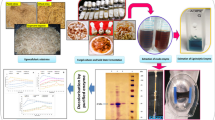
Abstract
In biotechnological methods for biodegradation, the effectiveness of detoxifying xenobiotics and recalcitrant substances in soil and water has sparked significant interest. Our study takes a unique approach by focusing on immobilizing the white-rot fungus (WRF) Pleurotus sajor-caju onto a Luffa cylindrica plant support. This innovative method aims to facilitate the mycoremediation of agro-industrial pollutant pulp wash generated by the orange industry. The immobilization process significantly increased MnP enzymatic activity, reaching 23 IU.mL−1 and Lac activity at approximately 40.5 IU.mL−1. Qualitative SEM and FTIR analyses provided insights into the microorganism’s attachment mechanism to the support, suggesting that aggregation occurs due to an affinity to the lignocellulosic structure, enhancing the production of polysaccharides responsible for biocatalyst adherence and potential reusability. Furthermore, the production of an enzymatic extract rich in ligninolytic enzymes from P. sajor-caju showcased its ability to reduce the toxicity of pulp wash. An exploration into the toxicity of citrus effluent revealed the generation of embryos with severe deformities and the inhibition of Lactuca sativa germination, even at low concentrations. Notably, post-treatment with the enzymatic extract resulted in a remarkable 90% reduction in toxicity to the trophic level of Danio rerio and lettuce seeds. This research significantly contributes to understanding fungal immobilization strategies in environmental biotechnology, emphasizing the potential of agricultural residues as sustainable inducers for enzyme production and their pivotal role in mitigating the environmental impact of agro-industrial waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, the use of biotechnological methods in biodegradation processes has gained more attention than conventional methods because it is a viable option to detoxify or remove xenobiotics and recalcitrants from soil and water [3, 15]. In this sense, white rot fungi are known for their efficiency in degrading aromatic structures and complex phenolic compounds due to their ability to excrete ligninolytic enzymes. [27].
Ligninolytic enzymes, such as lignin peroxidase, manganese peroxidase, and laccase, can catalyze the oxidation of complex xenobiotic substances present in dyes, pesticides, herbicides, and persistent organic pollutants [11]. By breaking down these complex molecules into simpler components, fungi facilitate their biodegradation and removal from the environment. It is common to use a commercial culture medium to stimulate the production of these enzymes during the fermentation process. This medium provides the necessary nutrients for the development of fungi, promoting the production of enzymes efficiently. In addition, phenolic and aromatic compounds, which have structures similar to lignin, are often used as inducers to increase the production of ligninolytic enzymes. [60].
Therefore, agro-industrial residues, rich in ligninolytic compounds, are used as inducers to stimulate the production of these enzymes. Among agroindustrial residues, orange-derived one has been gaining prominence due to the high concentration of organic matter, sugars, acids, and phenolic compounds that contribute to the induction of ligninolytic enzymes [20, 27, 64]. The biodegradation process of the orange residue contributes to reducing costs and the sustainable use of the toxic residue for different trophic levels [64]. The orange residue is obtained after extracting the leading juice, where a second wash is applied to the pulp, producing a low-quality juice with a high soluble solid content [53]. The washed pulp, as it is called, is a yellow liquid residue with an intense smell, high organic load, and presence of limonin substance, which entails low economic value in addition to its indiscriminate disposal in the environment due to genotoxic and phytotoxic implications [29].
Therefore, the high organic load present in orange residues makes the disposal of these residues unfeasible without prior treatment, which leads citrus industries to seek alternatives for managing these materials. Several studies have suggested using these residues to produce industrial enzymes. For example, (2020) highlighted the effectiveness of the pulp-washing system in conjunction with the fungus P. sajor-caju CCIBt 020 as a promising enzyme inducer for laccase, reaching activities of up to 4884 IU.L−1. In addition, this process demonstrated efficacy in mycoremediation by reducing the toxicity of pesticides. Another relevant approach was conducted by Mendoza et al. [39], who cultivated Galerina sp. using orange peels and sugarcane bagasse, resulting in a fourfold increase in enzymatic activity. The laccase extracted from this culture showed the ability to decolorize 60% of the Congo Red and Brilliant Blue G-250 dyes, indicating its potential application. However, it is important to emphasize that the assessment of the toxicity of these processes has not yet been addressed at different trophic levels.
Moreover, the induction of enzymes obtained through agricultural industry waste is a promising approach to replace synthetic inducers and reduce the toxicity of these residues in the environment. Thus, orange pulp wash is an attractive option because it is a lignocellulosic content rich in nutrients that can be used to induce the biosynthesis of Lac and MnP, in addition to being an approach of economic value and environmental importance, being an alternative for reducing costs and improve enzyme production [21, 38].
Although using these fungi in their free form is considered sustainable and efficient, they are often not perfectly adapted for industrial applications [27]. Thus, support for the immobilization of the fungus is a technique that facilitates the subsequent removal of the microorganism, enables the production of enzymes, and reduces stress during fungal growth [49].
Several support categories can be classified according to their origin, including organic, inorganic, and natural supports. Organic supports are created artificially, structured as polymers and hydrogels, with alginate polymer as an example widely used in this context [8]. Inorganic supports, such as glass, ceramics, and polyurethane foam, are also mentioned as means of cell immobilization in fermentation processes [30, 35]. However, it is important to note that these supports can induce changes in cells’ metabolism and enzymatic activity since these are not found in their natural state [41]. On the other hand, natural supports offer a versatile, economical, and highly compatible approach to microorganisms. Comparing environmental mitigation bioprocesses, it’s evident that using fungal-based bioremediation, specifically with white rot fungi immobilized on natural supports, provides a holistic and sustainable solution [44]. This approach harnesses the efficiency of ligninolytic enzymes in degrading pollutants while using of agricultural residues as inducers, which enhances the economic and environmental aspects of enzyme production. In contrast, other technologies like enzyme@MOFs systems may face challenges like specific working mediums, pore size compatibility issues, and limited research. At the same time, the synthesis and application of bismuth nanoparticles for environmental remediation also encounter challenges like the scarcity of research on bio-synthesized materials and the complexity of wastewater composition [17, 52]. Enzyme-based bioremediation processes, focusing on peroxidases and laccases, offer promising results but may require further exploration and optimization [51]. In this sense, agricultural by-products with a lignocellulosic structure, such as vegetable sponges [26, 28] and corn on the cob [69], emerge as promising alternatives for natural supports. These materials facilitate the immobilization of fungi through adhesion and contribute to improving the process’s yield.
Among the various support options, researchers have indicated that the vegetal loofah (Luffa cylindrica) stands out as a highly suitable natural matrix for immobilization. This is due to the remarkable porosity, high specific pore volume, physical stability, non-toxicity, and affordable cost of this material [2]. L. cylindrica, a material of lignocellulosic origin, consists of a natural mixture of hemicellulose (25 to 30%), cellulose (50 to 60%) and lignin (10 to 12%) [10, 40, 62]. This composition contributes to a favorable affinity with ligninolytic fungi, which simplifies the immobilization process and stimulates enzyme production, especially the MnP enzyme, since secondary metabolism plays a crucial role in lignin biodegradation [27, 64].
In this context, the immobilization of fungal cells has emerged as a promising strategy for managing microorganism growth while preserving its enzymatic activity in addition to emphasizing the importance of considering not only the efficiency of handling polluting substances but also the environmental and economic sustainability of the chosen bioprocess. This approach also ensures cells’ resilience when exposed to biotic and abiotic factors within industrial processes. With this perspective in mind, the primary aim of this research was to explore the utilization of agricultural residues as a viable solution for immobilizing P. sajor-caju and subsequently producing biotechnologically relevant enzymes. This study marks the inaugural investigation into the impact of fungal immobilization on the conversion of agricultural pollutants, alongside a comprehensive assessment of the cytotoxic and genotoxic effects associated with pulp washing during Lac and MnP production.
2 Materials and Methods
2.1 Microorganism and Culture Conditions
The substantial capacity of P. sajor-caju to generate commercially valuable lignocellulosic enzymes has been well-documented [26, 28]. Therefore, for this study, a strain originating from the CCIBt (Collection of Algae, Cyanobacteria, and Fungi Culture at the Institute of Botany) under the designation CCIBt 020 was chosen. The fungal culture was cultivated using MEA extract (Malt Extract Agar; EMD Chemicals®), following the protocol outlined by Campo Fernandes et al. [26, 28]. The fungus was subcultured periodically, and after 7 days of development of the microorganism, inoculums were removed for subsequent immobilization in L. cylindrica.
