Abstract
A process of transforming hydrotalcite, containing Cu–Zn–Al (2:1:1) to a catalysts for selective hydrogenolysis of glycerol to 1,2-propanediol has been studied. A two steps process, consisted of the calcination at increased temperature and subsequent reduction with hydrogen, as well as a one step process, in which the hydrotalcite was heated at hydrogen atmosphere so the calcination and reduction were joined to one operation, were verified experimentally. The influence of the temperature in the calcination step in the two steps process on composition, texture and catalytic properties of the active form of catalyst has been studied in detail. X Ray diffraction, thermogravimetric analyses, nitrogen physisorption, mercury porosimetry, temperature-programmed reduction and high-resolution transmission electron microscopy were used for the catalysts characterization. A study of calcination of a Cu–Zn–Al containing hydrotalcite at different temperatures showed, that at temperature ending with 350 °C, predominantly amorphous, mixed oxides containing phase was formed. The most active and most selective catalyst was obtained by the reduction of this amorphous oxide phase. In contrary, highly crystalline material was obtained at higher calcination temperatures, i.e at 450 or 700 °C respectively, but the activity of the reduced form of these crystalline oxides was lower. It was also proven, that by simultaneous calcination and reduction, a catalyst of the same activity and even better selectivity towards 1,2-propanediol could be prepared, comparing with the catalyst prepared by the two steps method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Selective hydrogenolysis of glycerol to 1,2-propanediol (propyleneglycol) is one of the most promising ways of utilization of glycerol generated as a by-product from the biodiesel production. Maximizing of the catalyst selectivity is the main assumption for the production process to be competitive with the petrochemical route, therefore main attention is to be paid to the catalyst research. Many types of catalysts for the hydrogenolysis of glycerol and higher polyols have been described in the literature but following main trends can be observed in this branch of catalysis: catalysts with noble metals as main active components and catalysts containing Cu in combination with another transition metals. A survey can be found in recent reviews [1, 2].
Glycerol can be converted to 1,2-propanediol by a catalytic reaction with hydrogen, according to a mechanism in which hydroxyacetone (acetol) takes part of the key intermediate, ethyleneglycol, ethanol, isopropanol being the main by-products [1, 2]. Acetol can be formed by the dehydration of glycerol on acidic centers [2,3,4], but some recent studies [2,3,4,5] point out that the reaction mechanism can be more complicated as acetol is also formed by dehydrogenation of glycerol to glyceraldehyde, followed by dehydration to pyruvic aldehyde, which is subsequently hydrogenated to acetol as shown in Online Resource 1. Basic active centers [5] and mainly metallic [3, 4, 6] active centers are important for the glyceraldehyde route to take place. In the glycerol to 1,2-propanediol hydrogenolysis, the role of specific types of active centers of the catalysts is rather unclear. It seems likely that acidity or basicity of a catalyst is not decisive for its activity as acetol formation proceeds effectively also over metals on neutral supports like carbon [7]. For the Cu containing catalysts, the amount of active Cu on the catalysts surface is supremely important for its activity [4, 6].
The Cu based catalysts have usually the form of mixed oxides prepared by co-precipitation [3, 4] or oxalate gel method [8, 9], though also impregnated Cu containing catalysts have been studied [10]. In recent years, the mixed oxides catalysts containing Cu, Al and very often another bivalent metal oxides, prepared by thermal decomposition of hydrotalcite-like structures, were described several times as promising catalysts for the glycerol hydrogenolysis [1, 2, 11,12,13,14,15]. Said mixed oxides have large specific surface area, basic properties, homogeneous dispersion of MeII and MeIII metals and their structure and properties can be influenced by the cationic composition [15].
Hydrotalcite is a mineral with a double-layered structure, the composition of which can be summarized as Mg6Al2(OH)16CO3·4H2O [16]. Octaedrically coordinated metal hydroxides create electrically positive layers, water and exchangeable anion (usually CO3−) are concentrated in a negatively charged layer in between the electrically positive layers [16]. In synthetic hydrotalcite-like materials, Mg can be substituted for other metals without any substantial changes in the structure of the resulting material. This is very important for the utilization of this materials in catalysis. The presence of CuII in the starting hydrotalcite-like material, possibly combined with other MeII metal content, is very important for the catalytic activity in the glycerol hydrogenolysis and also for selectivity to 1,2-propanediol [11, 12]. Zn has been referred as the most efficient MeII catalyst modifier [12, 13]. Anyway, Zn has been mentioned also as a good modifier for the Cu catalysts prepared by some alternative routes [3].
