Abstract
Carbon fiber-reinforced polymer (CFRP) materials are widely used in aerospace and recreational equipment, but there is no efficient procedure for their end-of-life recycling. Ongoing work in the chemistry and engineering communities emphasizes recovering carbon fibers from such waste streams by dissolving or destroying the polymer binding. By contrast, our goal is to depolymerize amine-cured epoxy CFRP composites catalytically, thus enabling not only isolation of high-value carbon fibers, but simultaneously opening an approach to recovery of small molecule monomers that can be used to regenerate precursors to new composite resin. To do so will require understanding of the molecular mechanism(s) of such degradation sequences. Prior work has shown the utility of hydrogen peroxide as a reagent to affect epoxy matrix decomposition. Herein we describe the chemical transformations involved in that sequence: the reaction proceeds by oxygen atom transfer to the polymer’s linking aniline group, forming an N-oxide intermediate. The polymer is then cleaved by an elimination and hydrolysis sequence. We find that elimination is the slower step. Scandium trichloride is an efficient catalyst for this step, reducing reaction time in homogeneous model systems and neat cured matrix blocks. The conditions can be applied to composed composite materials, from which pristine carbon fibers can be recovered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
1.1 Composite Materials Recycling
Carbon fiber-reinforced polymer (CFRP) composites are structural materials that offer higher specific mechanical properties [1, 2], longer service life, and improved efficiency and versatility compared to traditional structural metals [3]. FRPs contain long, continuous fibers (e.g., carbon, glass, aramid) encased within a thermoset polymer (e.g. epoxy) matrix (thermoplastic matrices are also used). The thermoset matrix is formed through an irreversible cure reaction of a resin, typically converting it from a viscous liquid to a stiff, glassy solid, whether the resin is infused into the fiber bed as liquid or pre-impregnated with the fibers in a prepreg sheet material. CFRPs are commonly used in applications including aerospace, wind turbines, and marine products, with emerging large-scale use in automotive manufacturing and civil engineering. This growth in the composites market creates a major sustainability problem, as there is no efficient strategy for recycling or reusing composite material waste at the end of the life of the material. This leads to millions of tons of non-degradable waste and impedes the even wider adoption of this class of materials in large-scale manufacturing. This issue is compounded by the inefficiency of CFRP manufacturing methods. For example, 10–30% of purchased prepreg material is typically scrapped in processing [4], and no adequate approaches exist for reusing production scrap. Furthermore, environmentally conscious public policies, such as the European end-of-life vehicle directive (2000/53/EC), which dictates that new vehicles must be at least 85% recyclable by weight [5], apply additional pressure to solve recycling of scrap and end-of-life composite materials to enable the industry’s continued growth.
Three decades of work in FRP composite recycling have not resulted in a tenable solution to the recycling of composite waste and scrap [6]. Although both the embedded fibers and the polymer resin are highly engineered materials with substantial value, current recovery research emphasizes reclaiming only the fibers by thermal or chemical means [7]. Thermal degradation (pyrolysis) involves high temperatures to decompose the polymer matrix. While effective, it is energy intensive and can degrade the fibers and produce undesirable (residual) solid and gaseous byproducts. Solvolysis methods dissolve the matrix in one or more solvents at near- or super-critical conditions. This allows for recovery of resin components for some processes, but crosslinked polymeric materials are very difficult to process in solution.
1.2 Known Methods
Hitachi Chemical illustrated an excellent example of monomer recovery in the depolymerization of a composite matrix by using alkoxide conditions to realize solvolysis of a polyester-based composite matrix [8] (Scheme 1). This type of depolymerization may ultimately enable the recovery of useful small molecules from the crosslinked polymer, which would preserve partial end value for the first time [9]. The chemical mechanism for this reaction involves a simple transesterification: a potassium alkoxide adds to a linking ester group in the matrix, thus cleaving the polymer and protecting the carboxylate group as a benzyl ester. Benzyl alcohol is a convenient solvent for this reaction, because it both enables intercalation of the reagents into the matrix and provides the requisite alkoxide nucleophile.