2.2 Pulp Wash
The unadulterated pulp wash derived from the orange juice production process was acquired before the industry’s dilution for disposal, exhibiting the characteristics outlined in Table 1. This material was carefully preserved within a refrigerated chamber at 4 °C to forestall microbial activity until its utilization. For experimental purposes, the pulp wash was diluted at ambient temperature using ultra-pure water, employing the same ratio employed by the industry (1:12). Considering findings from a study conducted by Cruz et al. (2018), the optimal pH for cultivating P. sajor-caju within the pulp wash was identified as 5. Consequently, the effluent’s alkalinity was rectified by adding a NaOH solution (2 M), fostering an environment conducive to fungal proliferation.
2.3 Luffa Cylindrica
The plant loofah (L. cylindrica) was implemented as an immobilizing support for the microorganism, providing resistance to external factors and operational ease. To improve the aggregation of fungal hyphae on the plant support, 1 cm3 cube were extracted from the inner part of the dried fruit of L. cylindrica, washed with distilled water for 40 min at 30 °C and then dried in an oven at 100 °C for 24 h.
2.4 Immobilization of P. sajor-caju cells in L. cylindrica Using Pulp Wash as a Culture Medium
Following the preceding treatment of L. cylindrica cubes, around 1 g (approximately ± 10 cubes) of the prepared supports were introduced into 250 mL Erlenmeyer flasks, each containing 100 mL of the pulp wash. Subsequently, the medium and the plant-based supports underwent autoclaving at 121 °C and 1 atm for 15 min. After cooling to room temperature, the flasks were inoculated with five mycelial discs (1.0 cm ø) from the fungal culture. The control setup involved the inoculation of P. sajor-caju in the residual medium without any immobilizing support. These flasks were then incubated for up to 12 days within an incubator (Tecnal, TE 420) under orbital agitation at 180 rpm, maintaining a temperature of 28 °C (± 2 °C) and darkness. Samples were extracted every 2 days for analysis [26, 28]. The whole graphic mechanism to treat pulp wash using the fungus P. sajor-caju immobilized on Luffa cylindrica for the mycoremediation process can be shown in Scheme 1.
2.5 Enzymatic Activity
Enzyme activity was assessed in triplicate within 2 days. The samples underwent vacuum filtration to segregate the biomass from the supernatant. Ligninolytic enzyme activities (Lac and MnP produced by both the free and immobilized fungus) were quantified from the supernatant and expressed as international units per liter of enzyme broth (UI.L−1), following the method described by Ferreira et al. [50].
For laccase enzymatic activity determination, 1.8 mL of supernatant (crude enzyme solution) was combined with 0.3 mL of the syringaldazine initiator solution (prepared using 0.05 g of syringaldazine dissolved in 50 mL of ethanol), along with 0.9 mL of 0.05 M citrate–phosphate buffer (pH 5.0), and incubated at 30 °C. The oxidation of syringaldazine was monitored by measuring the increase in absorbance at 525 nm after a 10-min reaction period [61]. A negative control was established using boiled samples to account for enzyme denaturation.
MnP activity was determined within a reaction mixture containing 1.8 mL of supernatant (crude enzyme solution), 0.15 mL of MnSO4 solution (2.0 mmol. L−1), 0.3 mL of sodium lactate buffer (0.25 M), 0.15 mL of H2O2 within 0.2 M sodium succinate buffer (pH 4.5), and 0.6 mL of bovine albumin (0.5%). The reaction was initiated with 0.3 mL of phenol red (0.1%). After an initial reading, the reaction ceased using 0.12 mL of 2.0 N NaOH 10 min later, and the absorbance was measured at 610 nm. The enzyme activity was determined through calculations based on the method by Kuwahara et al., [36].
In calculating the kinetic activity of the production of Lac and MnP, the average values of the absorbance triplicates were used in Eq. 1.
where: UI.L−1 = International unity, where international means μmol min.−1
∆Abs = Absorbance [Absfinal (T 10)—Absstart (T 0)].
ε = molar absorption coefficient (L mol−1 cm−1); Lac (ε525 nm) is 65,000 L mol−1 cm−1; MnP (ε610 nm) = 4460 L mol−1 cm.−1
V = Sample solution volume (mL).
t = Reaction time (minutes).
2.6 Physical Analysis of Free and Immobilized Fungal Biomass in L. cylindrica
2.6.1 Scanning Electron Microscopy (SEM)
To understand immobilization mechanism, the morphological structure of the mycelia in L. cylindrica was analyzed by scanning electron microscopy through the microscope JEOL-JSM-6390LV. After filtration, the immobilized biomass was washed in distilled water to remove non-adhered cells. Then, excess moisture was removed at 50 °C for 4 h in a forced convection oven. Microscopy was performed with a voltage acceleration of 10 kV, with secondary electrons (SEI) and a spot size of 50. The samples were metalized with gold with an exposure time of 8 min. Images were obtained at different magnifications in the range of 600–800x.
2.6.2 Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transform infrared spectroscopy (FTIR) analyses were performed to evaluate the possible functional groups responsible for the immobilization. Analyzes of dry fungal biomass from free cells and immobilized in L. cylindrica were performed in a spectrometer (NICOLET 6700, Thermo Scientific, USA). The spectra of the biomass samples were analyzed in wave numbers between 4000 and 650 cm−1 with 32 accumulations, with a maximum resolution of 2 cm−1.
2.6.3 Thermogravimetric Analysis (TGA)
Thermogravimetric analysis of the biomass of the free and immobilized fungal cells in L. cylindrica was carried out in the temperature range between 25 and 1000 °C under a continuous flow of nitrogen at a speed of 50 mL.min−1 through the equipment of SHIMADZU model DTG-60H (simultaneous DTA-TG), with a heating rate of 10 ºC. min−1. This analysis allows for the identification of biomass and evaluation of the interaction of microorganisms with support for immobilization.
2.7 Chemical Analysis of Pulp Wash Before and After Fermentation.
2.7.1 Determination of Chemical Oxygen Demand (COD)
For the determination of COD, the method recommended by APHA (2012) was used. The analysis started with the addition of 1.5 mL of the digester solution to the COD tube, 2.5 mL of the pure sample (sample to be analyzed), and 3.5 mL of catalyst solution in the same tube. Later, the tubes were homogenized and heated at 150 ºC for 120 min in a digester.
At the end of the procedure, the tubes acquire different shades due to the formation of the Cr3+ ion caused by the excess potassium dichromate that reacts with sulfuric acid (H2SO4). The absorbance was quantified in a UV/VIS spectrophotometer with a wavelength of 600 nm. The blank test correctly zeroed the device, replacing 2.5 mL of the pure sample with distilled water. The calibration curve was plotted with different concentrations of the standard solution of Potassium Biphthalate. With the absorption reading, it was possible to monitor the organic matter content of the raw samples (Eq. 2). The analysis was performed for the pulp wash and the enzymatic extracts of free and immobilized fungal cells in L. cylindrica.
2.7.2 Determination of Total Organic Carbon (TOC)
The analytical control for the degradation of the pulp wash effluent was evaluated using TOC readings performed in the Shimadzu device, TOC-LCSN, with an oven operating at 680 ºC. For this, an aliquot of 1 mL was collected, which was diluted in a 500 mL flask with ultra-pure water, at times 0, before the fermentation process and 12 days after finishing the fermentation with free fungal cells and immobilized in L. cylindrica. At first, the samples’ total carbon content (TC) was quantified, and later, the air was purged for TC to extract the dissolved CO2 and measure the inorganic carbon (IC) rate. The TOC was calculated by subtracting the TC from the IC.
2.8 Ecotoxicological Assay
2.8.1 Bioassay with Lactuca sativa
The bioassays with lettuce seeds were carried out according to the methodology proposed by Dutka (1989), where the seeds were obtained commercially, previously without treatments with any herbicide or growth inhibitor, to avoid cross-contamination. The pulp wash samples before and after fermentation with P. sajor-caju free and immobilized in L. cylindrica were diluted by adding distilled water, leaving the samples for testing with the following concentrations: 50%, 25%, 12.5%, 6.25%, 3.12%, and 1.56%.
This range of concentrations indicates results with greater precision, and it is expected that the less concentrated the pollutants are, the more the seed germinates and grows. The negative control is done with NaCl. For the saline solution of NaCl, 2.92 g of the salt was weighed, which was dissolved in a volume of 250 mL, resulting in a solution with a concentration of 0.2 M. It is expected that in the negative control, the growth will be null. As a positive control, distilled water was used, and only the tests that obtained a germination percentage greater than or equal to 90% were valid [56].