Preparation of the hydrotalcite based catalyst for the hydrogenolysis of glycerol proceeds in principle in following steps: co-precipitation at the conditions under which the hydrotalcite-like precipitate is formed, washing and drying the precipitate, calcination at higher temperatures and reduction of the resulting mixed oxides by hydrogen at higher pressure. Conditions in each of these steps can influence the properties as well as the performance of the active form of the catalyst, the calcination of the original precipitate in which mixed oxides are formed, being most probably the key operation [3,4,5,6, 11,12,13,14].
Calcination of hydrotalcite-like compounds at higher temperatures, carried out most frequently at the temperature between 400 and 500 °C, leads to mixed oxides from which active metallic catalysts can be prepared by the reduction with hydrogen [11,12,13]. The influence of the calcination temperature in the range between 300 and 500 °C on the catalyst structure and performance has been studied before on Cu–Al bimetallic system [16]. According this study, the best catalyst activity and selectivity was achieved if the calcination temperature was of 500 °C as the best crystallinity and the highest average pore diameter was achieved at this temperature. A detailed study of the influence of the calcination temperature on the catalyst structure and the amount and strength of active sites in resulting oxides phase has been made [14]. Cu–Zn–Al (1:1:1) hydrotalcite was calcined at 300, 500 and 800 °C respectively. The resulting oxides were tested in dimethyl carbonate synthesis. It was found that both at 300 and at 500 °C mixed oxides of very similar composition were obtained, the differences in surface area being also insignificant. In contrary, calcination at 800 °C led to the formation of the oxides of spinel type with remarkably lower specific surface area and narrower pores. Also the cumulative pore volume was much lower in the sample calcined at 800 °C. Jean et al. [3] studied calcination of Cu, Zn, Ba catalysts prepared by co-precipitation in the range of temperatures between 350 and 500 °C and found that calcination at 350 °C led to bigger particles which were more difficult to reduce by hydrogen in comparison with catalysts calcined at higher temperatures. It is also a conclusion of this study [3] that 300 °C is sufficient temperature for complete reduction of said mixed oxides. On the other hand, Fomasari et al. [17], who studied calcination of Ni/Mg/Al hydrotalcites at the temperatures 450, 660, 750 and 900 °C respectively, found by TPR that the higher the calcination temperature is, the higher temperature is necessary for the reduction of resulting mixed oxides by hydrogen. Reducibility of the mixed oxides depends not only on the calcination temperature but also on composition and atomic ratio among the metals [18].
Major part of previous studies about hydrotalcites as the precursors of hydrogenation catalysts [11,12,13,14,15, 17, 18] is focused on the HTC systems in which MgII is partially or entirely substituted by another bivalent metal or group of metals. Some recent studies [19, 20] also reveal an interesting potential of improvement the catalysts activity and selectivity by modification of the source hydrotalcite structure with another trivalent metal, besides AlIII. Mainly some noble metals can have a positive effect. These metals can be mainly Pd [20], Ru and Re [21].
Nanoscale catalyst represent another promising type of catalysts for the selective hydrogenolysis of C–O bonds in polyols and other biomass derived oxygenates. These catalysts are most frequently based on noble metals, though also Cu can be one of the metallic components [22].
An interesting novel method of the catalyst containing Cu has been described [21]. According to this method, an effective catalyst for glycerol hydrogenolysis has been prepared by the impregnation of Cu–Mg–Al hydrotalcite with a water solution of Cu(NO3)2 or by ion exchange between said hydrotalcite with CuCl2 solution. The hydrotalcite precursors processed these ways were finally calcined at 300 °C.
This study is focused on transforming Cu–Zn–Al hydrotalcite precursor into active catalysts for the hydrogenolysis of glycerol to 1,2-proipanediol, namely on analyzing of the influence of the conditions in calcination procedure on the performance of resulting catalysts. The Cu–Zn–Al system was selected, based on our previous work in which Zn was proven as the most effective bivalent hydrotalcite modifier [13].
