Abstract
Structure and electrocatalytic reactivity of cobalt mono-phosphosulfide nanoparticles supported on carbon nanotubes are investigated. Employing two different synthetic methods, we successfully synthesize cobalt mono-phosphosulfide nanoparticles adopting either the CoP crystal structure (CoP|S with the S/P ratio < 93%) or the CoS crystal structure (CoS|P with the P/S ratio < 73%). S substitution in the CoP structure makes the surface P atoms more oxidized whereas the S atoms stay completely reduced. P substitution in the CoS structure makes the surface S atoms more reduced but the Co atoms more oxidized whereas the P atoms stay completely oxidized. All the cobalt phosphosulfide nanoparticles exhibit relatively P-rich surface. The electrocatalytic performance of the nanoparticles for the hydrogen evolution reaction is studied. In acidic electrolyte, CoP|S nanoparticles show negligible dependence of activity on composition, whereas CoS|P nanoparticles are not stable enough to give any steady activity. In alkaline electrolyte, the activity of CoP|S nanoparticles decreases as the S substitution level increases, whereas that of CoS|P nanoparticles is weakly composition dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Storage, conversion and utilization of renewable energy involve many important electrochemical reactions such as water electrolysis to produce hydrogen, oxygen reduction and hydrogen oxidation to enable fuel cells, carbon dioxide reduction to fuels and chemical products, and nitrogen hydrogenation to ammonia [1,2,3,4,5,6,7,8,9,10]. While the thermodynamics of these reactions are straightforward, their kinetics are complicated. It is central to develop high-performance catalysts to drive these reactions at high rates toward desirable products. Among all the strategies that have been adopted to synthesize better electrocatalyst materials, doping or partial substitution, mostly cationic, is effective in tailoring electronic structure or surface chemical properties and thus enhancing catalytic performance [11,12,13,14,15].
Recently, transition metal phosphosulfides become attractive electrocatalyst materials for the hydrogen evolution reaction (HER) as the P/S substitution offers a new degree of freedom for optimizing the catalytic properties [16,17,18,19,20]. For example, our group found that partial P substitution for S in CoS2 nanoparticles can drastically improve the catalytic stability while maintaining the high activity [19]. Toward understanding the chemistry of this class of new electrocatalyst materials, particularly the synthesis, solid state chemistry, surface structure and electrocatalytic reactivity, our group performed the first systematic study on iron phosphosulfide nanoparticles [20]. To further expand the knowledge, it is important to investigate phosphosulfides of other metals.
Here we report our study on structure and electrocatalytic reactivity of cobalt mono-phosphosulfide nanoparticles supported on carbon nanotubes (CNTs). Cobalt mono-phosphosulfides can adopt the CoP crystal structure (CoP|S) with the S/P ratio < 93%, or take the CoS crystal structure (CoS|P) with the P/S ratio < 73%. Different synthetic methods are required to access the two structures. All the cobalt phosphosulfide nanoparticles possess a higher P/S ratio on the surface than that in the bulk, with P in a substantially oxidized state whereas S in its reduced state. Composition of the cobalt phosphosulfide nanomaterials affects their HER electrocatalytic properties differently in acidic and basic media. The activity of CoP|S is insensitive to the S/P ratio in acid; in base the activity decreases with increase of the S/P ratio. CoS|P is not stable in acid; in base the activity is relatively insensitive to the P/S ratio.
2 Results and Discussion
2.1 Sulfur-Substituted Cobalt Phosphide (CoP|S) Nanoparticles
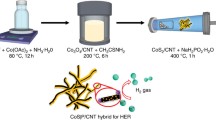
CoP|S nanoparticles were synthesized by converting Co3O4 nanoparticles (Figs. S1, S2) in a mixed atmosphere of PH3 and H2S generated in situ from NaH2PO2·H2O and NaHS·xH2O decomposition respectively. The synthesis is illustrated in Fig. 1 and the experimental details are available in the supplementary material. This method was adopted because directly reacting CoP with H2S cannot lead to successful S substitution for P in the CoP structure (Table S1), likely due to the stronger Co–P bonding than the Co–S bonding [20,21,22]. In the synthesis, 10 mg of Co3O4–CNT was used as the Co precursor; the amount of the P precursor NaH2PO2·H2O was fixed at 200 mg; the amount of the S precursor NaHS·xH2O was adjusted to access different levels of S substitution. The material obtained with X mg of NaHS·xH2O was named as CoP|S-Xmg. Scanning electron microscopy (SEM) images show that the CoP|S nanoparticles supported on CNTs are in the size range of 10–30 nm (Fig. S3). The compositions of the materials, as measured by energy dispersive X-ray spectroscopy (EDX), are presented in Table 1. As the NaHS·xH2O amount is increased from 3 to 100 mg, the atomic S content in the product material monotonically increases from 3 to 26%, reaching a maximum substitution level of ~ 48%.
