Abstract
Activity of heterogeneous catalysts for synthesis of Guerbet alcohols from hexanol was evaluated. Commercial synthetic hydrotalcite (HT) was used in the aldol condensation reaction, which is a part of the Guerbet reaction network. HTs were calcined at different temperatures in order to modify their basicity and additionally, NaOH was applied in some experiments as a homogeneous base. The homogeneous base proved to be more efficient in aldol condensation experiments, while HT also performed in an acceptable way. Bi-functional metal containing HTs were synthesized by wet impregnation and co-precipitation methods employing different active metals. The materials were characterized with a number of methods, including CO2-TPD, pyridine-FTIR, nitrogen physisorption, transmission electron microscopy, SEM–EDX and XRD. Copper containing catalysts produced hexyl-hexanoate with a very high selectivity, while Ni-containing counterparts exhibited the highest selectivity towards the Guerbet alcohol. The co-precipitated catalysts were more active in the current study than the ones produced by wet-impregnation. Nevertheless, synthesis of Guerbet alcohols from hexanol with a one-pot method proved to be challenging, with the best yield of the Guerbet product in 24 h being 5%. This is proposed to be largely due to thermodynamic limitations, which was confirmed by calculations of thermodynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The interest in fuels and chemicals derived from biological origins has increased greatly in the recent years. Alcohols from renewable sources are becoming more available in industrial quantities. The most easily produced alcohol is ethanol, therefore a large part of the current research concentrates on using it as a feedstock in further valorization [1,2,3,4].
Compared to ethanol, hexanol is more difficult to produce through either fermentation or chemical routes. Recent research indicates, however, that chemo-catalytic conversion of wood based cellulose to hexanol could be possible [5]. This reaction is very sensitive and requires careful optimization of reaction conditions for producing hexanol selectively.
One potential way to upgrade hexanol and other alcohols is the Guerbet reaction, which generates 2-alkyl alcohols from shorter alcohol monomers. These compounds, called Guerbet alcohols, have a unique branching pattern which can provide many interesting properties. Much research has been performed using the Guerbet reaction for the production of butanol from ethanol [6,7,8,9,10,11].
The current production capacity of Guerbet alcohols is limited. As an example, it can be mentioned that 2000–3000 tons/year of Guerbet alcohols were produced as specialty chemicals in 2010 by Exxon and Henkel [12]. However, with the increased potential production of renewable alcohols, the interest in the reaction has increased. Currently, one of the most produced Guerbet alcohols is 2-ethyl-hexanol which is widely applied in synthesis of plasticizers [13].
The most appealing way to produce Guerbet alcohols would be a so called one-pot reaction starting from short chain alcohols, where the overall reaction takes place in one reactor vessel. Most studied catalysts and those that can be found in patents are either completely homogeneous or homo-/heterogeneous. These kinds of systems cause some difficulties, e.g. separation of the products can be difficult. Because of these reasons, the present work aims to use a heterogeneous catalyst in the Guerbet-reaction with hexanol.
In general, in the Guerbet reaction two alcohols react to form an alcohol with a longer and often more branched carbon chain. The alcohol monomers can be identical or different, e.g. two ethanol molecules can form n-butanol, or propanol can react with methanol to form isobutanol. The compounds formed in the Guerbet reaction have lower melting points than the corresponding unbranched compounds with the same carbon number, partly because of the branching, which enables them to be used in applications where e.g. lubrication is important [14]. They are also saturated, which makes them very stable, even at elevated temperatures.
The reaction is traditionally performed in the presence of an alkoxide or some other base, e.g. KOH. Recently, several studies have concentrated on heterogeneous or combined homogeneous/heterogeneous systems [6,7,8,9,10,11, 15,16,17,18] due to, e.g., the easier separation of the catalyst from the reactants. A challenge in developing a system completely based on heterogeneous catalysis is that the reaction produces water, which deactivates many catalysts, or it promotes side reactions. This problem is also present in some systems with a homogenous base such as an alkoxide, as the water produced in the reaction neutralizes the base [19]. There exists no research reported in the open literature focusing on the Guerbet reaction of C6 alcohols utilizing heterogeneous catalysts in liquid phase systems.
The Guerbet reaction has been proposed to most probably proceed through three basic reaction steps, which can be seen in Fig. 1. The first step (1) is dehydrogenation of the alcohol-hexanol, releasing hydrogen. This is, at least at low temperatures (130–140 °C), the rate limiting step. The first and last steps can be catalyzed by an active metal that promotes both dehydrogenation and hydrogenation. The aldol condensation is typically catalyzed by a base, e.g. different hydroxides or alkoxides, whereas acid sites are required for dehydration. With the increased interest in solid base catalysis, recent studies often concentrate on completely heterogeneous catalytic systems, often with bifunctional catalysts, however, the literature on the subject is still limited [20].