2 Experimental
2.1 Catalysts Preparation
Cu–Zn–Hl hydrotalcites (HTC) were prepared by co-precipitation. A mixed solution of Al, Cu and Zn nitrates was precipitated by a mixed solution of KOH and K2CO3 in a flow regime in a glass reactor (Syrris Globe precipitation apparatus) under continuous pH control. Precipitation conditions were as follows: temperature of 60 °C, metal salts solution input of 30 cm3/min, KOH/K2CO3 solution input of 50 cm3/min. pH was maintained at the value of 9.5. All chemicals of “per analyses” quality had been delivered by Lachner Co.
The reaction mixture was maintained for 1 h after precipitation at the reaction temperature without mixing; the precipitate was then filtered out of the liquid, then spread on a stainless steel plate and dried overnight in a laboratory oven at 65 °C. Dry precipitate was than calcined in a laboratory oven at the programmed temperature, increasing by the speed of 2°C/min to the maximum (350, 450 or 700 °C) and maintained at this temperature for a period of 10 h.
2.2 Catalysts Characterization
X Ray diffraction (XRD) was performed using Cu-Kα radiation, wave length λ = 0.154056 nm, scanned in 2θ interval from 5 to 70° with the rate of 0.02°/s, voltage of 40 kV, current of 25 mA. D8 Advance Eco (Bruker) diffractometer was used. Evaluation of XRD patterns was done by using the Diffrac.Eva software with Powder Diffraction File database (PDF 2-2002, International Center for Difraction Data).
Thermogravimetric (TGA) analyses were performed in inert (N2) atmosphere on the Discovery series Texas Instrument analyzer. Following temperature program was used: starting temperature of 40 °C, temperature increase velocity of 10 °C/min, final temperature of 850 °C, isotherm held for 30 min.
The surface area and pore character of the catalysts were determined by nitrogen physisorption for which Autosorb IQ Station 1 (Quantachrome) device was used. The samples were outgassed at 473 K under vacuum for a period of 2 h, prior to the measurement.
Pore size distribution was determined by Non-Local-Functional Theory (NLDFT), which describes the behavior of absorptive molecules in gas phase and pores insides as well as interactions of absorptive and adsorbent surface.
Mercury porosimetry measurements were performed on Micromeritics AutoPore IV 9510 mercury porosimeter. All samples were dried before the analysis in a glass-cell at 110 °C under vacuum for 16 h.
Temperature-programmed reduction (TPR) experiments were performed on Micromeritics Auto Chem II 2920 (Micromeritics Corp., USA). The device was equipped with a thermal conductivity detector. Approximately 100 mg powdered sample was placed in a quartz reactor and the sample was thermally pre-treated at the temperature gradually increasing by the speed of 10°C/min up to 300 °C. The final temperature value was maintained for 10 min. The sample was consequently cooled down to 40 °C and the reduction was carried out in a flow of reducing gas, containing 10 vol% of H2 in Ar (50 ml/min).
High-resolution transmission electron microscopy (HRTEM) analysis samples for HRTEM analyses were dispersed in isopropanol and were intensively stirred in ultrasound before use. A small drop of the dispersion was gently spread on standard TEM Ni grid covered with thin carbon foil and dried.
Morphological and diffraction analyses were performed by means of a high resolution TEM (HRTEM) microscope JEOL JEM 3010 (Japan) equipped with a thermo-emission LaB6 cathode and operated at 300 kV. For better imaging of crystal particles, dark field (DF) images were used by selecting appropriate diffracted beams originated from analyzed crystal particles using electron diffraction (ED) patterns and the smallest objective aperture.
Electron diffraction patterns, obtained by selected area electron diffraction (SAED) method and HRTEM micrographs of crystal particles were interpreted using free SW Process Diffraction V_7.3.2Q [23,24,25]. The diffraction patterns were evaluated using JCPDS-2 database [26].
Chemical composition was analyzed simultaneously with morphological analyses using an EDS system (Inca, Oxford Instruments, UK) equipped with silicon (Li) crystal X-ray detector suitable for detection of light elements from carbon. Detector was connected directly to the column of the microscope. For investigation of distribution of elements on analyzed area, the element maps regime with focused electron beam in scanning mode was used.
Temperature-programmed desorption of ammonia (TPD) was measured by Micromeritics AutoChem II 2920 device (Micromeritics Corp., USA) equipped with a thermal conductivity (TCD) detector. A small sample (approximately 100 mg) was put into the quartz reactor and then heated up to 400 °C by the speed of 10 °C/min, then the maximum temperature was maintained for a period of 1 h in a flow of helium (25 ml/min). After that, the sample was cooled down to 50 °C and saturated with a gas mixture containing 10 vol% of ammonia in helium for 30 min. The physically adsorbed ammonia was then purged out from the sample in a flow of 25 ml/min He for 30 min. Finally, the sample was heated up to 900 °C in a flow of helium (25 ml/min), at the programmed temperature increasing by the speed of 10°C/min.