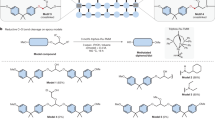
An important aspect of the Hitachi approach is that the mechanism of depolymerization is specific to matrices that are linked through ester groups. The overwhelming majority of high-performance composite materials currently in use are thermoset resins based on aniline/epoxide curing chemistry. Curing such resins results in alkylated aniline linkages that are largely inert to alkoxide conditions. Thus, orderly deconstruction of epoxy-based matrices is a much more challenging problem than that of polyesters, because epoxies lack a linkage that is vulnerable to solvolysis. To demonstrate this point, we prepared epoxy matrix 3 (Scheme 2) and applied Hitachi’s conditions to its depolymerization. The reaction occurred much more slowly than for the polyester matrix, because the transesterification mechanism is not available for these amine-cured systems. To combat this problem, conditions have appeared in the literature involving acidic hydrogen peroxide solutions for degradation of amine-linked epoxy matrices [1].
1.3 A Role for Catalysis
New methods must be developed to target the specific linking groups present in amine-cured epoxies if these processes are to be employed efficiently on an industrial scale. Herein, we show a mechanistic path for the depolymerization of amine-cured epoxies under oxidative acidic peroxide conditions. We propose this mechanism proceeds by oxygen atom transfer (OAT), followed by imine formation and imine hydrolysis (Scheme 3). It stands to reason, therefore, that catalysts capable of accelerating either the O-atom transfer or elimination step (or both) could accelerate the degradation of these composite matrices. Ultimately, validating (or refuting) this mechanism and understanding the kinetics of the OAT reaction could help in identifying conditions that enable the use of an oxygen atom source that is more sustainable than peroxide.
2 Experimental Section
2.1 Resin Preparation
The resin monomers used for the amine-cured epoxy resins DDS (1) and DGEBA (2) were acquired from Huntsman Corporation. For degradation studies, matrices were prepared from resins using a 2/5 amine/epoxy molar ratio and were mixed at room temperature in clean aluminum cans until fully homogenized. By 2/5 we mean to imply that the molar ratio of epoxy groups (in epoxy monomers) to NH groups (in the amine curing agent) is 2/5. The mixture was then heated to 120 °C in a pre-heated convection oven to further improve the mixing quality, yielding a clear homogenous mixture.
N,N,N′,N′-Tetramethyl DDS (Me4DDS) was prepared by combining 1.01 g (4.06 mmol, 1 equiv) DDS, 2.50 g (83.3 mmol, 20.5 equiv) paraformaldehyde and 50 mL acetic acid in a 250 mL round bottom flask. The flask was purged with N2, and 2.60 g (41.4 mmol, 10.2 equiv) sodium cyanoborohydride was added. The flask was stirred at 60 °C for 22 h. The resulting cloudy white solution was basified to pH 11 with 25% sodium hydroxide, extracted with 75 mL portions of dichloromethane, dried over anhydrous sodium sulfate and concentrated to yield 288 mg (23%) of product.
N,N′-dibutyl DDS (Bu2DDS) was prepared by mixing 0.50 g (2.01 mmol, 1 equiv) DDS, 1.40 g (10.2 mmol, 5.1 equiv) 1-bromobutane and 0.80 g (10.1 mmol, 5.0 equiv) pyridine in 15 mL tetrahydrofuran. The solution was allowed to react at 60 °C for 48 h, giving an orange solution with a dark-orange oil. The mixture was concentrated and extracted with 15 mL water and three 20 mL portions of ethyl acetate. The combined organic layers were washed with 20 mL of brine, dried over anhydrous sodium sulfite and concentrated. The product was isolated by silica column chromatography using a gradient of hexane–ethyl acetate (100:0–20:80), lyophilized with benzene to yield 25 mg (3%) of pristine, white solid. The balance of the material remained in mixed chromatography fractions.