A disc of Whatman No. 1 filter paper (85 mm ø) was placed in each Petri dish, previously labeled with its corresponding dilution, performed in triplicate. The filter paper was saturated with 4 ml of the respective dilution solution, and with the help of tweezers, 20 lettuce seeds were placed on the paper, with space between them to allow the roots to grow. The plates were closed and packed with cling film to prevent evaporation of the solutions and then placed in the dark for 5 days (120 h) at a temperature of 22 °C ± 1 °C. After this incubation period, the residue’s average effective concentration (EC50) was calculated using the trimmed Spearman-Karber method (Hamilton et al., 1977).
In addition to the lethality effects, the phytotoxic results were also evaluated through the relative and absolute quantification of the germinated seeds (RG) and (AG), the relative root growth (RRG), and the germination index (IG), according to Eqs. 3, 4, 5 and 6, respectively.
2.8.2 Bioassay with Zebrafish embryos (Danio rerio)
To execute of the bioassays, the embryos of D. rerio were obtained through random crosses between sexually mature individuals. Fertilized eggs were collected the day after mating with a defined age of 1 day. Test organisms were exposed to different concentrations of the material under study. The positive control was performed only with the culture medium to validate the experiment and enable the comparison between the natural development of the embryo and those exposed to contaminants. After performing the initial test, the concentration ranges to perform the definitive test in each group were defined for pulp wash in natura, pulp wash after fermentation with P. sajor-caju, and after fermentation with P. sajor-caju immobilized in L. cylindrica respectively:
-
Pulp wash: 0.0007%; 0.003%; 0.012%; 0.19%; 0.047%
-
P. sajor-caju: 0.19%; 0.39%; 0.78%; 1.56%; 3.12%
-
P. sajor-caju + L.C: 0.19%; 0.39%; 0.78%; 1.56%.
For each concentration, the dilutions were made with an embryo culture medium.
Embryos were individually placed in 2 mL of solution from each group within 24-well polystyrene plates, with 24 organisms per group, allowing precise control over the environment and exposure levels for each embryo. The experiment was conducted at a temperature of 26 °C under a light/dark cycle of 14/10 h, following the OECD (2013) guidelines. Mortality assessments were performed after 96 h of exposure. Live larvae (10 organisms per group) were photographed at 2x magnification using Optika View Version 7.1.1.5 software, which had been calibrated with a millimeter scale. Observations of embryos and larvae were conducted every 24 h using a stereomicroscope (Optika Model SMZ 2 LED) to monitor hatching and identify any malformations by OECD 236 (2013) standards. To analyze mortality percentages, the Statgraphics Centurion software was employed along with the Probit Analysis method to estimate the LC50 (lethal concentration for 50% of the organism population).
Analysis of variance was utilized to compare organism measurements and growth rates. Statistical significance was determined when 95% confidence intervals did not overlap.
3 Results and Discussion
3.1 Enzymatic Activity and Fungal Biomass
Enzymatic activities of Lac and MnP were assessed in suspended cultures of P sajor-caju and in cultures where the fungus was immobilized within L. cylindrica (referred to as P. sajor-caju immobilized), utilizing pulp wash as an agro-industrial inducing substrate (see Fig. 1). The results show that both cultures showed relevant activity from the sixth day of cultivation, reaching the eighth-day peak. However, it was observed that immobilization in L. cylindrica caused a reduction in Lac activity. When the microorganism was cultured in suspension in the pulp wash, the maximum Lac enzyme activity reached 120 IU.mL−1, while for the immobilized fungus, the maximum activity of Lac was 40.5 IU.mL−1 (Fig. 1). Despite the decline in Lac activity due to immobilization, plant support increase in MnP enzymatic activity. After the incubation period of the 10-day incubation period, the immobilized P. sajor-caju showed an MnP activity of 23.4 IU.mL−1, while the microorganism in suspension showed an activity of approximately 16.3 IU.mL−1 in the same period (as shown in Fig. 1).
The enzymatic activity of Lac and MnP was evaluated in correlation with the dry mass (g.L−1) generated by P. sajor-caju, both when immobilized on L. cylindrica using pulp as growth medium (referred to as immobilized P. sajor-caju) and when in the free fungal form in the pulp (referred to as P. sajor-caju). This evaluation was conducted over a 12-day incubation period under shake flask conditions. The waste-washing pulp was diluted at a ratio of 1:12, maintaining a pH of 5 for fungal cultivation
Furthermore, it can be deduced that despite the reduction in Lac activity resulting from the immobilization of P. sajor-caju within L. cylindrica, the utilization of pulp wash has demonstrated itself as a robust enzyme inducer. The observed enzyme activity in this study surpasses that reported in the existing literature [25, 54, 65]. These results substantiate the proposition that pulp wash stands as a promising residual substrate capable of inciting ligninolytic activity in the white rot fungus P. sajor-caju, as previously substantiated in the investigations conducted by Cruz et al. (2018) and Fernandes (2020). In parallel, supplementary investigations have documented analogous findings. For instance, when employing agro-industrial residues such as orange peel and sugar cane, Lac activity exhibited a notable augmentation, potentially escalating up to fourfold, as witnessed in the fungal species Galerina sp [42]. Notably, in the case of P. chrysosporium cultivated on wheat straw, an MnP activity of 993.9 ± 18.4 IU.mL−1 was achieved. Concurrently, the observations of Yehia e Couto [65] demonstrated a pronounced surge in MnP production when cultivating P. sajor-caju on substrates such as orange and banana peels, culminating in an MnP activity of 6.3 IU.mL−1 following 12 days of incubation.
The modulation of enzymatic activities is linked to the adaptive response of the microorganism towards the complex substrates, namely the pulp wash residue and the porous lignocellulosic matrix of the vegetable loofah. The immobilization of the microorganism on the porous lignocellulosic support facilitates pivotal benefits such as enhanced cellular oxygenation, mitigated shear stress, and heightened enzymatic stability in the face of pH variations and fluctuations in carbon or nitrogen availability, as evidenced by Copete-Pertuz et al. [19]. These outcomes align harmoniously with findings from studies conducted by Pérez-Grisales et al. [46], where the immobilization of the white rot fungus Leptosphaerulina sp. within a sponge environment induced notable increments in the activities of the triad of ligninolytic enzymes, namely Laccase (Lac) at 5.56 U mg−1, Versatile Peroxidase (VP) at 8.29 U mg −1, and Manganese Peroxidase (MnP) at 5.75 U mg −1. It is pertinent to highlight that the presence of the antibiotic CPD led to attenuated enzymatic activities, lending support to the conjecture that not solely the substrate support but also the encompassing milieu wields a discernible influence over the enzymatic conduct of white rot fungi. This phenomenon stems from the compositional attributes of the agro-industrial pulp wash effluent, enriched with carbon and nitrogen constituents, thereby instigating the robust production of Lac. Conversely, within the presence of L. cylindrica, a substrate characterized by a rich lignocellulosic composition (comprising cellulose at 50–60%, hemicellulose at 25–28%, and lignin at 10–12%), which concurrently hampers efficient aeration, the proclivity towards MnP production is accentuated [46].
The observed phenomenon herein substantiates findings documented by Sindhu et al. [55] and Ummalyma et al. [63], wherein the pivotal role of aeration in modulating the activity of ligninolytic enzymes in white rot fungi was substantiated. As lignin degradation involves oxidative mechanisms, meticulous aeration regulation contributes significantly to the enhancement of delignification processes. This premise rationalizes that immobilizing microorganisms onto a plant-based support system may not inherently serve as an effective strategy for augmenting Laccase (Lac) production. Such argument is attributed to the immobilization approach leading to a reduction in cellular contact surface area vis-à-vis the inducing substrate. Consequently, this configuration introduces complexities in achieving uniform dispersion and assimilation of the requisite nutrients indispensable for orchestrating the intricate oxidation–reduction reactions characteristic of the enzyme’s catalytic cycle. It is noteworthy, however, that the productivity of Manganese Peroxidase (MnP) is not notably perturbed by aeration dynamics [26, 28].
Supplemental validation is attained through scanning electron microscopy (SEM) to scrutinize the development of the microorganism on the botanical substrate and within the pulp wash. SEM examinations offer insights into the structural characteristics of L. cylindrica and the associated microorganisms. Initial observations reveal the intricate cross-sectional profile of L. cylindrica, exposing a porous and robust cell fiber architecture devoid of any microorganism presence (Fig. 2a). Subsequent examination on the eighth day of fermentation, coinciding with the zenith of enzymatic activity, unveils the complete adhesion of P. sajor-caju onto the L. cylindrica support (as depicted in Fig. 2b). The evident delignification of the cell wall (indicated by the white arrow) underscores the fungus’s utilization of the support as a conveniently accessible substrate. This mechanism triggers an escalation in Manganese Peroxidase (MnP) enzymatic activity, as MnP is the pivotal agent for disintegrating lignin and cellulose constituents intrinsic to the botanical support. This phenomenon is parallel in comparable assessments of hemicellulose, cellulose, and lignin degradation, as Bari et al. [9] reported during the decomposition of wood by Pleurotus ostreatus. Concomitantly, Sunardi et al. [59] have highlighted the transformative influence of substrate modifications on fungal behavior, thus signifying the potential for alterations in ligninolytic enzyme activity during the breakdown of lignocellulosic substrates. Terminating the 12-day fermentation period (as illustrated in Fig. 2c) showcases a discernible shift in the morphological constitution of the fungal biomass. This stage is characterized by many intertwining hyphal structures exhibiting self-aggregation tendencies.