2.3 Reaction Products Analyses
Products of glycerol hydrogenolysis were analyzed by gas chromatography (GC) method using Agilent 7890 device with Nukol™ (Supelco) capillary column 30 m long with inner diameter of 0.32 mm and film thickness of 0.25 µm. Analyses conditions were as follows: carrier gas He, column input pressure of 30 kPa, gasifier temperature of 320 °C, flame ionization detector (FID) temperature of 320 °C. Oven temperature program started at 60 °C, then followed by a temperature ramp of 5°C/min up to 160 °C, then a temperature increase by the speed of 10°C/min up to 200 °C, with final isotherm at 200 °C maintained for 30 min.
2.4 Catalysts Testing
In situ activation (reduction), of the catalyst was performed in situ, prior to the reaction. The reactor—a stainless steel autoclave (Parr) was equipped with rotating stirrer, a wire gauze basket for catalyst, temperature and pressure measurement and control, sampling valve enabling sampling of liquid in the course of reaction time, gas (N2 or H2) input tubing and an additional pressure vessel—a reactant input container. In the activation procedure, the catalysts were reduced in a continuous flow of hydrogen at the pressure of 7 MPa and gradually increasing temperature by the speed of 2 °C/min, up to the final temperature of 350 °C with hydrogen flow of 30 dm3h. The catalyst was then maintained at 350 °C for a period of 4 h. After that the reduction was complete. Then the testing started at the same reactor.
The catalyst testing started after the inner temperature had been established at the required value i.e. 230 °C. Glycerol was then injected from the reactant input container into the reactor by means of hydrogen overpressure. The stirrer was then switched on and the reaction thus started out. Usually five samples were withdrawn during each experiment to be analyzed off-line by GC method.
3 Results and Discussion
TGA analyses (Fig. 1) of prepared hydrotalcite, containing Cu, Zn, Al in atomic ratio of 2:1:1 revealed gradual destruction of the hydrotalcite structure and phasing out water and CO2 from the material. MS detector analyzing the effluent gas gave a clear figure of this. Most remarkable weight loss, which represents mainly a release of physically adsorbed water as well as the water from anionic layer (interlayer), can be observed around 100 °C. The second peak on the differential curve, between 200 and 400 °C with the maximum at 250 °C, can be attributed to dehydration of the cationic layer. Formation of metal oxides also took place at these temperatures. With further heating-up, the double layered structure was completely destroyed between 500 and 700 °C, the anion (CO32−) was decomposed and CO2 was released.
With regard to the TGA analyses results, it was decided to compare catalyst precursors obtained by the calcination of HTC to final temperatures of 350, 450 or 700 °C respectively. It could have been expected, based on TGA analyses and also on some previously published results [14, 16], that there would be no significant differences in composition and structure of the materials calcined at 350 or 450 °C respectively, while different character of the material calcined at 700 °C could have been supposed.
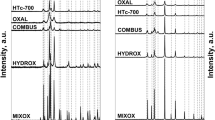
HTC calcined at the temperatures up to 350 or 450 °C respectively (HTc-350, HTc-450) feature similar composition, containing predominantly Cu, Zn and Al oxides in mixture, according to XRD analyses (Fig. 2). However, the diffraction signals, mainly for HTc-350, are very weak because of predominantly amorphous character of these samples with low contents of crystalline phase. There are most apparent lines at 2θ = 35.7°, 38.8°, 48.8° characteristic for CuO in the patterns of these samples.
In contrary to the samples calcined at lower temperatures, the sample calcined at higher temperature with the maximum at 700 °C, is apparently highly crystalline material with sharp lines (Fig. 2) at 2θ = 31.8°, 34.4°, 36.2°, 56.8° which identify the presence of ZnO. Besides this, the lines for 2θ = 32.6°, 35.7°, 38.8°, 48.8°, 53.7°, 58.4°, 61.6°, 66.3°, 68.3°, belonging to CuO, as well as the diffraction line for 2θ = 36.9°, characteristic for CuAl2O4 and the lines for 2θ = 31.3°, 36.9°, 55.7°, 59.4°, 65.1°, identifying ZnAl2O4, are also observed, the last two oxides being of spinel type. This is not surprise as it is in good agreement with previously published results [14]. The most important question was how these differences in the calcined product composition can influence the catalyst structure and performance of the active (i.e. reduced) form of the catalyst.