In the second step (2) two aldehydes from the previous step react in an aldol condensation reaction, where the carbonyl group in one aldehyde reacts with the α-carbon in the other aldehyde forming 2-butyl-2-octenal (2B2O) and a water molecule. The final step (3) is hydrogenation of 2B2O to 2-butyl-octanol (2BO), which requires hydrogen. The overall reaction being an example of so-called hydrogen borrowing reactions is theoretically self-sufficient in hydrogen. High concentrations of added molecular hydrogen have been observed to influence the overall reaction rate negatively, as dehydrogenation is hindered [18, 21]. On the other hand, Moteki and Flaherty [22] have recently reported that in ethanol condensation over Ca-hydroxyapatite at 275 °C under atmospheric pressure with varying amounts of hydrogen the rate for C–C bond formation followed the zero order. Furthermore, a minor positive effect of hydrogen facilitating enhanced butanol formation was observed in ethanol condensation over Pd/Mg–Al and Ru/Mg–Al mixed oxides [6]. It was additionally reported in [23] that the rate for the reaction between ethanol and deuterium at 300 °C under atmospheric pressure in the gas phase was enhanced in the presence of Cu0.5Mg5CeOx, while in the absence of copper the gas phase reaction of D2 was very slow and hydrogen atoms were originating from the catalyst surface. This result indicates that hydrogen cannot be efficiently used in the subsequent hydrogenation step after dehydrogenation over mixed oxide catalysts [24].
In the current work aimed primarily at catalyst screening the option of additional supply of hydrogen was not pursued.
At low temperatures dehydrogenation is the rate-limiting step. At higher temperatures of about 160–180 °C the aldol condensation limits the overall rate. When using reaction temperatures higher than 180 °C undesired side reactions start to occur. The most used and most reactive raw materials applied in the Guerbet reaction are unbranched primary alcohols, as branching can obstruct compounds from reacting through steric hindrances [14].
A side product of the reaction is an ester, in this case hexyl-hexanoate (HH), which may be produced through the so-called Tishchenko reaction, catalyzed by the Lewis acid sites that may also be present in solid bases [25]. MgO can be an efficient catalyst for the Tishchenko reaction, therefore it may not be an optimal one in the Guerbet reaction, at least in the liquid phase. Hydrotalcite (HT) in turn should be, if not completely inactive, but at least not efficient in esterification [25, 26].
As the aldol condensation reaction in the Guerbet reaction is base catalyzed, using a solid base would be beneficial. Alkali oxides are much stronger bases than e.g. HT, being weaker than Na-doped superbases [19]. Weaker bases may be still useful, as they may have other beneficial properties such resistance to water or lower selectivity to side products. It is also known that CaO reacts to the much less reactive Ca(OH)2 when exposed to water [20].
One of the supports used in this work was mesoporous HT, which was reported by Carlini et al. [15] to be active in the Guerbet reaction. It belongs to a group of layered double hydroxides having formula Mg6Al2(OH)16CO3·4H2O. The structure is formed by substituting Mg2+ cations in brucite-like Mg(OH)2 with Al3+ cations. This creates a charge imbalance in the layers, which is compensated by anions such as \({\text{CO}}_{3}^{{2 - }}.\) The structure enables HTs to be used as bifunctional catalysts, as the cations can be substituted with other cations, e.g. Cu or Ni. Mg2+ or Al3+ cations can be even completely substituted by other cations, such as Zn2+, keeping general structure the same [15, 27].
Calcined HT is active in the aldol condensation reaction, which can be acid or base catalyzed [28], while its basicity is not as strong as e.g. of MgO. However, it is more resistant to water, justifying its application in the Guerbet reaction [15]. HTs were reported to be resistant to deactivation, according to Marcu et al. [29], who investigated Cu-containing HTs.
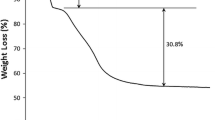
Calcination is a very important step to activate HT. When thermogravimetric analysis was performed for HT [28], the first weight loss has been observed to occur between 82 and 217 °C, because of the release of adsorbed and interlayer water. The second region of weight loss occurred between 227 and 440 °C. This indicates that the HT should be calcined at temperatures at least over 440 °C allowing the interlayer to be completely devoid from hydroxyl and carbonate molecules forming thereby the basic sites [28]. HT obtains its basic nature only after calcination at appropriate temperatures to release water and \({\text{CO}}_{3}^{{2 - }}\) from the interlayer and adsorbed water bound to the surface. Strong Lewis basic sites can be formed because of the presence of O2−, and weak acid sites because of Al3+ ions. If HT is rehydrated with decarbonated water Brønsted basic sites may appear because of OH− groups [30].
The aim of the current work was to explore the possibilities to produce the Guerbet alcohol 2BO from hexanol with heterogeneous catalysis in the liquid phase. The methodology has been applied previously rather successfully to reactions involving methanol, ethanol and propanol [6, 15], but higher alcohols have been very scarcely studied and reported in literature, especially utilizing purely heterogeneous catalysis [31].
2 Experimental
2.1 Materials
The materials used were hexanol (Sigma-Aldrich, 98%), hexanal (Sigma-Aldrich, 98%), dodecane (Sigma-Aldrich, ≥ 99%), dihexylether (Sigma-Aldrich, 97), HH (Sigma-Aldrich, ≥ 97), HT (synthetic, Sigma-Aldrich), copper(II) nitrate (Fluka, 99–104%), nickel(II) nitrate (Fluka, ≥ 97), CaO (Merck), NaOH (Merck), magnesium nitrate (J.T. Baker), aluminium nitrate (Merck), pyridine (Sigma-Aldrich, > 99,5%).