The progressive evolution of the fungus P. sajor-caju within a substrate constituted by waste citrus pulp wash: a Microscopic depiction of untreated L. cylindrica utilized as the immobilizing matrix for fungal cell entrapment; b Micrograph illustrating the co-culture of P. sajor-caju and L. cylindrica, captured after an incubation period of 8 days; c visualization of P. sajor-caju at the culmination of a 12-day fermentation process. These images collectively illuminate the developmental dynamics and temporal transformations undergone by P. sajor-caju in the context of waste citrus pulp wash immobilized in L. cylindrica
3.2 FTIR
The spectra analysis allows us to understand and identify the biochemical composition of the fungal biomass and the agents responsible for the immobilization. The FTIR spectra of free biomass grown in citric waste and immobilized in L. cylindrica in the culture medium after 12 days of fermentation are shown in Fig. 3. and detailed in Table 2.
FTIR spectra of free biomass cultivated in citric waste (P. sajor-caju) at time zero, and with 12 days of cultivation (P. sajor-caju 12 days) and immobilized biomass in L. cylindrica fermented in washing pulp at time zero (P. sajor-caju + L.C) and with 12 days of immobilization (P. sajor-caju + L.C 12 Days) in shaken flask conditions. The wavelengths are divided into I Polysaccharides, II Lipids, III Proteins, IV Lipids, and V Cell wall
The peaks in the region of 1800–600 and 3000–3600 cm−1 regions represent lignocellulolytic organic compounds. In an investigation conducted by Zervakis et al. [68], peaks found in these areas were cited as characteristic of the taxonomy of Pleurotus since they present information about the fungal cell wall and cell membrane-associated compounds.
The scientific literature delineates that filamentous fungi possess cell walls of an extensive linear polymer composed of N-acetylglucosamine. This distinctive structural attribute is notably evident in basidiomycete fungi, such as those belonging to the genus [58]. The functional groups identified at approximately 3250 cm−1 indicate stretching vibrations associated with hydroxyl (–OH) and amino (–NH) derivatives. These hydroxyl radicals play a pivotal role in the oxidation process of recalcitrant compounds inherent within the pulp wash milieu [6, 31]. Other peaks associated with the fungal cell membrane were found at 1640 cm−1, representing the primary amide, and at 1370 cm−1, represented by a characteristic shoulder of aldehydes. (C–O–H) [67].
Important macromolecules such as proteins, lipids, and carbohydrates were found in the spectral profile of free and immobilized cells. Peaks around 1450–1260 cm−1 and between 2915–2845 cm−1 (Fig. 3) are reported by the characteristic absorbance of fatty acids that are related to lipid chains (CH2/CH3) [31, 34]. The bands of these regions show an increase in the transmittance peak with the time of culture for immobilized cells. According to Kosa et al. [34], this behavior indicates that the main biochemical changes were related to accumulating of intracellular lipids (Table 2).
The accumulation of lipids coincided with an enhancement in transmittance related to protein peaks at 1642 and 1537 cm−1 (amides I and II) during cell immobilization. Amide I peaks often indicate of specific secondary structures in a protein sample. In contrast, the amide II band offers insights into hydrogen bonding and the overall conformation of the protein, commonly utilized in conjunction with amide I for a more comprehensive structural analysis. This observation suggests a potential increase in the protein percentage of the cells immobilized on L. cylindrica. A similar trend was noted by Haneef et al. [32], who observed an augmentation in proteins and lipids in P. ostreatus samples cultivated in cellulose-potato-dextrose broth compared to those cultivated in pure cellulose.
The absorption peak at approximately 1050 cm−1 is ascribed to the asymmetrical stretching vibrations inherent to C–O–R and C–O–C bonds. The aromatic spectral region corresponds to the characteristic signatures of lignin, constituting the principal constituent of L. cylindrica [7, 70]. Conversely, the secondary peak potentially signifies the presence of chitin alongside hemicellulose and cellulose structures, which are integral components within the fungal cell wall and the L. cylindrica structure, respectively. At the end of cell immobilization, changes were observed in the region of 1110 cm−1, as observed by Naumann [43], demonstrating characteristic peaks of carbohydrates. We can assume that this increase is related to the action of cell wall polysaccharides (β-glucan, chitin, mannan, chitosan, etc.), with transmittance peaks representing C–O, which were stimulated by the presence of lignin in the immobilizing support. The addition of this peak supports the weighting made by García (2017), where polysaccharides are cited as fixing agents responsible for immobilization on the surface of the support. Therefore, we can conclude that the increase in this region confirms the overlapping of cells in the plant support and is directly correlated with the performance of the biocatalyst in lignin degradation, as observed in the SEM images, in addition to interfering with the relevant enzymatic activities (LAC and MnP).
3.3 TGA
Thermogravimetric analysis determines the decomposition, dehydration, and oxidation of organic compounds. Figure 4 shows the TGA curves of samples of L. cylindrica, free fungal biomass (P. sajor-caju), and immobilized in the structure of L. cylindrica (P. sajor-caju + L.C) at the end of fermentation.
TGA analysis of L. cylindrica (L.C), biomass of free P. sajor-caju cultured in Pulp wash (P. sajor-caju) with 12 days of culture and immobilized in L. cylindrica in Pulp culture medium wash (P. sajor-caju + L.C) with 12 days of immobilization under shaken flask conditions. The residue was diluted 1:12 and pH 5
The analysis of the samples shows that the first stage of thermal degradation occurs between 50 and 200 °C, which corresponds to the loss of absorbed and bound water. It is possible to observe that after fermentation in pulp wash, new peaks appeared at a temperature range of 200 °C, both for the free fungus (P. sajor-caju) and for the immobilized (P. sajor-caju + L.C) fungus. This behavior may indicate that the minerals and simple organic compounds that were adsorbed from the pulp wash are being degraded at this first moment.
The decomposition pattern of the free fungal structure (P. sajor-caju) was different over time, especially from 180 °C to 498 °C, with a significant mass loss between this temperature range, which can be attributed to the degradation of carbon chains present in fungi [22]. In this range, the mass loss was divided into two intervals, where possibly the decomposition of the fungal cell wall, composed of polysaccharides and glycoproteins, occurs. The outer wall composed of proteins decomposes at between 261 to 270 °C, leaving a residual mass of 41%. Soon after, the inner wall, composed of chitin and glucans, decomposes between 341.8 to 498 °C with a residual mass of 30%. This loss may be associated with the decomposition of some organic polymer synthesized by the microorganism during its development. Degradation ends at 961 °C with a residual mass of 15%. Some studies propose that the final combustion is associated with the decomposition of carbonates or fixed carbons of the polysaccharide structure [1]. The analysis shows that fungal biomass supports temperatures close to 200 °C of degradation and consequently promotes the expansion of its application in several biotechnological areas.
The immobilization of the microorganism in the L. cylindrica structure (P. sajor-caju + L.C.) led to a predominance of characteristic fungal spectra attributed to the higher density of the microorganism within the sample. There is a loss of 50% of the mass in the thermogravimetric profiles up to 270 °C, characterizing the decomposition of the external fungal wall composed of glycoproteins. Chitin degradation, the microorganism’s inner cell wall, occurs at 450 °C with a 25% mass loss. The apparent spectrum at 340 °C is similar to the cellulose in L. cylindrica, with a mass loss of 50%. This result indicates the presence of the microorganism in the inner part of L. cylindrica, which corroborates the increase in MnP activity, where the microorganism is using the support to boost enzymatic development. A similar result was observed by Barton-Pudlik et al. [10], who described the impact of A. niger on commercial Lignocel C120 wood composites.
3.4 Chemical Analysis
Effluent treatment effectiveness can be defined as the process that eliminates or reduces the levels of important physical–chemical parameters before its disposal (COD and TOC) [48]. Figure 5 shows the results of the chemical parameters of the raw pulp wash after the submerged fermentation with immobilized P. sajor-caju (P. sajor-caju + L.C) and free. The free fungus reduced 96% of the TOC and 94% of the COD, while the immobilized microorganism caused a reduction of 94% of the TOC and 92% of the COD present in the citrus effluent. The decrease in COD indicates the change of chemical species, while the decrease in TOC points to the mineralization of carbon compounds. This result indicates a robust metabolic activity of P. sajor-caju, capable of using the organic compounds in the pulp wash as a source of carbon and metabolic energy. Similar behavior was described by Cruz et al. (2018) after biological treatment of the pulp wash with P. sajor-caju reduced the COD parameter by 99%.