TEM analyses of samples calcined at different final temperatures (Fig. 3) showed that all the samples contain relatively thin flat plates with sizes in order of hundreds of nm. The sample calcined at 350 °C contained small phases of amorphous material the characteristic size of which was predominantly between 3 and 10 nm These phases contained small seeds of crystals of the size below 2 nm In contrary, the sample calcined at 450 °C was partially crystalline while the sample calcined at 700 °C was basically fully crystalline, the last one containing also discrete particles formed by disintegration of the plates present in the samples calcined at lower temperatures, said discrete particles being also in the form of stamens.
EDS point analyses disclosed that Cu, Zn, Al, O distribution was relatively homogeneous in the samples calcined at 350 and 450 °C respectively which was confirmed in all points measured. However, remarkable differences among measured points, including an absence of some of the metals, were noticed in the sample HTc-700, calcined at 700 °C. This can be attributed to disintegration of its structure. More details can be found in Online Resource 1, Figs. S1–S3.
Cu crystallites dimensions were estimated from TEM analyses of activated (reduced) samples depended on the temperature in calcination step as can be seen in Table 1. The source TEM images—see Online resource 1 It is apparent that the higher the temperature in calcination is the bigger Cu particles were obtained after reduction.
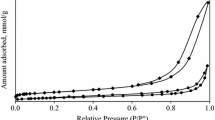
N2-izotherms for the oxides phases obtained by calcination at different temperatures are depicted in Fig. 4a. The adsorption isotherms with the hysteresis loops, characteristic for the samples calcined up to 350 or 450 °C, are classified as the IV type of isotherms (IUPAC) and identify mesoporous materials. Said type of hysteresis loop shows on the plate-like materials with slit-shaped pores which have non-rigid aggregates. This was also confirmed by HRTEM (Fig. 3). In contrary, the isotherm of HTc-700 shows a great difference in comparison with the isotherms of the samples calcined at lower temperatures. It can be seen that the hysteresis loop is very narrow, which indicates a lower proportion of mesopores. The shapes of the curves show, that the materials have a large distribution of the pore size values. The distribution curves (Fig. 4b) for all the materials studied follow approximately the same pattern the sample HTc-350, calcined at lowest temperature, having the biggest proportion of narrow pores, below 5 nm. However, the entire pores volume values are different. The pores of diameter between 12.6 and 26.3 nm have the highest proportion in the entire pore volume for all the materials studied, the diameter of 14.6 nm representing the maximum on the distribution curve.
Internal surface area (Table 2) was comparable for of all the catalysts samples, slightly lower value being observed in HTc-700 both in calcined and reduced form. But it can be concluded that the calcination temperature doesn’t affect the surface area substantially. This is however in some disagreement with previously published results [14], according which the spinel compounds containing catalyst featured significantly lower internal surface area containing also narrower pores.
The macropores distribution was measured by Hg-porosimetry. It can be seen (Fig. 5), that an intensive signal was observed for the diameter of 8588 nm in case of HTc-350. In comparison, the maximum for HTc-450 and HTC-700 was of 6636 and 6556 nm respectively. Cumulative volume of macropores in the range between 1000 and 10,000 nm was higher in the catalysts calcined at lower temperatures with the temperature maximum of 350 or 450 °C respectively. The pores, which have the pore size higher than 16,000 nm, that can be observed in all samples, could be interpreted as a free space among particles. In spite of the differences in texture of the calcined materials, it is noticeable that all samples were basically mesoporous materials.
TPR of the calcined samples -see Fig. 6- revealed that the sample containing spinel oxides (HTc-700), calcined at highest temperature, was easier to reduce in comparison with the samples calcined at lower temperatures. Its reduction took place in narrower temperature interval while the reduction peaks of the samples calcined at lower temperatures (HTc-350, HTc-450) were broader with the maximum around 250 °C and indicated stepwise reduction process in which reduction of CuO to Cu2O and subsequent reduction of Cu2O to Cu could be distinguished.