2.2 Catalyst Preparation
The catalysts were prepared by two methods. In the wet impregnation procedure predetermined amounts of nitrates were dissolved in distilled water. Ten grams of HT (Sigma) calcined at 600 °C for 5 h were added to the nitrate solution. This mixture was left to age at 60 °C for 24 h. After aging water was evaporated under vacuum and the resulting powder was dried at 100 °C overnight and then calcined [32].
In the coprecipitation procedure predetermined quantities of the metal nitrates were dissolved in 150 ml of deionized water to achieve concentration of the metal ions 1 M. The metals were Mg and Al, and either Ni or Cu. The amounts of Mg and Al were selected to give the same Mg/Al ratio as in the commercial HT ca. 2.25. The amount of metals was calculated to give 10% nominal loading if all other metals were in the oxide form. Equimolar amounts of Al2(CO3)3 and Na2CO3 were dissolved in 300 ml of deionized water to achieve a concentration of approximately 0.67 M. The carbonate solution was placed in a five-necked round flask, which was then put in a heating jacket and equipped with a stirrer and a combined pH and temperature probe. The solution was kept at 60 ± 3 °C during precipitation as the nitrates were added dropwise during approximately 30 min and the pH was controlled by manual dropwise addition of a 2 M NaOH solution to keep the solution pH at 10 ± 0.1. After complete addition of nitrates, the mixture was the aged for 1 h with stirring and 18 h without stirring at 60 °C. Thereafter the precipitated material was filtered and washed with deionized water, dried at 100 °C overnight and calcined at a chosen temperature for 5 h [33,34,35]. The co-precipitated (CP) Cu/HT catalyst was synthesized to give the molar percentages of Cu/Mg/Al metals 11/60/29, respectively [15, 16, 32].
2.3 Catalyst Characterization
2.3.1 Nitrogen Physisorption
BET specific surface area measurements were performed with a Sorptometer 1900 (Carlo Erba Instruments) at 77 K. The specific pore volume was calculated with the Dollimore–Heal equation.
2.3.2 CO2-TPD
CO2-TPD was done using Micromeritics Autochem 2010. The sample was placed in a glass vial which was positioned in an oven with the appropriate gas lines connected, and then pretreated in He-flow at the temperature of calcination for each sample (400–600 °C) to remove water and other contaminants, and then cooled to ambient temperature. Thereafter the catalysts where reduced ex situ in 40 ml/min hydrogen at 400 °C for 3 h. The samples were then exposed to a flow of CO2 at 100 °C allowing CO2 adsorption on the surface. A flow of He was used to flush away physisorbed CO2. Temperature was increased linearly by 10 °C/min under He flow to the calcination temperature, measuring the amount of desorbed CO2. The area under the curve was integrated and the desorption amounts were calculated with the previously calibrated values. Only the results below the calcination temperature of the materials will be shown.
2.3.3 Pyridine Adsorption and Desorption with FTIR
Acidity was measured by pyridine-FTIR. The samples were analyzed by pressing the catalyst into self-supporting thin pellets weighing 15–25 mg. The pellets were prepared for analysis by outgassing at 450 °C for 1 h in vacuum in situ. Pyridine was then adsorbed on the surface in He flow for 30 min. The samples were heated from 100 to 150, 250 and 350 °C and cooled back to 100 °C between each temperature raise. Spectra measured several times after each respective temperature rise were compared to the background spectrum. Spectral bands at 1450 and 1545 cm− 1 were used to identify Lewis and Brønsted acid sites. The areas of the peaks at respective sites were integrated, and the amount of sites was quantified with the extinction coefficient reported by Emeis [36].
2.3.4 Transmission Electron Microscopy
The morphology and metal particle size of some catalysts were studied with transmission electron microscopy (TEM). The equipment used for TEM was JEM 1400 plus with an acceleration voltage of 120 kV. The particle sizes were measured from the TEM pictures with the software ImageJ.
2.3.5 Scanning Electron Microscopy
The SEM analysis was performed with a Zeiss Leo Gemini 1530 microscope, which was equipped with secondary electron and backscattered electron detectors. To get the composition of some of the materials, EDX analysis was used to perform elemental analysis, with an acceleration voltage of 15 kV. A carbon coating was applied increasing conductivity and enabling more accurate pictures with high magnification. At the same time such carbon coating prevents determination of carbon already present in the catalysts in the form of interlayer carbonates.
2.3.6 XRD
A Philips X’Pert Pro MPD X-ray powder diffractometer was used in the XRD measurements. The diffraction mode used was Bragg–Brentano, and the monochromatized Cu Kα X-ray beam was generated with a voltage of 40 kV and a current of 45 mA. A fixed 0.25° divergence slit and a fixed 15 mm mask were used to collimate the primary X-ray beam. A 7.5 mm anti-scatter slit was used in the diffracted beam side prior to the proportional counter. The measured diffractograms were analyzed with Philips X’Pert HighScore and MAUD programs. HighScore together with MAUD was used for the phase analysis and MAUD for the Rietveld refinement. The phase detection threshold of this measurement is approximately 5%. The measured 2θ angle range was 6.6°–80.0°, with a step size of 0.026° and a measurement time of 90 s per measurement. The Rietveld refinement method was used to estimate the crystal size dc and the amount of MgO phase in the sample. HT ((Mg4Al2)(OH)12(CO3)(H2O)3)0.5 with the trigonal crystal system was used as a HT reference.