White-rot fungi are exodigesters, whose excreted ligninolytic enzymes are essential for processing aromatic compounds, including biomolecules with lignin-like organic carbon sources [26, 28]. Due to its chemo-organotrophic metabolism (ability to use diverse organic chemical compounds to obtain energy), the simultaneous depletion of TOC and COD means that P. sajor-caju was processing the organic compounds of the effluent as additional sources of nutrients. Pulp wash is a citrus effluent composed primarily of limonene and linalyl acetate [37], and therefore, it is possible to assume that part of the limonene and linalyl acetate is being transformed into secondary terpenes with a simpler carbon chain.
Lotito et al. [37] and Ponezi et al. [47] performed chromatographic analyses of citric effluent after a batch process using activated sludge. They identified that part of the limonene present in the effluent was transformed into α-terpineol, in addition to a 78% reduction in COD. On the other hand, Onken and Berger [45] identified that limonene was biotransformed into carveol and carvone after fermentation of Pleurotus sapidus in citrus effluent from orange extract. The lack of studies in this area indicates that future chromatographic analyses will be crucial to understanding the compounds’ biodegradation in the pulp wash.
The limonene in this effluent can work as a natural bactericidal barrier to combat external agents, disrupting microbial cell membranes and preventing the proliferation of harmful bacteria. However, high concentrations of limonene cause disturbances in the aquatic ecosystem and irritation to the skin and eyes of humans (Cruz et al., 2018). Thus, the toxicity of the pulp wash was evaluated before and after the production of the fungal enzymatic extract to ensure safety in its applicability, bringing an understanding of the balance and potential ecological impact of limonene in the effluent being crucial for effective wastewater management.
3.5 Ecotoxicity Test on Citrus Effluent (Pulp Wash)
Toxicity tests allow detecting toxic pollutants through the changes caused in the test organism precisely because they are more sensitive to environmental stress [14]. This section discuss the results of using bioassays with L. Sativa and Zebrafish (Danio rerio) embryos to test the toxicity of pulp wash before and after biological treatment with free and immobilized fungal biomass. The results were analyzed using relative germination (RG), absolute germination (AG), relative root growth (RRG), germination index (GI), and average effective concentration (EC50).
3.5.1 Effect of Pulp Wash on Germination of Lactuca Sativa Seeds
The effects on germination and root growth were used as parameters by counting the number of germinated seeds and the average length of the samples after five days of cultivation (120 h). As can be seen in Fig. 6, it was found that in the negative control (with NaCl) total inhibition of seed germination was observed, probably motivated by the salinity of the NaCl solution that made it difficult for the seeds to absorb water, while allowing the entry of ions at toxic levels in the soaked seeds [23]. On the other hand, in the positive control (with distilled water), the effects of RG and AG were verified, as the water facilitated the dissociation of several types of ionic molecules, such as salts and sugars, causing the chemical interaction between the different substances to be inside the vegetable seed were facilitated, promoting energy and nutrients necessary for the growth of the embryonic axis [12].
Regarding germination (Fig. 6), it was noticed that the percentages of RG and GA of the seeds tend to increase with the decrease of the pulp wash concentration. For the samples treated by P. sajor-caju free, the effects were higher than those of samples treated with the immobilized fungus. This suggests that immobilization is repressing the oxidation of toxic substances present in the residue that affect the normal development of seeds.
The results indicated that in low concentrations (1.56 and 6.25%) of the effluent treated with immobilized and free P. sajor-caju, it was possible to obtain high germination rates, reaching 90 and 97% of RG, respectively. Cruz et al. (2018) evaluated the toxicity effect of the pulp wash treated with the free basidiomycete P. sajor-caju through the L. sativa seed and also reached high values of AG (98 and 93%) for concentrations of 1.56 and 3.12% of the effluent, identifying the high level of toxicity of the effluent studied. These results corroborate those found in this study, where the highest germination values were found in the lowest pulp wash concentrations (1 and 3%) in the two treatment conditions.
3.5.2 Effect of Pulp Wash on Relative Root Growth and Germination Index of Lactuca sativa Seeds
Other parameters analyzed were the percentage of relative root growth (RRG) and germination index (GI) of L. sativa in the presence of pulp wash (Fig. 7). It is noticed that the RRG values for concentrations up to 6.25% of the residue biologically treated with free and immobilized cells are higher concerning the positive control with water. Such statement can be explained by the presence of nutrients in the pulp wash, which promoted the stimulation and consequent root growth.
These analyses confirm that untreated pulp wash contains toxic and inhibitory properties even at low concentrations, which makes root growth impossible. Regarding the GI of the seeds, it is noted that, regardless of the morphology of the fungal cells, the treatments favor better conditions for seed germination due to the removal of toxic compounds present in the residue.
According to studies by Cesaro et al. (2015) and Viana et al. (2018), who evaluated the phytotoxicity of fertilizers and effluent from the brewing industry, respectively, using the L. sativa seed, all GI results below 40% indicate sensitivity of seed inhibition, between 40 and 80% slight inhibition, while values between 80 and 120% are not considered significant and values above 120% are considered growth stimulus. The results of this study indicate that the concentrations of 6.25%, 12, 50%, 25.00%, and 50.00% of the pulp wash caused high effects of toxicity to the seeds, concomitantly with the treatment with free and immobilized cells in the concentrations of 1.56%, 3.12%, and 6.25%, promoted the stimulus of seed growth.
Following exposure to unbound fungal mycelia, a statistically significant reduction (p < 0.05) in toxicity was observed, resulting in a complete absence of lethality and efficacious toxicity concentration towards the test organism. The mean effective concentration (EC50), which signifies the concentration of the toxic agent capable of inducing 50% mortality following the exposure period, exhibited a 24-fold decrease (from 1.4% to 34.3%) for the unconstrained microorganism and a 13-fold reduction (from 1.4% to 18.7%) when the fungus was immobilized. Comparative toxicity assessment demonstrated that the pulp wash demonstrated heightened toxicity relative to agro-industrial effluents, exemplified by the oil extract appraised in T. repens (EC50 = 8.68%) and T. aestivum (EC50 = 11.58%) seeds, as examined by Elisashvili et al. [24]. These findings resonate with the investigation conducted by Young et al. [66], which probed cereal remnants in an anaerobic bioreactor and employed L. sativa seeds to probe toxicity levels. Their study unveiled a twofold reduction in toxicity (57.61% to 100%). Utilizing L. sativa seeds as a test organism is paramount in delineating the biological and toxicological dimensions of chemical constituents present within diverse effluents and residues. Given its well-established status, L. sativa serves as a standard species for toxicity assays, and these bioassays offer a cost-effective approach [18].
3.5.3 Effects of Pulp Wash on the Development of Zebrafish (Danio rerio) Embryos
The calculated LC50 values for zebrafish embryos exposed to raw pulp wash and after using it as a substrate inducing the fungus P. sajor-caju free and immobilized in L. cylindrica are shown in Fig. 8. The embryos of the control group showed regular development, as described by the OECD (2013), without significant mortality or detection of malformations (Fig. 8a).
Development of embryos exposed to a control solution. b raw pulp wash. c pulp wash after fermentation with P. sajor-caju to produce crude enzymatic extract. d pulp wash after fermentation process with P. sajor-caju immobilized in L. cylindrica (P. sajor-caju + L.C) for the production of crude enzymatic extract. Arrows indicate the malformation found. Arrow 1, fin fold; arrow 2, Swim bladder; arrow 3, neurocranial skeleton; arrow 4, Yolk sac; arrow 5, Cardiac edema
During the period of ontogenesis, represented by the embryo’s development, the raw pulp wash induced numerous effects that impaired the development and survival of D. rerio at a significance level of p < 0.05 (Fig. 8b). In the first 96 h of exposure to raw effluent, the concentration that caused lethality in 50% of the organisms (LC50) was 0.06%. Additional effects were observed, showing that embryos exposed to raw pulp wash showed abnormality in the fin fold, characterized by the caudal portion, which can cause difficulties in the locomotion of the fish (Fig. 8b, arrow 1), suppression of the swim bladder (Fig. 8b, arrow 2) which interferes with the balance of hydrostatic pressure increasing the swimming energy cost. The images also showed deformity in the neurocranial skeleton (Fig. 8b, arrow 3) and in the yolk sac (Fig. 8b, arrow 4), the pouch responsible for the nutrition of the embryo, which indicates a toxic effect caused by the malabsorption of nutrients necessary for normal vertebrate development. Identifying of edema in the pericardium, the outer portion of the heart, indicates the consequence of a cardiovascular abnormality, possibly due to an increase in the permeability of blood vessels (Fig. 8b, arrow 5).