Composition and texture of the catalysts in active (reduced) form were also studied. According TEM analyses, the catalysts activated by calcination and subsequent reduction with hydrogen (two steps activation method)—see Fig. 7—consisted of sintered particles having mostly a stick-like shape. These sticks did substantially differ neither in morphology nor in chemical composition. In all samples, rounded Cu particles were present the size of which was dependent on the temperature in the calcination step. This size reached ~ 10 to 50 nm for the calcination temperature of 350 °C, and 100–400 nm when the temperature in the calcination step was 450 or 700 °C respectively.
TEM/ED analyses of the catalysts after calcination and reduction by H2 (two steps activation). Maximum calcination temperature a 350 °C, b 450 °C, c 700 °C, reduction at 7 MPa and maximum temperature of 350 °C. Diffraction patterns (in corners) were obtained from bigger amount of samples then visible in images. The arrows indicate Cu particles
All the reduced samples were crystalline, according to SAED analyses. Diffraction patterns can be interpreted as compositions of the patterns of cubic ZnAl2O4 (Pdf 71–0968) and cubic Cu (Pdf 04-0836). Also the presence of rhomboedric CuAlO2 is probable in the sample calcined at 700 °C.
EDS spot analysis confirmed the presence of Cu particles (almost 100% of Cu) in the matrix containing predominantly Zn and Al. Elemental EDS mapping confirmed the existence of separated spots containing predominantly Cu. For more details, see Online Resource 1, Figs. S4–S7.
TPD-NH3 (see Table 3 and On-line Resource 1) analyses disclosed that activation (reduction) of the catalyst had remarkable impact on the increase of amount of acidic active centers on the surface of the catalyst calcined at 700 °C. This catalyst in reduced form has much higher amount of acidic centers in comparison with the catalysts calcined at lower temperatures.
Batch tests of all catalysts, calcined at different temperatures and reduced in situ, using the same reduction procedure, disclosed that all the samples were active in glycerol hydrogenolysis (see Fig. 8). The catalyst HTc-350, calcined at lowest temperature, exhibited highest activity while HTc-700, calcined at highest temperature, was least active. However, the difference between HTc-450 and HTc-700 was not significant. This can be explained by the presence of bigger Cu particles present in the catalysts calcined at higher temperatures (HTc-450, HTc-700) and, in case of HTc-700, also by poorer and non-homogeneous distribution of Cu particles. HTc- 350 showed highest activity in hydrogenolysis and also highest selectivity towards 1,2-propanediol (Table 4). Its internal surface area (in reduced form) was equal to that of the sample calcined at 450 °C (HTc-450) and only slightly above HTc-700, calcined at 700 °C. But the calcined intermediate (precursor) for HTc-350 was a material of predominantly amorphous character (Fig. 3) that gave finest Cu particles in reduction.
The calcination of hydrotalcite with the maximum temperature of 350 °C proceeded at the same temperature regime like the in situ pre-reduction (activation) of the catalyst. Therefore the idea of joining these two processes into a single operation was experimentally studied. In this operation, raw hydrotalcite was directly reduced in hydrogen atmosphere (7 MPa) at the temperature increasing by the sped of 2°C/min, up to 350 °C. The oxides resulting from thermal decomposition of HTC were thus immediately reduces and possible re-crystallization of the oxides was supposed to be suppressed or eliminated.
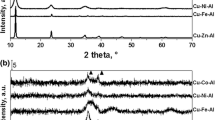
Figure 9 shows XRD patterns of two catalysts obtained by the two step activation, i.e. calcination + reduction and the one step activation, i.e. calcination and reduction in one go. The both patterns contain sharp, symmetric peaks, characteristic for crystalline material The presence of four phases can be identified from the patterns: ZnO for which signals at 2θ = 31.8°, 34.4°, 44.6°, 56.8° and 62.9° are characteristic, metallic Cu characterized by signals at 2θ = 43.3° and 50.5°. Also the oxides of spinel type (CuAl2O4 and ZnAl2O4), characterized by signals at 2θ = 31.2°, 36.5°, 38.9°, 55.5°, 59.0°, 64.9° are present. The presence of spinel oxides in both samples, with no regard to the way of their activation means that these spinel oxides, usually formed at high temperatures, are created also at relatively low temperatures in reduction environment, under higher pressure of hydrogen.
It can be observed in TEM images that the sample of catalyst activated by the one-step activation method with the maximum activation temperature of 350 °C (Fig. 10) contained crystalline Cu particles, which were confirmed by electron diffraction, elemental mapping and by EDS as well. It also contained crystalline particles of ZnAl2O4. The sample was very similar to that obtained by two steps activation (HTc-350) by its morphology, crystallinity as well as by particles size. Also the pore structure of the samples obtained by the one step and the two step activation method was very similar as documented in On-line Resource 2.