2.4 Experimental Setup for Evaluation of Catalytic Activity
The reactor used in the catalyst screening process was a 300 ml Parr autoclave. Either hydrogen or argon could be introduced into the reactor through the gas inlet valve. The liquid sampling valve was connected to the gas inlet, which makes it possible to empty the sampling tube after the sample has been taken. The catalysts were activated ex-situ in hydrogen flow before the reaction if needed.
The reactor was filled with dodecane as a solvent (150 ml) and 3 ml of the reagent, mostly hexanol or hexanal. Reagents, reaction time and reaction temperature were varied. The majority of experiments were performed at 200 °C. Other conditions were also tested. The catalysts were activated at 400 °C in hydrogen flow for 3 h. Thereafter the catalyst was added into the mixture, the reactor was sealed, purged three times with argon and then pressurized with 11 bar of inert argon gas. The catalyst was ground to a fine powder below 63 µm to avoid intraparticle mass transfer limitations. A stirring speed of 800 rpm was used, which was sufficient to suppress external mass transfer limitations. The reactor was rapidly heated to the reaction temperature, 200 °C unless otherwise stated. The samples were taken through the liquid sampling valve at predetermined times, and filtered with a 7 µm filter to remove any catalyst particles that could harm the analyzing instruments. 0.5 g of the CP catalysts and 0.75 g of the neat HTs were used.
2.5 Product Analysis
Quantitative analysis was done with a gas chromatograph (Agilent 6890N) equipped with a flame ionization detector and a Agilent DB-1 column (length 30 m, diameter 0.250 mm, film thickness 0.5 µm). GC-MS (Agilent 6890N) was used for qualitative analysis of the side products. The following temperature program was used: 50 °C for 5 min, ramping 3 °C/min up to 110 °C and 20 °C/min to 240 °C and holding there for 2 min. For GC–MS the same column and the program for GC-FID were applied, giving the same elution order to simplify identification. Not all compounds could be identified due to overlapping of peaks and an insufficient spectral library, however, unidentified compounds were present in inferior concentrations. For quantitative analysis, calibration curves were made with external standards of known concentrations. Calibration for the aldol condensation product 2-butyl-2-octanol (2B2O) was approximated with the calibration curve of 2BO. The mass balances for experiments on only aldol condensation confirmed that this assumption is accurate enough for the purposes of this work, but should be used with care for modeling. Water could not be quantified with the instruments used. The yields of products were calculated taking into account reaction stoichiometry, which was equal to unity for hexane and two for other cases.
The gas phase samples were taken from the reactor by temporarily attaching a device, which was installed to a gas exit. It consisted of a pipe to lead the gas away, a valve to control the flow, and a septum through which a GC syringe could be injected without gas leaking. Samples were taken by injecting a needle into the device and flushing the syringe with the analyzed gas to remove contaminants. The sample was then taken and injected manually directly into the GC–MS, and analyzed with the same method and column as other samples.
3 Results and Discussion
3.1 Catalyst Characterization Results
3.1.1 Specific Surface Areas
The catalyst notation can be explained using an example CP 10 wt% Ni/HT 600 which corresponds to synthesis of the catalyst by coprecipitation (CP), 10 wt% stands for metal loading of a particular metal in weight percent with HT as a support while the number 600 reflects the catalyst calcination temperature in °C. The catalysts with the prefix Imp were synthesized by the impregnation method. CP Cu/HT was synthesized using a protocol reported previously by Carlini et al. [15], namely by precipitation of nitrates with sodium carbonate maintaining the pH equal to 10 by dropwise addition of 1 M aqueous solution of sodium hydroxide. Washing and drying of the precipitate overnight at 100 °C was followed by calcination at 500 °C for 5 h.
The specific surface areas of all of the measured catalysts were similar, as displayed in Table 1. The synthesis method does not seem to have any significant effect on the surface areas of the catalysts where HT is the support. A slight increase was noticeable when the calcination temperature was increased, at least when comparing Imp 5 wt% Ni/HT 400 and Imp 5 wt% Ni/HT 600, which are otherwise similar.
3.1.2 Acidity
The specific surface areas of all of the measured catalysts were similar. The synthesis method does not seem to have any significant effect on the surface areas of the catalysts where HT is the support.
The acidities of the catalysts producing either very large or very small amounts of ester were determined by FTIR spectroscopy with pyridine are shown in Table 2. No Brønsted acid sites were found in any of the catalysts, therefore only Lewis acid sites are presented.
The catalysts containing Cu exhibited slightly more acid sites measured at 250 and 350 °C, which are denoted as medium and strong sites. The amount of sites measured at 150 °C was in the same order of magnitude for all HTs, while Cu/MgO exhibited a lower amount of Lewis acid sites. It can also be observed that compared to neat calcined HT, the amount of acid sites increases more when Cu was used as an active metal instead of Ni. As will be presented later, the catalysts containing Cu also produced more esters than Ni based catalysts. This indicates that they catalyze the Tishchenko reaction more effectively. This was in particular visible for CP Cu/HT and Cu/MgO, even if latter catalyst contained less acid sites. The amount of acid sites is in the same order of magnitude with neat HTs reported by Liu et al. [37].