After the fermentation period with free P. sajor-caju, the toxicity of the pulp wash reduced its negative impact by 90-fold during zebrafish ontogenesis, presenting 96hCL50 of 5.04%. When the fungus was immobilized in L. cylindrica, the reduction in toxicity for the test organism under analysis was less significant (12 times), indicating an LC50 of 0.67%. Given this, we can extrapolate that the catalytic cycle of Lac is capable of mineralizing the organic compounds present in the effluent, also represented by the molecules of limonene and linalyl acetate (present in the pulp wash), into less toxic compounds, since this enzyme presented a better performance. More significant when P. sajor-caju is free (127 U.ml−1). This result also suggests that pulp wash fermentation for 8 days exposed to P. sajor-caju and P. sajor-caju + L.C avoided their lethal toxic effects and malformation of zebrafish embryos. However, genotoxicity tests should be performed and considered in future work to analyze possible long-term damage.
It is important to note that this raw effluent showed more significant toxicity for this trophic level (2nd consumers) when compared to other effluents, such as vinasse (CL50 = 0.34%) [57] and olive oil that has an EC50 of 7.05% [5]. No induced effects on zebrafish were found when the effluent was treated by P. sajor-caju and P. sajor-caju + L.C in other parameters evaluated, such as coagulation, head malformation, somites (vertebral column), tail and otoliths (caudal fin), eye development, and pigmentation. The results of the ecotoxicology analysis indicate that, despite the high reduction of COD and TOC for both fungal treatment settings, the free P. sajor-caju obtained a better response to zebrafish toxicity.
The consistency of these results can be compared to the study carried out by Kala et al. [33], who evaluated the efficiency of remediation of the effluent from carbonization of coal (coke) through zebrafish embryos to investigate the toxicity before and after treatment with O-Fenton oxidation. They showed that there was no significant toxicity after treatment, reducing 80% of the observed lethal effects. In another study carried out by Asgher et al. [4] and Bilal et al. [13], the toxicity was evaluated after mycoremediation of real textile effluents by different trophic levels, where they observed a 50% reduction in toxicity at the end of the bioprocess.
Our findings suggest that using organisms at different trophic levels holds promise as a method to assess contaminants’ toxic potential, environmental sensitivity, and potential reuse in industrial processes. Furthermore, our evaluation of the biodegradation and reduced toxicity of the enzymatic extracts we developed opens up new avenues for their application.
4 Conclusion
According to the analyses carried out in this chapter, it is observed that the process of concentration of Laccase and Manganese peroxidase of P. sajor-caju using citric waste pulp wash resulted in an enzyme extract with high activity (120 IU.mL−1 and 16 IU.mL −1 respectively). The addition of L. cylindrica reduces the activity of Lac 40.5 IU.mL−1. However, it induces an increase in MnP (23 IU.mL−1). The SEM images show delignification of the cellulose fibers of L. cylindrica, indicating that the microorganism causes degradation in the support when the immobilization is performed by adsorption. Therefore, fermentation time is relevant to maintain the integrity of the catalytic cell. Infrared spectra (FTIR) and thermal analysis (TGA) point to the formation of fixing agents such as polysaccharides responsible for immobilization.
When analyzing the crude enzymatic extract generated, it was noted that the free and immobilized P. sajor-caju fungus had good responses for the reduction of pulp wash COD (94 and 92% respectively), as well as for the reduction of TOC (96 and 94%, respectively). The enzymatic extract developed by P. sajor-caju free reduced the toxicity of the pulp wash by 90 times, presenting a 96hCL50 of 5.04% for Danio rerio. Based on these findings, it was feasible to ascertain the optimal timeframe for generating a crude enzymatic extract displaying potential efficacy in degrading of emerging effluents encompassing dyes, antibiotics, and pesticides.
Data availability
Not applicable.
References
Abdel-Galil EA, Abdel Aziz OA, Mostafa AZ, Amin M (2018) Characterization and sorption behavior of some toxic metal ions on Fusarium oxysporum as biomass adsorbent. Desalin Water Treat 133:134–145. https://doi.org/10.5004/dwt.2018.23010
Anastopoulos I, Pashalidis I (2020) Environmental applications of Luffa cylindrica-based adsorbents. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.114127
Aragão MS, Menezes DB, Ramos LC, Oliveira HS, Bharagava RN, Romanholo Ferreira LF, Teixeira JA, Ruzene DS, Silva DP (2020) Mycoremediation of vinasse by surface response methodology and preliminary studies in air-lift bioreactors. Chemosphere 244:125432. https://doi.org/10.1016/j.chemosphere.2019.125432
Asgher M, Wahab A, Bilal M, Nasir Iqbal HM (2016) Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal Agric Biotechnol 6:195–201. https://doi.org/10.1016/j.bcab.2016.04.003
Babić S, Malev O, Pflieger M, Lebedev AT, Mazur DM, Kužić A, Čož-Rakovac R, Trebše P (2019) Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci Total Environ 686:903–914. https://doi.org/10.1016/j.scitotenv.2019.06.046
Backa S, Gierer J, Reitberger T, Nilsson T (1992) Hydroxyl radical activity in brown-rot fungi was studied by a new chemiluminescence method. Holzforschung 46:61–67. https://doi.org/10.1515/hfsg.1992.46.1.61
Baharlouei A, Jalilnejad E, Sirousazar M (2018) Fixed-bed column performance of methylene blue biosorption by Luffa cylindrica: statistical and mathematical modeling. Chem Eng Commun 205:1537–1554. https://doi.org/10.1080/00986445.2018.1460364
Bardi A, Ciummei Y, Spennati F, Moga IC, Gregorio SD, Petroni G, Munz G (2022) Comparing carriers as a support media of white-rot fungi in natural tannins removal. Environ Adv 9:100311. https://doi.org/10.1016/j.envadv.2022.100311
Bari E, Mohebby B, Naji HR, Oladi R, Yilgor N, Nazarnezhad N, Ohno KM, Nicholas DD (2018) Monitoring the cell wall characteristics of degraded beech wood by white-rot fungi: Anatomical, chemical, and photochemical study. Maderas Cienc y Tecnol. https://doi.org/10.4067/S0718-221X2018005001401
Barton-Pudlik J, Czaja K, Grzymek M, Lipok J (2017) Evaluation of wood-polyethylene composites biodegradability caused by filamentous fungi. Int Biodeterior Biodegrad 118:10–18. https://doi.org/10.1016/j.ibiod.2017.01.014
Bautista-Zamudio PA, Flórez-Restrepo MA, López-Legarda X, Monroy-Giraldo LC, Segura-Sánchez F (2023) Biodegradation of plastics by white rot fungi: A review. Sci. Total Environment 901:1–33. https://doi.org/10.1016/j.scitotenv.2023.165950
Bertagnolli CM, de Menezes NL, Storck L, dos Santos OS, Pasqualli LL (2003) Desempenho de sementes nuas e peletizadas de alface (Lactuca sativa L.) submetidas a estresses hídrico e térmico. Rev Bras Sementes 25:7–13. https://doi.org/10.1590/S0101-31222003000100002
Bilal MH, Prehm M, Njau AE, Samiullah MH, Meister A, Kressler J (2016) Enzymatic Synthesis and Characterization of Hydrophilic Sugar Based Polyesters and Their Modification with Stearic Acid. Polym 2016(8):80. https://doi.org/10.3390/POLYM8030080
Charles J, Sancey B, Morin-Crini N, Badot P-M, Degiorgi F, Trunfio G, Crini G (2011) Evaluation of the phytotoxicity of polycontaminated industrial effluents using the lettuce plant (Lactuca sativa) as a bioindicator. Ecotoxicol Environ Saf 74:2057–2064. https://doi.org/10.1016/j.ecoenv.2011.07.025