2.2 Monomer and Matrix Digestion
Representative digestion experiments were performed by reacting 1.00 g prepared (cured) polymer matrix in a 1 L flask with 60.0 mL glacial acetic acid (EMD Millipore) and 10.0 mL 30% aqueous hydrogen peroxide (EMD Millipore). The resulting mixture was refluxed (110 °C bath) with additional 5.00 mL hydrogen peroxide solution portions added every hour. These reaction conditions were also applied to a ca. 5 g sample of an FRP panel based on resin monomers 1 and 2. The panel was an 8-ply composite laminate, fabricated using 2 × 2 twill weave carbon fiber fabrics (3K, 193 g/m2, FibreGlast) and a 1/1 amine/epoxy stoichiometric ratio matrix. Final polymer content was 45 ± 2 wt%. The cured laminates were cut into 100 × 20 mm coupons and subjected to digestion. Carbon fibers from the reaction mixture were separated and examined by SEM. The product mixture was screened for monomeric materials by GC/MS and MALDI mass spectroscopy, using sodium dihydroxybenzoate as the MALDI matrix.
The degradation of Me4DDS was studied by placing 5.1 mg (17 μmol, 1 equiv) Me4DDS, 700 μL acetic acid-d4 (Cambridge Isotope Labs) and 50 μL 30% hydrogen peroxide (490 μmol, 29 equiv) into a J-young NMR tube and letting react in an overnight VT NMR experiment at 40 °C.
The degradation of Bu2DDS was observed by placing 7.9 mg (22 μmol, 1 equiv) Me4DDS, 700 μL acetic acid-d4 and 50 μL 30% hydrogen peroxide (490 μmol, 22 equiv) into a J-young NMR tube and letting react in an overnight VT NMR experiment at 40 °C.
The dissolution rate of amine-cured epoxies was shown to be dependent on both the chemical reaction rate and the diffusion rate of the reagents through the material. To accelerate the diffusion limit, 1.00 g of the epoxy sample was placed in 100 mL acetic acid at 110 °C for 30 min; then 1 mol% selected catalyst and 2 mL 30% hydrogen peroxide were added. Epoxy dissolution time was defined as the time for the epoxy to completely dissolve into solution, determined visually. The products from these reactions were analyzed by GC/MS and MALDI mass spectrometry.
3 Results and Discussion
3.1 Digestion of Small Molecule Matrix Models
Small molecule models of amine-cured epoxy matrices, in which the linking glycerol fragments within the matrix were modeled respectively as methyl (6a) and n-butyl (6b) groups, were synthesized and digested under acidic peroxide conditions (Scheme 4). NMR kinetics studies on Me4DDS (6a) revealed rapid oxygen atom transfer to form N-oxide intermediates (6) within minutes, proceeding to 50% conversion in ca. 1.5 h at 40 °C. Prolonged heating of Me4DDS-N, N′-dioxide (7a) at 80 °C for 12 h resulted in degradation of ca. 60% of the dioxide. Dioxide intermediate 7a formed fully before any degradation product from this material could be formed. Thus, O-atom transfer is much faster than elimination in this sequence. Dioxide 7a appears to convert slowly to acetoxymethanol-d4 (10a), which would form by elimination of 8a’s N-oxide, and a DDS derivative that can go on to further oxidation. To accelerate the conversion of 7a to cleavage products, we screened a panel of oxophilic Lewis acid catalysts. Among an initial screen of sulfuric acid, gadolinium(III) triflate, yttrium(III) triflate, gallium(III) triflate, scandium(III) chloride, triphenylborane, and trifluoroborane, only ScCl3 enabled full conversion of 7a to decomposition products in 12 h at 80 °C. Safety note no transition metals with populated d orbitals (e.g. FeCl3, a d5 metal) can be used in this experiment. Such species will catalyze rapid decomposition of hydrogen peroxide, which could create an explosive oxygen/fuel mixture when combined with acetic acid vapor. Scandium(III) and trivalent lanthanides are d0 metals and can thus be used safely under these conditions.