3.1.3 Basicity
The results of the CO2-TPD measurements can be seen in Table 3.
The sum of sites between 100 and 400 °C is shown in Table 3 to make a comparison of the total amount of sites easier. For neat HTs the amount of basic sites, particularly stronger ones, decreased at higher calcination temperatures. The same trend is visible when comparing HTs with active metals such as Ni or Cu. The exceptions are CP 10 wt% Ni/HT 400 and CP 10 wt% Ni/HT 600, for which a large increase is visible. This could mean that CP catalysts reacted differently to thermal treatment. The amount of desorbed CO2 is in the same order of magnitude as in the work by Carlini et al., with the desorbed amount decreasing when a metal other than Mg and Al was introduced into the catalyst structure [15].
3.1.4 Cluster Size
TEM results are given in Table 4. Despite a large amount of metal, CP Cu/HT has the smallest cluster size. The effect of the calcination temperature on the cluster size is also visible. The average metal particle size increased from 3.5 to 4.0 nm when calcination temperature of CP 10 wt% Ni HT 400 was increased to 600 °C. TEM images of the catalysts which underwent thermal treatment of different temperature are depicted in Fig. 2. A small difference in cluster size visible from Fig. 2 could be explained by metal sintering.
Since the metal nanoparticles are formed in the reduction process after both the HT thermal treatment and the co-precipitation and the reduction temperature was the same for all synthesized catalysts it might be suggested that depending of the thermal treatment the interactions between the support and the nanoparticles during the activation process are somewhat different. This hypothesis requires further TPR analysis, which will be done in the future to assess interactions between HT support calcined at different temperatures and metal nanoparticles.
3.1.5 Scanning Electron Microscopy and EDXA Results
No significant changes were visible in CP 5 wt% (Fig. 3) or 10 wt% Ni/HT (Fig. 4). These catalysts contained needle like and round crystals after calcination (Fig. 4). The molar composition, excluding oxygen is displayed in Table 5.
Table 5 the CP catalysts contained large amount of Na indicating that washing of the catalysts was inadequate. It should be kept in mind, that large amounts of Na could influence catalyst properties. Only the CP Cu/HT CP catalyst did not contain any Na. Trace amounts of Si and Ca were also found. The molar Mg/Al ratio is approximately the same in all catalysts.
3.1.6 XRD Results
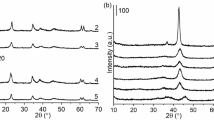
All of the patterns measured by XRD were very similar exhibiting relatively intensive peaks at ca. 2θ = 42° and 61.5° [crystal planes (200) and (220), respectively], which indicate the presence of MgO phase (Table 6). The peaks characteristic to HT were visible at approximately 2θ = 10.5°, 22.1°, 33.7°, 38.2°, 46.0° and 60.0° [38]. The HT peaks were less intensive than those of the MgO phase. The proportions between phases are shown in more detail in Table 6. All peaks were also relatively broad, indicating small crystal sizes and/or a high degree of crystal defects. The XRD patterns of all catalysts except CP 5 wt% Ni/HT 400 are characteristic for a Mg(Al)O mixed oxide phase with a periclase structure [6].
Presence of Cu or Ni was not detected, which can be explained by very small sizes of metal clusters not measurable by XRD, in line with TEM results.
3.2 Preliminary Experiments
Preliminary screening was performed based on findings in the literature for the Guerbet reaction and similar reactions. The conversion of reactant and the yield of the Guerbet product 2BO are presented in Table 7.
Reasonable activity was noticed for Cu in dehydrogenation reaction (Table 7, entries 1–4 and 7). Both BASF R3-12 and CuO, CrOx/SiO2 produced hexanal from hexanol with Cu being the main active metal in both cases. Table 7 illustrates that Ni could also be used, as it generated the Guerbet product and hexanal. These two metals were selected for further investigations. HT was chosen as the solid base as it was active in the aldol condensation per se, which was confirmed by formation of both hexanal and octanal (Table 7, entries 5 and 6), and did not produce undesired side products. MgO displayed poor selectivity towards 2BO undergoing also strong deactivation by reacting with water to form a less basic Mg(OH)2 (Table 7, entries 3 and 4). Alumina was observed to have poor selectivity, leading to large amounts of side products. Catalysts containing Ir had been moderately efficient in other works [17], but gave a poor yield in the current case (Table 7, entry 8).
3.3 Aldol Condensation Reaction with Hexanal on Hydrotalcite
HT was calcined at different temperatures and tested with hexanal in the aldol condensation reaction. The results are displayed in Fig. 5. As can be seen, the calcination temperature did not have a large impact on the reaction rate. HT 600 had a 10% higher initial rate, 1.85 × 10− 6 mol/(s g), compared to HT 400, 1.62 × 10− 6 mol/(s g). HT 500 had the lowest initial rate. To explore if water had a detrimental effect on the reaction rate, HT 600 was tested in identical reaction conditions, adding 0.54 g of water in the second experiment. This decreased the rate considerably, as can also be seen in Fig. 5.