Chen S, Zhu M, Guo X, Yang B, Zhuo R (2023) Coupling of Fenton reaction and white rot fungi for the degradation of organic pollutants. Ecotoxicol Environ Saf 254:114697. https://doi.org/10.1016/j.ecoenv.2023.114697
Cheng S, Huang A, Wang S, Zhang Q (2016) Effect of different heat treatment temperatures on the chemical composition and structure of Chinese fir wood. BioResources 11:4006–4016. https://doi.org/10.15376/biores.11.2.4006-4016
ChukwuemekaEkeoma B, NnamdiEkeoma L, Yusuf M, Haruna A, KosisochukwuIkeogu C, MericanAljunidMerican Z, Kamyab H, Pham CQ, Vo DVN, Chelliapan S (2023) Recent advances in the biocatalytic mitigation of emerging pollutants: a comprehensive review. J Biotechnol 369:14–34. https://doi.org/10.1016/j.jbiotec.2023.05.003
Colombo A, Módenes AN, Trigueros DEG, de Medeiros BL, Marin P, Monte Blanco SPD, Hinterholz CL (2018) Toxicity evaluation of the landfill leachate after treatment with photo-Fenton, biological and photo-Fenton followed by biological processes. J Environ Sci Heal A Tox Hazard Subst Environ Eng 54:1–8. https://doi.org/10.1080/10934529.2018.1544475
Copete-Pertuz LS, Pérez-Grisales MS, Castrillón-Tobón M, Correa Londoño GA, TafurtGarcía G, Mora Martínez AL, Copete-Pertuz LS, Pérez-Grisales MS, Castrillón-Tobón M, Correa Londoño GA, TafurtGarcía G, Mora Martínez AL (2018) Decolorization of Reactive Black 5 Dye by Heterogeneous Photocatalysis with TiO 2 /UV. Rev Colomb Química 47:36–44. https://doi.org/10.15446/REV.COLOMB.QUIM.V47N2.67922
Cruz YWG, Vieira YA, Vilar DS, Torres NH, Aguiar MM, Cavalcanti EB, Américo-Pinheiro JHP, Soriano RN, Bharagava RN, Lima ÁS, Ferreira LFR (2020) Pulp wash: a new source for the production of ligninolytic enzymes and biomass and its toxicological evaluation after biological treatment. Environ Technol 41:1837–1847. https://doi.org/10.1080/09593330.2018.1551428
Dedousi M, Melanouri EM, Diamantopoulou P (2023) Carposome productivity of Pleurotus ostreatus and Pleurotus eryngii growing on agro-industrial residues enriched with nitrogen, calcium salts, and oils. Carbon Resour Convers 6:150–165. https://doi.org/10.1016/j.crcon.2023.02.001
Ding C, Cheng W, Nie X, Yi F, Xiang S, Asiri AM, Marwani HM (2018) The reactivity of carbonized fungi supported nanoscale zero-valent iron toward U(VI) influenced by naturally occurring ions. J Ind Eng Chem 61:236–243. https://doi.org/10.1016/j.jiec.2017.12.021
Dodd GL, Donovan LA (1999) Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am J Bot 86:1146–1153. https://doi.org/10.2307/2656978
Elisashvili V, Kachlishvili E, Asatiani MD (2018) Efficient production of lignin-modifying enzymes and phenolics removal in submerged fermentation of olive mill by-products by white-rot basidiomycetes. Int Biodeterior Biodegrad 134:39–47. https://doi.org/10.1016/j.ibiod.2018.08.003
Farag AM, Hassan SW, Beltagy EA, El-Shenawy MA (2015) Optimization of production of anti-tumor l-asparaginase by free and immobilized marine Aspergillus terreus. Egypt J Aquat Res 41:295–302. https://doi.org/10.1016/j.ejar.2015.10.002
Fernandes CD, Nascimento VRS, Meneses DB, Vilar DS, Torres NH, Leite MS, Vega Baudrit JR, Bilal M, Iqbal HMN, Bharagava RN, Egues SM, Romanholo Ferreira LF (2020) Fungal biosynthesis of lignin-modifying enzymes from pulp wash and Luffa cylindrica for azo dye RB5 biodecolorization using modeling by response surface methodology and artificial neural network. J Hazard Mater 399:123094. https://doi.org/10.1016/j.jhazmat.2020.123094
Fernandes CD, Vilar DS, Torres NH, Bilal M, Iqbal HMN, Bharagava RN, Egues SM, Ferreira LFR (2021) Fungal Potential for the Degradation of Synthetic Dyes: An Overview of Renewable Alternatives for the Production of Lignin-Modifying Enzymes 153–181. https://doi.org/10.1007/978-981-16-1947-2_7
Fernandes JMC, Sousa RMOF, Fraga I, Sampaio A, Amaral C, Bezerra RMF, Dias AA (2020) Fungal biodegradation and multi-level toxicity assessment of vinasse from distillation of winemaking by-products. Chemosphere 238:124572. https://doi.org/10.1016/j.chemosphere.2019.124572
Garcia-Castello E, Rodriguez-Lopez A, Conidi C, Cassano A (2022) Valorization of citrus by-products by membrane processes. Membrane Engineering in the Circular Economy, Elsevier BV, pp. 413–436. https://doi.org/10.1016/B978-0-323-85253-1.00009-5
Gonçalves EV, Martins MD, Xavier dos Santos S, Borges LL, Caramori SS (2023) Immobilized fungi in commercial polyurethane foam remove short-time phosphorus from domestic effluents. Environ. Challenges 11:100693. https://doi.org/10.1016/j.envc.2023.100693
Gupta BS, Jelle BP, Gao T (2015) Application of ATR-FTIR Spectroscopy to Compare the Cell Materials of Wood Decay Fungi with Wood Mould Fungi. Int J Spectrosc 2015:1–7. https://doi.org/10.1155/2015/521938
Haneef M, Ceseracciu L, Canale C, Bayer IS, Heredia-Guerrero JA, Athanassiou A (2017) Advanced Materials from Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci Rep 7:1–11. https://doi.org/10.1038/srep41292
Kala S, Sogan N, Verma P, Naik SN, Agarwal A, Patanjali PK, Kumar J (2019) Nanoemulsion of cashew nut shell liquid bio-waste: Mosquito larvicidal activity and insights on possible mode of action. South African J Bot 127:293–300. https://doi.org/10.1016/j.sajb.2019.10.006
Kosa G, Kohler A, Tafintseva V, Zimmermann B, Forfang K, Afseth NK, Tzimorotas D, Vuoristo KS, Horn SJ, Mounier J, Shapaval V (2017) Microtiter plate cultivation of oleaginous fungi and monitoring of lipogenesis by high-throughput FTIR spectroscopy. Microb Cell Fact 16:1–12. https://doi.org/10.1186/s12934-017-0716-7
Kujawa J, Głodek M, Li G, Al-Gharabli S, Knozowska K, Kujawski W (2021) Highly effective enzymes immobilization on ceramics: Requirements for supports and enzymes. Sci Total Environ 801:149647. https://doi.org/10.1016/j.scitotenv.2021.149647
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracelluar H2 O2 -dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250. https://doi.org/10.1016/0014-5793(84)80327-0
Lotito AM, De Sanctis M, Pastore C, Di Iaconi C (2018) Biomethanization of citrus waste: effect of waste characteristics and of storage on treatability and evaluation of limonene degradation. J Environ Manage 215:366–376. https://doi.org/10.1016/j.jenvman.2018.03.057
Maguire NAP, Kuhmann T, Gerlach D, Fan R, Czermak P (2022) Statistical mixture designs for media development with agro-industrial residues—supporting the circular bioeconomy. EFB Bioeconomy J 2:100023. https://doi.org/10.1016/j.bioeco.2022.100023
Mendoza L, Ibrahim V, Álvarez MT, Hatti-Kaul R, Mamo G (2014) Laccase production by Galerina sp. and its application in dye decolorization. Journal of Yeast and Fungal Research 5:13–22. https://doi.org/10.5897/JYFR12.025
Moin S, Abbasi MW, Naeem A, Rauf A et al (2021) Short term exposure with ultraviolet radiations: a strategy to improve resistance against root-infecting fungi in Luffa cylindrica (L) Roem. Acta Ecol Sin 41:157–163. https://doi.org/10.1016/j.chnaes.2021.02.013
Moreno-García J, García-Martínez T, Mauricio JC, Moreno J (2018) Yeast immobilization systems for alcoholic wine fermentations: Actual trends and future perspectives. Front Microbiol 9:241. https://doi.org/10.3389/fmicb.2018.00241
Munir N, Asgher M, Tahir IM, Riaz M, Bilal M, Shah SMA (2015) Utilization of agro-wastes for production of ligninolytic enzymes in liquid state fermentation by Phanerochaete chrysosporium-IBL-03. Iternational J Chem Biochem Sci 7:9–14
Naumann A (2015) Advanced microscopy in mycology. Springer, pp 61–88. https://doi.org/10.1007/978-3-319-22437-4