Catalyst activated by one-step method at 7 MPa (H2) and maximum temperature of 350 °C. a TEM bright field image of a group of particles, b dark field image of identical place demonstrating crystal particle of cubic Cu, c diffraction pattern of sample imaged in a, with indication of beam, used for DF image b, d interpretation of a typical diffraction pattern obtained from a bigger portion of the sample. Arrows indicate the same Cu particle
A comparison of batch testing of the catalysts activated by the one step activation and by the two step activation respectively is shown in Fig. 11. It can be seen from Fig. 11 that the initial reaction rate was slightly higher when the catalyst activated by the two steps method was used, but after certain time, at higher glycerol conversion, approximately equal reaction rate could be observed. The catalyst activated by the one step method had not probably been reduced to the same degree prior to the reaction as the one activated by the two steps method and its reduction was finished during the hydrogenolysis of glycerol. Nevertheless, it can be concluded, that both activation methods led to catalysts of approximately the same activities. But after 120 min of reaction time, at the glycerol conversion around 80%, the selectivity of the catalyst activated by the one step method was inevitably higher (Table 4).
Activity of the catalysts correlates with the size of Cu particles. The smaller Cu particles are, the higher the activity is, the catalyst activated by the one step method being the only exception. Though its Cu particles were a bit larger (see Table 1) than those of the catalyst activated by the two steps method at the same temperature (350 °C), its activity was practically equivalent.
Similar relation can be observed between Cu particle size (Table 1) and cumulative pore volume (Fig. 4b). With increasing calcination temperature an increase of Cu particles can be observed (Table 1) while the cumulative pore volume and activity decrease.
The acidity of the catalyst surface evidently didn´t positively influence the catalysts activity. In the activated form of catalysts, the amount of acidic active centers increases with increasing calcination temperature, while the catalysts activity decrease in the same order. This shows on that the mechanism starting with dehydration of glycerol to acetol on acid centers [1] doesn´t take place. Dehydration of glycerol to glyceraldehyde on metallic centers is more probable first step of the reaction mechanism [3] over the catalyst studied.
4 Conclusions
The conditions of the preparation of an active form of catalyst for the selective hydrogenation of glycerol to 1,2-propanediol from its hydrotalcite precursor have considerable impact on the catalyst internal structure, texture, phase composition and also on the catalyst properties. A study of calcination of Cu–Zn–Al (2:1:1) hydrotalcite at different temperatures showed, that at temperature ending at the value of 450 °C highly crystalline material was obtained, while at lower temperature (350 °C), though HTC destruction also took place, predominantly amorphous mixed oxides phase was formed. Despite the fact that the samples calcined at 450 and 700 °C respectively, contained well developed crystals, their chemical compositions differed. In contrary to the catalyst calcined at 450 °C, the sample calcined at 700 °C contained first of all oxides of spinel type. However, these differences in composition had only minor, if any, effect on the catalysts activity. Also said spinel oxides were well reducible with hydrogen, yielding metallic Cu. The dimensions of the crystal conglomerates in the oxide phase after calcination seems to be more significant as the have an impact on the size of Cu particles after reduction with hydrogen and also on homogeneity of Cu dispersion in the catalyst mass. However, the best catalyst in terms of activity and selectivity was obtained from the amorphous mixed oxides prepared by calcination of the HTC at lowest temperature, i.e. up to 350 °C.
The possibility of preparation an active form of the catalyst for the hydrogenolysis of glycerol by a one-step calcination—hydrogenation method was also experimentally verified. In this method, the hydrotalcite was heated up at high pressure (7 MPa) of hydrogen, so calcination and reduction proceeded at the same time, without previous calcination in an inert atmosphere. The method was verified at the activation temperature up to 350 °C. It was proven, that by this method, a catalyst of the same activity and even better selectivity towards 1,2-propanediol can be prepared, comparing with the catalyst prepared by the two steps method. It means that the calcination step can be omitted and hydrotalcite can be directly activated in hydrogen atmosphere without any loss of the catalyst performance. This can simplify the catalyst preparation process.