The X-ray diffraction (XRD) patterns of the synthesized CoP|S-CNT materials are presented in Fig. 2. All the patterns agree well with that of the pure CoP (PDF# 00-029-0497, orthorhombic lattice, space group No. 62 Pnma). It is observed that as the S substitution level increases, the major diffraction peaks shift to lower 2θ angles except for the (211) peak whose position remains almost unchanged, indicating formation of a solid solution type phase and overall expansion of the unit cell volume, and thus confirming the successful S substitution for P in the CoP crystal structure [23,24,25]. As the amount of the S precursor is further increased to 200 mg, the (211) peak shifts to lower 2θ angles and its position matches that of the (102) diffraction peak of CoS (Fig. S4), suggesting that a different phase has formed.
X-ray photoelectron spectroscopy (XPS) measurements were performed to study the surface structures of the CoP|S nanomaterials. Figure 3a–c shows the Co 2p, P 2p and S 2p core level XPS spectra of the CoP|S nanoparticles supported on CNTs. In the Co 2p3/2 region, the two peaks at 779.1 and 781.9 eV correspond to Co bonded with P/S and O [26, 27], respectively. The presence of the Co–O component indicates the existence of a surface oxidation layer, which is known for 3d transition metal sulfides and phosphides [26, 28, 29]. The intensity ratio of the two components remains nearly unchanged as the S substitution level increases (Fig. 3a). It is noted that an additional feature appears at 778.8 eV in the Co 2p3/2 spectrum of the CoP|S-200 mg sample (Fig. S5). The binding energy coincides with that of CoS, suggesting occurrence of phase separation for the product material as the S precursor amount is increased to 200 mg, which is consistent with the XRD results. The P 2p spectra exhibit a P–O component at 134.3 eV and a P–Co component at 129.8 eV, and the oxidation becomes increasingly significant as the S substitution level increases (Fig. 3b), whereas the S 2p spectra of all the CoP|S materials show only the reduced S–Co component at 162.0 eV (Fig. 3c). Figure 3d compares the surface S/P ratios derived from the XPS spectra with the bulk S/P ratios derived from the EDX spectra. The nanoparticle surface possesses a significantly higher P/S ratio than its bulk. Taken together, these results reveal that CoP|S nanoparticles are relatively P rich on the surface and the surface P atoms are oxidized to keep the S atoms in a reduced state. Previously we have observed similar phenomena for iron phosphosulfide nanoparticles [20].
We use the HER as a model reaction to investigate the composition-dependent electrocatalytic properties of the CoP|S nanoparticles. Figure 4 presents the linear sweep voltammograms (LSVs) together with the corresponding Tafel plots for the CoP|S-CNT materials with various S substitution levels in both acidic and alkaline electrolyte. The HER catalytic activity of the CoP|S nanoparticles in 0.5 M H2SO4 shows negligible composition dependence. The catalysts with various S substitution levels reach the current density of 10 mA/cm2 within a narrow overpotential range of 91–97 mV (Fig. 4a, b and Table S2). However, in 1 M KOH, the HER catalytic activity of the CoP|S nanoparticles decreases as the S substitution level increases. The overpotential to drive the HER at 10 mA/cm2 increases from 130 to 173 mV (Fig. 4c, d and Table S3).
2.2 Phosphorus-Substituted Cobalt Sulfide (CoS|P) Nanoparticles
As illustrated in Fig. 5, CoS|P nanoparticles were synthesized by reacting pre-synthesized CoS nanoparticles with PH3 gas generated in situ from thermal decomposition of NaH2PO2·H2O (see the supplementary material for experimental details). In the P substitution reaction, 5 mg of CoS was used as the Co precursor and the amount of the P precursor NaH2PO2·H2O was adjusted to realize different levels of P substitution. The reaction temperature was set to 170 °C because higher temperature would cause phase separation into CoP and CoS (Fig. S6). Following the convention of the CoP|S case, the material obtained with X mg of NaH2PO2·H2O was named as CoS|P-Xmg. The CoS|P nanoparticles are in the size range of 10–30 nm and are well anchored on CNTs (Fig. S7). As the NaH2PO2·H2O amount is increased from 10 to 600 mg, the atomic P content in the product material, as measured by EDX, monotonically increases from 5 to 23% (Table 2), reaching a maximum substitution level of ~ 42%.