3.4 Bifunctional Hydrotalcite-Based Catalysts
Experimental results with bifunctional catalysts are displayed in Table 8. Catalysts containing Cu exhibited poor selectivity towards 2BO (Table 8, entries 1–4) and produced large amounts of ester, HH such behaviour was displayed by both impregnated and CP copper catalysts. This is different from the results reported in [12], where the Guerbet product was produced in large quantities with good selectivity using methanol and propanol as substrates. Fe containing catalyst (entry 5 in Table 8) also demonstrated a similar low yield of 2BO, 0.7%, with low conversion. Among impregnated catalysts (Table 8, entries 1–9) all Ni containing ones had better selectivity towards the Guerbet alcohol than Cu catalysts with the yield of 2.4% after 72 h. An increase of the calcination temperature of Imp 5 wt% Ni/HT 400–600 °C increased the yield of the aldol condensation product from 0 to 1.6%. The latter catalyst had still a similar yield of the Guerbet alcohol. On the other hand, the CP Ni catalysts resulted in much larger yields with the best catalyst (Table 8, entries 10, 11), CP 10 wt% Ni/HT 400, giving a yield of 5% of 2BO in 24 h. This catalyst exhibited relatively mild basicity and no strong basic sites opposite to CP 10 wt% Ni/HT 600, which catalyzed also formation of large amounts of unknown products. Thus it should be concluded that mild basicity is beneficial for formation of 2BO. The yield of 2BO obtained by CP 10 wt% Ni/HT 400 is somewhat below the values (about 10%) recently reported for the Guerbet coupling of pentanol [31]. Increasing the calcination temperature to 600 °C lowered the yield of 2BO to 3.5%, also causing a loss of mass balance of 33%, most probably because of the gas phase products. The results in terms of conversion and yield are analogous to the work of Marcu et al. [6], where other metals were used, with ethanol as a substrate.
Figure 6 shows concentration profiles in the presence of different catalysts. The results from blank test with neat HT 600 are also displayed. As can be seen, neat HT produces some aldol condensation product 2B2O, but does not have enough hydrogenative properties to form 2BO (Fig. 6b). As it can also be seen, the catalyst containing Cu, generated HH through the Tishchenko reaction much faster than other catalysts, e.g. it produced 75% more ester in 24 h than CP 10% Ni/HT 600. This copper containing catalyst did not show any sign of deactivation. With other catalysts the reaction rate decreases with time, probably due to deactivation. One possible reason for catalyst deactivation might be formation of water. It has been reported in [39] that because of experimentally observed formation of CO already at 127 °C the cleavage of C–C bond of aldehydes by carboxylates intermediates can occur in the presence of water over Al2O3 and Pt/Al2O3 catalysts. These catalysts exhibit Lewis acidity similarly to the catalysts in the current work (Table 2). The concentration of hexanal reached a maximum very quickly, possibly limited by thermodynamics. The mechanism for the Tishchenko reaction catalyzed by Cu catalysts forming an ester has been proposed in [40] to occur via a nucleophilic attack by basic oxygen in an adsorbed alkoxide or acyl with an electrophilic carbon in the carbonyl group of the adsorbed alkanal.
Figure 7 shows selectivities towards different products as a function of conversion in the presence of CP 10% Ni/HT 400. Selectivity towards 2BO increases with increasing conversion, while the selectivities to other products decrease in line with the consecutive reaction network depicted in Fig. 1.
The experimental results reported here strongly indicate thermodynamic limitations. To address the issue of reaction thermodynamics calculations were performed using ASPEN Plus. The results presented in Fig. 8 clearly support presence of severe thermodynamic limitations.
3.5 Effects of Water Removal and Temperature Increase on the Guerbet Reaction
To investigate if removal of water would have a positive impact on the reaction rate, CaO was added as a water scavenger. While reactive distillation could have been more effective in a larger scale, utilization of a water scavenger is more practical in the case of preliminary testing performed in the current work. The amount of CaO was calculated to be sufficient to adsorb all water formed in the reaction, with a surplus of approximately 10% to adsorb moisture from the solvent.
The role of CaO is, however, not only restricted to scavenging water as addition of CaO can influence the overall basicity of the catalyst in apparently a nonlinear fashion. This makes assessment of the role of CaO addition far from being straightforward.
As can be seen from the results displayed in Table 9, addition of CaO increased the yield of the target compound 2BO from 5 to 5.6%. An experiment with otherwise similar parameters at elevated temperature 250 °C further increased the yield to 8.6%. The added basicity from CaO can possibly also contribute to the result. CaO may also cause an increase in the yield of HH from 1 to 2.2%.
In the experiment at 250 °C the mass balance was not complete, which implies that some compounds were transferred to the gas phase or to heavy products, which are not eluting from GC. To investigate the gaseous products, the gas phase was analyzed by GC–MS for a qualitative assessment, as the used column was not optimized for analyzing very light gases, such as methane and carbon monoxide. Some gases could have also condensed before reaching the sampling syringe due to cooling in the tubing used for sampling. Only 1-pentene and pentane were observed in the gas phase. This result indicates that decarbonylation took place giving pentane followed by dehydrogenation to pentene. Decarbonylation of aldehydes occurs on Ni surfaces, e.g. Ni(111) catalyzed decarbonylation of propanal occurred already at − 178 °C confirmed by HREELS and TPD methods [41]. Heavy alcohols in refluxing hexanol were reported to be formed over Raney nickel catalyst in the presence of Na and CaO along with formation of 2BO [42]. Thus it can be concluded that a lack mass balance can be attributed to formation of gaseous and heavy products.