A dibutylated analog of DDS (6b, Bu2DDS) behaves analogously to 6a. This extended model system is used to probe the cleavage sequence available to singly-alkylated DDS anilines, whether formed as such upon resin curing or generated following the first dealkylation of a fully-alkylated aniline. Use of the butyl group as a model of the polymer was also chosen to facilitate identification of the C4 degradation products. We observe that under conditions analogous to the oxidation of 6a, this system undergoes initial conversion to nitrone intermediate 7b somewhat slower than oxidation of 6a, compare 3.8 versus 1.5 h to reach 50% conversion, respectively. Cleavage of 7b proceeds to yield products 9b and 10b; the former (major) species was identified by appropriate patterns in the 1H NMR and phase-gated HSQC spectra appearing at δ = 5.1 (1H) and 101 (13C) ppm. No evidence of olefin formation was observed. Unlike its tetramethylated congener, cleavage of 7b proceeded at a rate similar to the oxidation of 6b: only modest portions of 7b (< 10%) accumulated in the NMR experiment.Footnote 1 Thus, in this system, it is not obvious which step of the sequence should be the subject of catalyst development. In the overall sequence of degradation of a fully alkylated DDS, it appears that elimination of the initially-formed N-oxide intermediate is the slowest step.
3.2 Digestion of Amine-Cured Matrix
Based on the proposed mechanism, two possible rate-limiting steps in the digestion are O-atom transfer and elimination. Catalysts with activity for each of these respective steps were screened for their effect on epoxy matrix dissolution time. Methyltrioxorhenium (MTO)Footnote 2 and ascorbic acid (VC) were applied as OAT catalysts while Lewis acids scandium trichloride (ScCl3) and aluminum trichloride (AlCl3, a ScCl3 analog that appears elsewhere in the composites degradation literature [14, 15]) were tested as elimination catalysts.
Figure 1 shows that neither MTO nor ascorbic acid modulate the rate of polymer degradation beyond measurement error. This is consistent with the behavior of our Me4DDS model, because we find that 6a undergoes O-atom transfer rapidly relative to elimination of 7a. For the elimination catalysts, the effectiveness of AlCl3 is comparable to the background reaction. ScCl3 reduced dissolution time significantly compared to background, from which we infer that in the present polymerized matrix, elimination, rather than OAT, is rate-limiting.
3.3 Digestion of Composite Materials
We next applied our scandium-based conditions to the degradation of carbon fiber-reinforced epoxy panels. Because of the stiff nature of the fibers, it is difficult to determine precisely the time at which matrix is completely degraded away from its imbedded fibers, so experiments with and without scandium were performed through the same time duration.
Degradation of FRP panels based on resin monomers 1 and 2 (1:1 ratio) under peroxide/ acetic acid conditions allowed for recovery of clean carbon fibers. SEM images (Fig. 2) revealed that fibers isolated from the reaction conditions are unaffected and remain in pristine condition. This finding is significant, as these carbon fibers, highly engineered materials of substantial value that drive both the cost and the energy demand of production [16,17,18], can be recovered and repurposed without resorting to pyrolysis. While in these experiments we recovered fibers in disordered orientations, which would limit their direct usability to applications appropriate for short fiber mats, we are working to develop conditions to remove the matrix without disrupting the fiber weave.
Mass spectral studies of the panel degradation solution by GC/MS and MALDI MS did not reveal significant portions of any expected monomer [e.g., bis(phenol A), DDS, or a derivative thereof]. This is unsurprising, because we observe by NMR that peroxide/acetic acid conditions destroy these aromatic moieties in less than 12 h at 80 °C in our model studies. Thus, as we expect, most of these materials are destroyed quickly under the composite panel decomposition conditions, which require minimally 6 h at a temperature of 110 °C. Identifying and accelerating the rate-limiting step in this catalysis is key for applying milder digestion conditions, ultimately to realize successful monomer re-isolation.
4 Conclusion
CFRP composites are commonly employed in a range of different fields due to their light weight and versatility, but their expanded and continued use is restricted by the lack of standard procedures for the deconstruction and reuse of these materials. Prior work that has enabled recovery of resin monomers in this field is limited to Hitachi’s solvolysis conditions for acid anhydride-linked epoxies. The Hitachi chemistry relies on the ester-based chemistry around which that resin is designed. Ester solvolysis conditions should not affect degradation of the more common amine-cured epoxies, since these structures lack a solvolysis-labile functionality. Some peroxide-based conditions are known to degrade these epoxy-based systems, but there is no prior explanation of how this chemistry might work.