Navina BK, Velmurugan NK, Senthil Kumar P, Rangasamy G, Palanivelu J, Thamarai P, Vickram AS, Saravanan A, Shakoor A (2024) Fungal bioremediation approaches for the removal of toxic pollutants: Mechanistic understanding for biorefinery applications. Chemosphere 350:141123. https://doi.org/10.1016/J.CHEMOSPHERE.2024.141123
Onken J, Berger RG (1999) Effects of R-(+)-limonene on submerged cultures of the terpene transforming basidiomycete Pleurotus sapidus. J Biotechnol 69:163–168. https://doi.org/10.1016/S0168-1656(99)00040-1
Pérez-Grisales MS, Castrillón-Tobón M, Copete-Pertuz LS, Plácido J, Mora-Martínez AL (2019) Biotransformation of the antibiotic agent cephadroxyl and the synthetic dye Reactive Black 5 by Leptosphaerulina sp. immobilised on Luffa (Luffa cylindrica) sponge. Biocatal Agric Biotechnol 18:101051. https://doi.org/10.1016/J.BCAB.2019.101051
Ponezi AN, Duarte MCT, Coraucci Filho B, de Figueiredo RF (2005) Análise da biodegradação dos componentes do óleo cítrico por CG/EM e análise da população microbiana de um reator de lodo ativado no tratamento de água residuária de uma indústria cítrica. Eng Sanit e Ambient 10:278–284. https://doi.org/10.1590/s1413-41522005000400003
Prabakar D, Suvetha KS, Manimudi VT, Mathimani T, Kumar G, Rene ER, Pugazhendhi A (2018) Pretreatment technologies for industrial effluents: Critical review on bioenergy production and environmental concerns. J Environ Manage 218:165–180. https://doi.org/10.1016/j.jenvman.2018.03.136
Przystaś W, Zabłocka-Godlewska E, Grabińska-Sota E (2018) Efficiency of decolorization of different dyes using fungal biomass immobilized on different solid supports. Brazilian J Microbiol 49:285–295. https://doi.org/10.1016/j.bjm.2017.06.010
Romanholo Ferreira LF, Aguiar MM, Messias TG, Pompeu GB, Queijeiro Lopez AM, Silva DP, Monteiro RT (2011) Evaluation of sugar-cane vinasse treated with Pleurotus sajor-caju utilizing aquatic organisms as toxicological indicators. Ecotoxicol Environ Saf 74:132–137. https://doi.org/10.1016/J.ECOENV.2010.08.042
Saddique Z, Imran M, Javaid A, Latif S, Kim TH, Janczarek M, Bilal M, Jesionowski T (2023) Bio-fabricated bismuth-based materials for removal of emerging environmental contaminants from wastewater. Environ Res 229:115861. https://doi.org/10.1016/j.envres.2023.115861
Saddique Z, Imran M, Javaid A, Rizvi NB, Akhtar MN, Iqbal HMN, Bilal M (2024) Enzyme-linked metal organic frameworks for biocatalytic degradation of antibiotics. Catal Letters 154:81–93. https://doi.org/10.1007/S10562-022-04261-3/TABLES/2
Schalow S, Baloufaud M, Cottancin T, Fischer J, Drusch S (2018) Orange pulp and peel fibres: pectin-rich by-products from citrus processing for water binding and gelling in foods. Eur Food Res Technol 244:235–244. https://doi.org/10.1007/s00217-017-2950-y
Seyide S, Karaduman AB, Yamaç M (2017) Increasing of laccase and manganese peroxidase activity by co-culture of immobilized pleurotus ostreatus and lentinus tigrinus mycelia. Mantar Dergisi 8(2):152–162. https://doi.org/10.15318/Fungus.2017.46
Sindhu R, Gnansounou E, Binod P, Pandey A (2016) Bioconversion of sugarcane crop residue for value added products—an overview. Renew Energy 98:203–215. https://doi.org/10.1016/j.renene.2016.02.057
Sobrero MC, Ronco A (2004) Ensayos de toxicidad aguda con semillas de lechuga 55 Ensayo de toxicidad aguda con semillas de lechuga Lactuca sativa L.
Sousa RMOF, Amaral C, Fernandes JMC, Fraga I, Semitela S, Braga F, Coimbra AM, Dias AA, Bezerra RM, Sampaio A (2019) Hazardous impact of vinasse from distilled winemaking by-products in terrestrial plants and aquatic organisms. Ecotoxicol Environ Saf 183:109493. https://doi.org/10.1016/j.ecoenv.2019.109493
Sriharsha DV, Kumar RL, Savitha J (2017) Immobilized fungi on Luffa cylindrica: An effective biosorbent for the removal of lead. J Taiwan Inst Chem Eng 80:589–595. https://doi.org/10.1016/j.jtice.2017.08.032
Sunardi, Tanabe J, Ishiguri F, Ohshima J, Iizuka K, Yokota S (2016) Changes in lignocellulolytic enzyme activity during the degradation of Picea jezoensis wood by the white-rot fungus Porodaedalea pini. Int Biodeterior Biodegrad 110:108–112. https://doi.org/10.1016/j.ibiod.2016.02.022
Suryadi H, Judono JJ, Putri MR, Eclessia AD, Ulhaq JM, Agustina DN, Sumiati T (2022) Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 8:e08865. https://doi.org/10.1016/j.heliyon.2022.e08865
Szklarz GD, Antibus RK, Sinsabaugh RL, Linkins AE (1989) Production of Phenol Oxidases and Peroxidases by Wood-Rotting Fungi. Mycologia 81:234. https://doi.org/10.2307/3759705
Tanobe VOA, Sydenstricker THD, Munaro M, Amico SC (2005) A comprehensive characterization of chemically treated Brazilian sponge-gourds (Luffa cylindrica). Polym Test 24:474–482. https://doi.org/10.1016/j.polymertesting.2004.12.004
Ummalyma SB, Supriya RD, Sindhu R, Binod P, Nair RB, Pandey A, Gnansounou E (2019) Biological pretreatment of lignocellulosic biomass—Current trends and future perspectives. Second and Third Generation of Feedstocks, Elsevier, pp 197–212. https://doi.org/10.1016/b978-0-12-815162-4.00007-0
Vilar DS, Fernandes CD, Nascimento VRS, Torres NH, Leite MS, Bharagava RN, Bilal M, Salazar-Banda GR, Eguiluz KIB, Romanholo Ferreira LF (2021) Hyper-production optimization of fungal oxidative green enzymes using citrus low-cost byproduct. J Environ Chem Eng 9:105013. https://doi.org/10.1016/J.JECE.2020.105013
Yehia RS, Rodriguez-Couto S (2017) Discoloration of the azo dye Congo Red by manganese-dependent peroxidase from Pleurotus sajor caju. Appl Biochem Microbiol 53:222–229. https://doi.org/10.1134/S0003683817020181
Young BJ, Riera NI, Beily ME, Bres PA, Crespo DC, Ronco AE (2012) Toxicity of the effluent from an anaerobic bioreactor treating cereal residues on Lactuca sativa. Ecotoxicol Environ Saf 76:182–186. https://doi.org/10.1016/j.ecoenv.2011.09.019
Zdarta J, Jesionowski T (2016) Luffa cylindrica sponges as a thermally and chemically stable support for Aspergillus niger lipase. Biotechnol Prog 32:657–665. https://doi.org/10.1002/btpr.2253
Zervakis GI, Bekiaris G, Tarantilis PA, Pappas CS (2012) Rapid strain classification and taxa delimitation within the edible mushroom genus Pleurotus through the use of diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy. Fungal Biol 116:715–728. https://doi.org/10.1016/j.funbio.2012.04.006
Zhang A, Wang G, Gong G, Shen J (2017) Immobilization of white rot fungi to carbohydrate-rich corn cob as a basis for tertiary treatment of secondarily treated pulp and paper mill wastewater. Ind Crops Prod 109:538–541. https://doi.org/10.1016/j.indcrop.2017.09.006
Zou W, Bai H, Gao S, Li K (2013) Characterization of modified sawdust, kinetic and equilibrium study about methylene blue adsorption in batch mode. Korean J Chem Eng 30:111–122. https://doi.org/10.1007/s11814-012-0096-y
Acknowledgements
This investigation received partial financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—under Finance Code 001, a Brazilian foundation operating within the purview of the Ministry of Education (MEC); Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brazil (CNPq), through grant numbers 314150/2021-8 and 405797/2022-2, a Brazilian foundation affiliated with the Ministry of Science and Technology (MCT); and FAPITEC/SE (Foundation for Support to Research and Technological Innovation of the State of Sergipe), under grant number 88887.157371/2017-00.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernandes, C.D., de Castro, V.L.S.S., Vallim, J.H. et al. Enzymatic Extract from Luffa-Immobilized Pleurotus sajor-caju: A Promising Biocatalyst for Agro-Industrial Pollutant Reduction and Toxicity Mitigation. Top Catal (2024). https://doi.org/10.1007/s11244-024-01970-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-024-01970-4