The XRD patterns of the CoS|P nanoparticles with different P substitution levels supported on CNTs are shown in Fig. 6. The diffraction patterns are all consistent with that of CoS (PDF# 03-065-3418, hexagonal lattice, space group No. 194 P63/mmc). As the P substitution level increases, all the diffraction peaks shift to higher 2θ angles, indicating shrinkage of the unit cell volume. This indicates successful P substation for S without altering the crystal structure of CoS. The decrease in unit cell volume can be explained by stronger bonding between Co and P than that between Co and S. As the P precursor amount is increased to 1000 mg, additional diffraction peaks start to appear (Fig. S8), suggesting emergence of new phases in the CoS|P-1000 mg material.
XPS measurements were performed to analyze the surface compositions and oxidation states of the CoS|P materials. For the CoS nanoparticles, the major component of the Co 2p3/2 spectrum is at 778.8 eV and that of the S 2p spectrum is at 162.1 eV (Fig. 7a, b), both corresponding to Co–S bonding [28]. The additional features at higher binding energies (781.5 eV for Co 2p3/2 and 168.0 eV for S 2p) indicate that the surface of the CoS nanoparticles is slightly oxidized. As the P substitution level increases, the surface S atoms become fully reduced (Fig. 7b) whereas the Co atoms become increasingly oxidized (Fig. 7a). It is noteworthy that all the surface P atoms are in a high oxidation state (Fig. 7c), which is different from previously studied P-substituted FeS nanoparticles [20]. Similar to CoP|S, CoS|P nanoparticles also possess relatively P-rich surface (Fig. 7d).
Unlike CoP|S, CoS|P nanoparticles are highly catalytically unstable in 0.5 M H2SO4. Under constant potential operation, the HER current density drops drastically and the CoS|P-CNT catalyst almost loses all the activity within 200 s (Fig. S9). This contrasts the previously reported CoS2 and FeS cases where P substitution for S substantially improves the catalytic durability [19, 20]. The difference is likely correlated with the fact that the surface P atoms of the CoS|P nanoparticles are all in an oxidized state which is not able to persist under strong acidic conditions. The CoS|P nanoparticles are much more stable in 1 M KOH so that their catalytic activity for the HER can be reliably measured (Fig. 8). The CoS|P-CNT catalysts with various P substitution levels reach an HER catalytic current density of 10 mA/cm2 at overpotentials ranging from 254 to 272 mV, showing weak composition dependence (Table S4).
HER catalytic activity of metal phosphosulfide nanoparticles can be influenced by P or S substitution in multiple aspects. First, as anionic ligands, P and S atoms can alter the binding energy of hydrogen atoms (H*), which are the key reaction intermediate for the HER, on the metal sites, and thus affect the catalytic activity [18]. Second, as basic sites, P and S atoms may interact with protons and affect their binding and reduction [30, 31]. Third, P and S atoms themselves may serve as active sites for HER catalysis [32, 33]. It is understandable that HER catalytic activity and its composition dependence will be influenced by pH of electrolyte, because the Volmer–Heyrovsky pathway can be controlled by different steps in different electrolyte solutions, which is evidenced by the substantially different Tafel slopes measured in acidic vs alkaline electrolyte (Fig. 4b, d and Table S4). It is also understandable that crystal structure of metal phosphosulfides will play a role, since different atomic arrangement and electronic structures will be rendered by changing the crystal structure. Furthermore, metal phosphosulfide nanoparticles are subject to restructuring under HER conditions, for instance, changing of surface oxidation state and composition. The restructuring, which should be electrolyte-dependent, will strongly affect, if not determine, the catalytic activity. Further experiments in combination with computational simulations are strongly desired to elucidate the structure–reactivity correlations in more depth.
3 Conclusion
We have synthesized and characterized S-substituted CoP and P-substituted CoS nanoparticles supported on CNTs to study the structure and electrocatalytic reactivity of cobalt mono-phosphosulfide nanomaterials. These materials can take either the CoP or the CoS crystal structure within certain composition (S/P ratio) ranges. The nanoparticle surface possesses a significantly higher P/S ratio than its bulk, with the P atoms being substantially oxidized whereas the S atoms being largely reduced. Increasing the S substitution level decreases the HER catalytic activity of CoP|S nanoparticles in base, whereas no obvious composition dependence is identified for the activity of CoP|S nanoparticles in acid or CoS|P nanoparticles in base.