4 Conclusions
The aim of the current study was to enhance the one-pot production of Guerbet alcohol (2BO) from hexanol with the aid of heterogeneous catalysis. Based on an extensive literature review, HT based bi-functional catalysts containing Cu, Ni, and Fe prepared with impregnation and co-precipitation with varying post-treatment were chosen for the liquid phase batch experiments. Detailed catalyst characterization was performed.
The CP catalysts were more efficient, most probably because of smaller active metal clusters, which was confirmed by TEM. Calcination temperature of neat HT was found to have a minor impact on its catalytic behavior in aldol condensation. Selectivity towards the aldol condensation product was excellent not changing significantly with different calcination temperatures.
Fe on HT was ineffective in the key reaction step - dehydrogenation of the alcohol. Fe on HT was ineffective in dehydrogenation and hydrogenation. Cu was more active when applied as copper chromite supported on silica, while in the case of copper on HT, selectivity towards the ester increased substantially, and selectivity towards the Guerbet product declined. Ni containing catalysts exhibited the highest yields of the Guerbet product. Nevertheless, 5 wt% Ni/HT 400 afforded just 5% of the Guerbet product in 24 h at 200 °C.
Water was confirmed to have a detrimental effect on the reaction rate. CaO was used as a water scavenger, slightly increasing the yield, which could at least partially be related to additional basicity. When the temperature was increased to 250 °C in an experiment with CaO as a water scavenger, combined with the most active catalyst found, the yield of the Guerbet product increased to 8.9%, up from 5.6%. Since there were no additional products in the liquid phase and ca. 8% was missing from the mass balance, apparently lighter degradation products were released to the gas phase. Analysis of the gas phase showed presence of 1-pentene and pentane, which were the only detected hydrocarbons, indicating selective transformations of hexanol to liquid.
Overall, it can be stated that the rate of the one-pot Guerbet reaction is slow being severely limited by thermodynamics. Reactive separation or other methods for product removal, including water, could be recommended to shift equilibrium to the desired products.
References
Riittonen T, Toukoniitty E, Kumar N, Madnani D, Leino AR, Kordas K, Szabo M, Sapi A, Arve K, Wärnå J, Mikkola JP (2012) One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide—the effect of the active metal on the selectivity. Catalysts 2:68–84
Wu X, Fang G, Liang Z, Leng W, Xu K, Jiang D, Ni J, Li X (2017) Catalytic upgrading of ethanol to n-butanol over M–CeO2/AC (M = Cu, Fe, Co, Ni and Pd) catalysts. Catal Commun 100:15–18
Quesada J, Faba L, Diáz E, Ordónez S (2018) Enhancement of the 1-butanol productivity in the ethanol condensation catalyzed by noble metal nanoparticles supported on Mg–Al mixed oxide. Appl Catal A 563:64–72
Quesada J, Arreola-Sánchez R, Faba L, Díaz E, Rentería-Tapia VM, Ordónez S (2018) Effect of Au nanoparticles on the activity of TiO2 for ethanol upgrading reactions. Appl Catal A 551:23–33
Liu S, Okuyama Y, Tamura M, Nakagawa Y, Imai A, Tomishige K (2015) Production of renewable hexanols from mechanocatalytically depolymerized cellulose by using Ir–ReOx/SiO2 catalyst. ChemSusChem 8(4):628–635
Marcu IC, Tanchoux N, Fajula F, Tichit D (2013) Catalytic conversion of ethanol into butanol over M–Mg–Al mixed oxide catalysts (M = Pd, Ag, Mn, Fe, Cu, Sm, Yb) obtained from LDH precursors. Catal Lett 143:23–30
Tsuchida T, Kubo J, Sakuma S, Takeguchi T, Ueda W, Yoshioka T (2008) Reaction of ethanol over hydroxyapatite affected by Ca/P ratio of catalyst. J Catal 259:183–189
Ogo S, Onda A, Iwasa Y, Hara K, Fukuoka A, Yanagisawa K (2012) 1-Butanol synthesis from ethanol over strontium phosphate hydroxyapatite catalysts with various Sr/P ratios. J Catal 296:24–30
Coville NJ, Ndou AS, Plint N (2011) Dimerisation of ethanol to butanol over solid-base catalysts. Appl Catal A 251:337–345
Birky TV, Kozlowski JT, Davis RJ (2013) Isotopic transient analysis of the ethanol coupling reaction over magnesia. J Catal 298:130–137
Yang C, Meng C (1993) Bimolecular condensation of ethanol to 1-butanol catalyzed by alkali cation zeolites. J Catal 142:37–44
NPCS Board of Consultants and Engineers (2010) Industrial alcohol technology handbook. Asia Pacific Business Press, Inc., New Delhi
Weissermel K, Arpe HJ (2003) Industrial organic chemistry. Wiley, Darmstadt
O’Lenick A Jr (2001) Guerbet chemistry. J Surfactant Deterg 4(3):311–315