We report the first investigation into the molecular mechanism of the digestion of amine-cured epoxy CFRP composite materials under acidic peroxide conditions. We have shown that dissolution times of these matrices can be affected by treatment with acetic acid and 30% hydrogen peroxide, reaching completion in as few as 6 h. Furthermore, we have shown that this reaction time can be accelerated by scandium(III) chloride, which is functioning as an elimination catalyst in a small-molecule model system. We show that these reaction conditions are too oxidative for small molecule re-isolation, because our monomers are not stable to the acidic, oxidative conditions. However, we demonstrate that high-value, pristine carbon fibers can be recovered from digested CFRP materials. Our investigation reveals that the digestion mechanism proceeds by an O-atom transfer reaction, in which an N-oxide or nitrone species forms, followed by a sequence to break a key polymeric C–N linkage. We find that oxygen atom transfer is rapid relative to elimination of the initially-formed N-oxide intermediate, which presages the possibility of using a less aggressive oxygen atom source, perhaps air itself, for this step.
Ongoing work in this area should focus on (1) identification of conditions to affect the requisite oxidation step of the degradation sequence in a sustainable, environmentally benign way that preserves the aromatic moieties present in the composite resin monomers, and (2) acceleration of the requisite downstream elimination/cleavage events. Identification of such conditions will enable both large-scale implementation of oxidative depolymerization of epoxy composites and re-isolation of high-value monomers for resin recycling, thereby opening a route to recycling of both fibers and matrices.
References
Xu P, Li J, Ding J (2013) Composites Sci Technol 82:54–59
Das M, Varughese S (2016) ACS Sustain Chem Eng 4:2080–2087
Campbell FC (2006) Manufacturing technology for aerospace structural materials. Elsevier, London
Asmatulu E, Twomey J, Overcash M (2013) J Compos Mater 48:593–608
European Parliament, Council of the European Union. (2000) Directive 2000/53/EC of the European Parliament and of the Council; OJ L 269:L0053; 2013
Oliveux G, Dandy LO, Leeke GA (2015) Prog Mater Sci 72:61–99
Piment S, Pinho ST (2011) Waste Manag 31:378–392
Shibata K, Nakagawa M (2014) CFRP Recycling technology using depolymerization under ordinary pressure. Hitachi Chemical Technical Report No. 56
Maekawa K, Shibata K, Kuriya H, Nakagawa M (2011) Proceedings of 60th the Society of Polymer Science Japan Annual Meeting, p 6
Gella C, Ferrer È, Alibés R, Busqué F, de March P, Figueredo M, Font J (2009) J Org Chem 74:6365–6367
Murray RW, Iyanar K (1996) J Org Chem 61:8099–8102
Aurich HG, Franzke M, Kesselheim HP (1992) Tetrahedron 48:663–668
Hudson A, Betz D, Kühn FE, Jiménez-Alemán GH, Boland W (2013) Methyltrioxorhenium. In Fuchs PL (ed) Encyclopedia of reagents for organic synthesis. Wiley, New York
Wang Y, Cui X, Ge H, Yang Y, Wang Y, Zhang C, Li J, Deng T, Qin Z, Hou X (2015) ACS Sustain Chem Eng 3:3332–3337
Liu T, Zhang M, Guo X, Liu C, Liu T, Xin J, Zhang J (2017) Polym Degrad Stab 139:20–27
Pimenta S, Pinho ST (2011) Waste Manag 31:378–392
Witik RA, Gaille F, Teuscher R, Ringwald H, Michaud V, Månson J-AE (2012) J Clean Prod 29–30:91–102
Witik RA, Payet J, Michaud V, Ludwig C, Månson J-AE (2011) Composites Part A 42:1694–1709
Acknowledgements
Financial support from the USC Zumberge fund, the M.C. Gill Composites Center at USC, the National Science Foundation (CHE-1566167), and George Olah’s Hydrocarbon Research Foundation are gratefully acknowledged. We thank the NSF (DBI-0821671, CHE-0840366) and the NIH (S10 RR25432) for NMR spectrometers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the late George Olah, who taught us to identify and confront the central problems of molecular enterprise.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navarro, C.A., Kedzie, E.A., Ma, Y. et al. Mechanism and Catalysis of Oxidative Degradation of Fiber-Reinforced Epoxy Composites. Top Catal 61, 704–709 (2018). https://doi.org/10.1007/s11244-018-0917-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-0917-2