References
Roger I, Shipman MA, Symes MD (2017) Nat Rev Chem 1(1):0003
Zou X, Zhang Y (2015) Chem Soc Rev 44(15):5148–5180
Boldrin P, Ruiz-Trejo E, Mermelstein J, Bermúdez Menéndez JM, Ramírez Reina T, Brandon NP (2016) Chem Rev 116(22):13633–13684
Zheng J, Zhuang Z, Xu B, Yan Y (2015) ACS Catal 5(7):4449–4455
Sheng W, Zhuang Z, Gao M, Zheng J, Chen JG, Yan Y (2015) Nat Commun 6(5):5848
Qiao J, Liu Y, Hong F, Zhang J (2014) Chem Soc Rev 43(2):631–675
Chang Z, Huo S, Zhang W, Fang J, Wang H (2017) J Phys Chem C 121(21):11368–11379
Zhang X, Wu Z, Zhang X, Li L, Li Y, Xu H, Li X, Yu X, Zhang Z, Liang Y, Wang H (2017) Nat Commun 8:14675
Weng Z, Jiang J, Wu Y, Wu Z, Guo X, Materna KL, Liu W, Batista VS, Brudvig GW, Wang H (2016) J Am Chem Soc 138(26):8076–8079
van der Ham CJM, Koper MTM, Hetterscheid DGH (2014) Chem Soc Rev 43(15):5183–5191
Kibsgaard J, Tsai C, Chan K, Benck JD, Nørskov JK, Abild-Pedersen F, Jaramillo TF (2015) Energy Environ Sci 8(10):3022–3029
Zhang X, Zhang X, Xu H, Wu Z, Wang H, Liang Y (2017) Adv Func Mater 27(24):1606635–1606647
Chhetri M, Gupta U, Yadgarov L, Rosentsveig R, Tenne R, Rao C (2015) Dalton Trans 44(37):16399–16404
Du C, Huang H, Jian J, Wu Y, Shang M, Song W (2017) Appl Catal A 538:1–8
Feng Y, OuYang Y, Peng L, Qiu H, Wang H, Wang Y (2015) J Mater Chem A 3(18):9587–9594
Liu P, Zhu J, Zhang J, Xi P, Tao K, Gao D, Xue D (2017) ACS Energy Lett 2(4):745–752
Kibsgaard J, Jaramillo TF (2014) Angew Chem Int Ed 53(52):14433–14437
Caban-Acevedo M, Stone ML, Schmidt JR, Thomas JG, Ding Q, Chang HC, Tsai ML, He JH, Jin S (2015) Nat Mater 14(12):1245–1251
Liu W, Hu E, Jiang H, Xiang Y, Weng Z, Li M, Fan Q, Yu X, Altman EI, Wang H (2016) Nat Commun 7:10771
Wu Z, Li X, Liu W, Zhong Y, Gan Q, Li X, Wang H (2017) ACS Catal 7(6):4026–4032
SpringerMaterials (2000) Springer: Berlin, Heidelberg. http://materials.bibliotecabuap.elogim.com/lb/docs/sm_lbs_978-3-540-46702-1_7. Accessed 20 April 2017
PAULING FILE Multinaries Edition (2012) Springer & Material Phases Data System (MPDS), Switzerland & National Institute for Materials Science (NIMS), Japan, 2016. http://materials.bibliotecabuap.elogim.com/isp/physical-property/docs/ppp_049551. Accessed 20 April 2017
Toraya H (1989) J Am Ceram Soc 72(4):662–664
Zou Z, Ye J, Sayama K, Arakawa H (2001) Nature 414(6864):625–627
Zhang J, Gao L (2004) Inorg Chem Commun 7(1):91–93
Tian J, Liu Q, Asiri AM, Sun X (2014) J Am Chem Soc 136(21):7587–7590
Li H, Yang P, Chu D, Li H (2007) Appl Catal A 325(1):34–40
Faber MS, Dziedzic R, Lukowski MA, Kaiser NS, Ding Q, Jin S (2014) J Am Chem Soc 136(28):10053–10061
Li X, Liu W, Zhang M, Zhong Y, Weng Z, Mi Y, Zhou Y, Li M, Cha JJ, Tang Z, Jiang H, Li X, Wang H (2017) Nano Lett 17(3):2057–2063
Shi Y, Zhang B (2016) Chem Soc Rev 45(6):1529–1541
Anantharaj S, Ede SR, Sakthikumar K, Karthick K, Mishra S, Kundu S (2016) ACS Catal 6(12):8069–8097
Xiao P, Sk MA, Thia L, Ge X, Lim RJ, Wang J-Y, Lim KH, Wang X (2014) Energy Environ Sci 7(8):2624–2629
Jaramillo TF, Jørgensen KP, Bonde J, Nielsen JH, Horch S, Chorkendorff I (2007) Science 317(5834):100–102
Acknowledgements
This work is partially supported by the Petroleum Research Funds from the American Chemical Society and the Global Innovation Initiative from the Institute of International Education. Q.G. thanks Nankai University for financial support. X.L. thanks the Chinese Scholarship Council for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gan, Q., Wu, Z., Li, X. et al. Structure and Electrocatalytic Reactivity of Cobalt Phosphosulfide Nanomaterials. Top Catal 61, 958–964 (2018). https://doi.org/10.1007/s11244-018-0954-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-0954-x