References
Zhou C, Zhao H, Tong D, Wu L, Yu W (2013) Catal Rev: Sci Eng 55:369–453. https://doi.org/10.1080/01614940.2013.816610
Ruppert AM, Weinberg K, Palkovits R (2012) Angewandte Chem Int Ed 51:2564–2601. https://doi.org/10.1002/anie.201105125
Jean D,. Nohair B, Bergeron J, Kaliaguine S (2014) Ind Eng Chem Res 53:18740–18749.
Du Y, Wang C, Jiang H, Chen C, Chen R (2016) J Ind Eng Chem 35:262–267. https://doi.org/10.1016/j.jiec.2016.01.002
Balaraju M, Jagadeeswaraiah K, Sai Prasad BS, Lingaiah N (2012) Catal Sci Technol 2:1967–1976
Xiao Z, Li C, Xiu J, Wang X, Williams CT, Liang C (2012) J Mol Catal A 365:24–31. https://doi.org/10.1016/j.molcata.2012.08.004
Chiu CW, Dasari MA, Suppes GJ, Sutterlin WR (2006) AICHE J 52:3543–3548. https://doi.org/10.1002/aic
Bienholz A, Schwab F, Claus P (2010) Green Chem 12:290–295. https://doi.org/10.1039/b914523k
Liu Y, Pasupulety N, Gunda K, Rempel G, Flora TT (2014) Top Catal 57:1454–1462. https://doi.org/10.1007/s11244-014-0318-O
Pudi S et al (2014) Int J Chem React Eng 12:1–12. https://doi.org/10.1515/ijcre-2013-0102
Valencia R, Tirado JA, Sotelo R, Trejo F, Lartundo L (2015) React Kinet Mech Catal 116:205–222. https://doi.org/10.1007/s11144-015-0885-5
Meher LC, Gopinath R, Naik SN, Dalai AK (2009) Ind Eng Chem Res 48:1840–1846. https://doi.org/10.1021/ie8011424
Kolena J. Soukupová L, Kocík J, Lederer J (2017) React Kinet Mech Catal. https://doi.org/10.1007/s11144-017-1239-2
Kumar P, Srivastava CV, Moshra IM (2015) Energy Fuels 29:2664–2675. https://doi.org/10.1021/ef502856z
Zhang LH, Zheng C, Li F, Evans DG, Duan X (2008) J Mater Sci 43:237–243. https://doi.org/10.1007/s10853-007-2167-8
Cavani F, Trifiro F, Vaccari A (1991) Catal Today 11:173–301
Fornasari G, Gazzano M, Matteuzzi D, Trifiro F, Vaccari A (1995) Appl Clay Sci 10:1069–1082
Casenave S, Martinez H, Guimon C, Auroux A, Hulea V, Dumitriu E (2003) J Therm Anal Calorim 72:191–198
Xia S, Yuan Z, Wang L, Chen P, Hou Z (2011) Appl Catal A 403:173–182. https://doi.org/10.1016/j.apcata.2011.06.026
Xia S, Zheng L, Nie R, Chen P, Lou H, Hou Z (2013) Chin J Catal 34:986–992. https://doi.org/10.1016/S1872-2067(11)60505-6
Yuan ZL, Wang JH, Wang LN, Xie WH, Chen P, Hou ZY, Zheng XM (2011) Appl Catal B 101:431–440. https://doi.org/10.1016/j.apcatb.2010.10.013
Yan N, Dyson PJ 2013, Curr Opin Chem Eng. https://doi.org/10.1016/j.coche.2012.12.004
Lábár JL (2008) Microsc Microanal 14:287–295. https://doi.org/10.1017/S1431927608080380
Lábár JL (2009) Microsc Microanal 15:120–129. https://doi.org/10.1017/S1431927609090023
Lábár JL (2012) Microsc Microanal 18:406–420. https://doi.org/10.1017/S1431927611012803
JCPDS PDF-2 database (2004) International Centre for Diffraction Data. Newtown Square, PA, Release 54
Acknowledgements
This publication is a result of the project Development of the UniCRE Centre (LO1606) which has been financially supported by the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme I. The results were achieved using the infrastructure of the project Efficient Use of Energy Resources Using Catalytic Processes (LM2015039) which has been financially supported by MEYS within the targeted support of large infrastructures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolena, J., Skuhrovcová, L., Kocík, J. et al. Catalyst for Selective Hydrogenolysis of Glycerol, Prepared from Hydrotalcite-Like Structures. Top Catal 61, 1746–1756 (2018). https://doi.org/10.1007/s11244-018-1005-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1005-3