Park JH, Baek MW, Lee HY, Kim DJ, Uhm HM, Park JH (2006) Specific activation of the human HSP70 promoter by copper sulfate in mosaic transgenic zebrafish. J Biotechnol 126(3):406–413
Seok SH, Baek MW, Lee HY, Kim DJ, Na YR, Noh KJ, Park SH, Lee HK, Lee BH, Ryu DY, Park JH (2007) Quantitative GFP fluorescence as an indicator of arsenite developmental toxicity in mosaic heat shock protein 70 transgenic zebrafish. Toxicol Appl Pharmacol 225(2):154–161
Stephanou A, Latchman DS (2011) Transcriptional modulation of heat-shock protein gene expression. Biochem Res Int 2011:1–8. doi:10.1155/2011/238601
Sun L, Xu W, He J, Yin Z (2010) In vivo alternative assessment of the chemicals that interfere with anterior pituitary POMC expression and interrenal steroidogenesis in POMC:EGFP transgenic zebrafish. Toxicol Appl Pharmacol 248:217–225
The Environmental (Protection) Rules (1986) Ministry of Environment and Forests, India. www.cpcb.nic.in/GeneralStandards.pdf
Tiemann KJ, Gardea-Torresdey JL, Gamez G, Dokken K, Sias S, Renner MW, Furenlid NR (1999) Use of X-ray absorption spectroscopy and esterification to investigate Cr(III) and Ni(II) ligands in alfalfa biomass. Environ Sci Technol 33(1):150–154
Townsend AT, Miller KA, McLean S, Aldous S (1998) The determination of copper, zinc, cadmium and lead in urine by high resolution ICP-MS. J Anal At Spectrom 13(11):1213–1219
Urasaki A, Morvan G, Kawakami K (2006) Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174(2):639–649
Verma N, Singh M (2005) Biosensors for heavy metals. Biometals 18(2):121–129
Viarengo A (1989) Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. Rev Aquat Sci 1(2):295–317
Wan H, Korzh S, Li Z, Mudumana SP, Korzh V, Jiang YJ, Lin S, Gong Z (2006) Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp Cell Res 312:1526–1539
Westerfield M (2000) The Zebrafish Book: A guide for the laboratory use of zebrafish (Danio rerio), 4th edn. Univ. of Oregon Press, Eugene
Wu YL, Pan X, Mudumana SP, Wang H, Kee PW, Gong Z (2008) Development of a heat shock inducible gfp transgenic zebrafish line by using the zebrafish hsp27 promoter. Gene 408(1):85–94
Zou J, Beermann F, Wang J, Kawakami K, Wei X (2006) The Fugu tyrp1 promoter directs specific GFP expression in zebrafish: tools to study the RPE and the neural crest-derived melanophores. Pigment Cell Res 19(6):615–627
Acknowledgments
The authors acknowledge the Department of Biotechnology, Government of India and the Indian Council of Agricultural Research, New Delhi for funding this research project; Dr. Dilip Kumar and Dr. W. S. Lakra, former Directors, ICAR-CIFE, Mumbai and Dr. Gopal Krishna, Director, ICAR-CIFE, Mumbai for providing facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pawar, N., Gireesh-Babu, P., Sabnis, S. et al. Development of a fluorescent transgenic zebrafish biosensor for sensing aquatic heavy metal pollution. Transgenic Res 25, 617–627 (2016). https://doi.org/10.1007/s11248-016-9959-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-016-9959-z