Carlini C, Marchionna M, Noviello M, Raspolli Galletti AM, Sbrana G, Basile F, Vaccari A (2005) Guerbet condensation of methanol with n-propanol to isobutyl alcohol over heterogeneous bifunctional catalysts based on Mg–Al mixed oxides partially substituted by different metal components. J Mol Catal A 220(2):13–20
Carlini C, Di Girolamo M, Marchionna M, Noviello M (2002) Selective synthesis of isobutanol by means of the Guerbet reaction Part 1. Methanol/n-propanol condensation by using copper based catalytic systems. J Mol Catal A 184:273–280
Cano R, Yus M, Ramón DJ (2012) First practical cross-alkylation of primary alcohols with a new and recyclable impregnated iridium on magnetite catalyst. Chem Commun 48:7628–7630
Xu G, Lammens T, Liu Q, Wang X, Dong L, Caiazzo A, Ashraf N, Guan J, Mu X (2014) Direct self-condensation of bio-alcohols in aqueous phase. Green Chem 16:3971–3977
Hattori H (1995) Heterogeneous basic catalysis. Chem Rev 95:3
Gabriëls D, Hernández WY, Sels B, Van Der Voort P, Verberckmoes A (2015) Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal Sci Technol 5(8):3876–3902
Veibel S (1967) On the mechanism of the Guerbet reaction. Tetrahedron 23(4):1723–1733
Moteki T, Flaherty DW (2016) Mechanistic insights to C–C bond formation and predictive models for cascade reactions among alcohols on Ca- and Sr-hydroxyapatites. ACS Catal 6:4170–4183
Gines MJL, Iglesia E (1998) Bifunctional condensation reactions of alcohols on basic oxides modified by copper and potassium. J Catal 176:155–172
Kozlowski JT, Davis RJ (2013) Heterogeneous catalysts for the Guerbet coupling of alcohols. ACS Catal 3: 1588–1600
Seki T, Hattori H (2003) Tishchenko reaction over solid base catalysts. Catal Surv Asia 7:145–156
Shen J, Kobe J, Dumesic J, Chen Y (1994) Synthesis and surface acid/base properties of magnesium/aluminum mixed oxides obtained from hydrotalcites. Langmuir 10:3902–3908
Suarez DR, Zeifert BH, Garduno MH (2007) Cu hydrotalcite-like compounds: morphological, structural and microstructural properties. J Alloys Compds 434–435:783–787
Bhat BM (2012) Synthesis and characterization of hydrotalcite and hydrotalcite compounds and their application as a base catalyst for aldol condensation reaction. Orient J Chem 28:1751–1760
Marcu IC, Tichit D, Fajula F, Tanchoux N (2009) Catalytic valorization of bioethanol over Cu–Mg–Al mixed oxide catalysts. Catal Today 147(3–4):231–238
Lutic D (2010) Tailoring the basic and acid sites by thermal treatments of Mg Al hydrotalcites for their use in aldol condensation. Acta Chem Iasi 18:147–158
Panchenko V, Paukshtis E, Murzin D, Simakova I (2017) Solid base assigned n-pentanol coupling over VIII group metals: elucidation of the Guerbet reaction mechanism by DRIFTS. Ind Eng Chem Res 56:13310–13321
Murzin DY (2013) Engineering catalysis. De Gruyter, Turku
Tsyganok AI, Inaba M, Suzuki K, Takehira K, Hayakawa T (2004) Combined partial oxidation and dry reforming of methane to synthesis gas over noble metals supported on Mg–Al mixed oxide. Appl Catal A 275:149–155
Daza CE, Gallego J, Moreno JA, Mondragon F (2008) CO2 reforming of methane over Ni/Mg/Al/Ce mixed oxides. Catal Today 133–135:357–366
Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Emeis CA (1993) Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J Catal 141:347–354
Liu Y, Lotero E, Goodwin J, Mo X (2007) Transesterification of poultry fat with ethanol using Mg–Al hydrotalcite derived catalysts. Appl Catal A 331:138–148
International Centre for Diffraction Data, ICDD. Powder Diffraction File 2 (PDF-2). 1996 release
Dömök M, Tóth M, Raskó J, Erdohelyi A (2007) Adsorption and reactions of ethanol and ethanol–water mixture on alumina-supported Pt catalysts. Appl Catal B 69:262–272
Sad ME, Neurock M, Iglesia E (2011) Formation of C–C and C–O bonds and oxygen removal in reactions of alkanediols, alkanols, and alkanals on copper catalysts. J Am Chem Soc 133:20384–20398
Myint MNZ, Yan Y, Chen JG (2014) Reaction pathways of propanal and 1-propanol on Fe/Ni(111) and Cu/Ni(111) bimetallic surfaces. J Phys Chem 118:11340–11349
Bolle J, Bourgeois L (1951) Condensation of alcohols by the Guerbet reaction at atmospheric pressure. Compte Rendu 233:1466–1467
Acknowledgements
This work is a part of the activities of the Johan Gadolin Process Chemistry Centre, a center of excellence financed by Åbo Akademi University. Financial support from Neste Oyj is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Storgårds, F., Mäki-Arvela, P., Kumar, N. et al. Catalytic Conversion of Hexanol to 2-Butyl-octanol Through the Guerbet Reaction. Top Catal 61, 1888–1900 (2018). https://doi.org/10.1007/s11244-018-1047-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1047-6