Abstract
Improvements in the fundamental understanding of electrocatalysis have started to revolutionize the development of electrochemical interfaces for the efficient conversion of chemical energy into electricity, as well as for the utilization of electrons to produce new chemicals that then can be re-used in energy conversion systems. Here, some facets of the role of trace level of impurities (from 10−7 to 10−6 M) in electrocatalysis of the oxygen reduction reaction, hydrogen oxidation and evolution reactions, and CO oxidation reactions are explored on well-characterized platinum single crystal surfaces and high surface area materials in alkaline and acidic environments. Of particular interest is the effect of anions (e.g., Cl−, \( {\text{NO}}_{ 3}^{ - } \)) and cations (i.e., Cu2+) present in the supporting electrolytes as well as surface defects (i.e., ad-islands) that are present on metal surfaces. The examples presented are chosen to demonstrate that a small level of impurities may play a crucial role in governing the reactivity of electrochemical interfaces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Advances in the field of electrocatalysis over the past several decades have been driven by the technological imperative to develop stable, active and cost-effective materials for efficient energy storage and energy conversion devices [1–5]. The term electrocatalysis has been commonly used to describe studies where the rates of electrochemical reactions have a strong dependence on the nature of the electrode materials. Not surprisingly, virtually every electrochemical reaction in which chemical bonds are broken or formed relies on electrocatalysis in some form, and the kinetics varies by many orders of magnitude for different electrode materials. This is true for a wide spectrum of electrochemical reactions, spanning from the “simple” hydrogen evolution reaction (HER) [6, 7] and hydrogen oxidation reaction (HOR) [8–10] to the more complex oxygen reduction reaction (ORR) [11] and oxygen evolution reaction (OER) [5, 12, 13] all the way to multi-electron processes that take place during the oxidation of organic molecules and the formation of intermediate species during such reactions [14–21]. In all cases, the development of new materials for electrocatalytic processes has been closely tied to the Sabatier Principle, whereby the binding energy between the catalyst and covalently-bonded reactants, intermediates and products has to be optimized for the reaction to proceed effectively [22].
Although an improved understanding and optimization of the interactions between covalently-bonded reaction intermediates is significant for future advances in electrocatalysis, an even more exciting subject that is at the frontier of current surface electrochemical knowledge relates to how solvated, non-specifically adsorbed ions located in the vicinity of the surface may influence the chemistry of covalently-adsorbed reactive and spectator species [9, 15, 23–28]. It has recently been shown that these solvated ions can modify the surface in a variety of ways; e.g., via direct energetic interaction with covalently-adsorbed species or via the formation of non-specifically adsorbed patterns of ion/water complexes that selectively control the reactivity of electrochemical interfaces. Current understanding of the rich phase space of synergy between covalent and non-covalent interactions at electrochemical interfaces is still in its infancy; however, trends are beginning to emerge that make it possible to make predictions about surface structure, the nature of double layer and their corresponding reactivity towards electrochemical processes in both alkaline and acid environments.
In addition to the clear role of covalent and non-covalent interactions as two key energetic descriptors in electrocatalysis, the importance of various types of impurities, either in the form of surface defects or various levels of anions and cations present in the supporting electrolytes, has long been evident. For example, covalently-adsorbed halides and bi-sulfate anions act as remarkable poison (spectator) species in the electrochemical conversion of hydrogen and oxygen in fuel cell reactions. Furthermore, adsorbed foreign metal cations on metal surfaces—so-called underpotentially deposited (UPD) ad-atoms [29–35]—may catalyze or inhibit many electrocatalytic processes on metal surfaces. The role of surface defects in electrocatalysis is also widely recognized, especially the role they play in the observed functional link between the activity and stability of metal and non-metal oxide surfaces during the OER [5, 12, 36, 37] or in the case of CO oxidation reaction on platinum single crystals [24, 38, 39]. Although these studies have helped to develop a conceptual understanding of the role of impurities in determining interfacial reactivity, many key questions concerning these relationships are still elusive, especially when the relevant impurities are present in trace levels. In this semi-review paper, we demonstrate that a small level of impurities, in fact, has a big role in guiding electrocatalysis of three key electrochemical reactions, e.g., the HER/HOR, ORR and CO oxidation reaction.
2 Experimental Section
2.1 Electrode Preparations
Pt single crystal electrodes (6 mm disc electrodes) were prepared by inductive heating for 15 min at ~1100 K in an argon hydrogen flow (3 % hydrogen). The electrode behavior is known to be significantly influenced by the preparation procedure, and specifically when prepared by inductive heating leads to formation of defects such as ad-islands on the surface. The annealed specimens were cooled slowly to room temperature under an inert atmosphere and immediately covered with a droplet of DI water. The Pt nanoparticles obtained from Tanaka (TKK) was mixed with water in the concentration of 1 mg/mL and ultrasonically mixed for 1 h. Known volumes of the suspensions were then added using a micro pipet onto the glassy carbon disk electrode.
2.2 Chemicals
The chemical solutions were prepared from KOH (Sigma Aldrich or JT Baker) and Milli-Q de-ionized (DI) water. The acid electrolytes were all purchased from Sigma Aldrich at highest purity levels available. Acid purification was performed by simple electrolysis using separate Pt mesh as working and counter electrodes, at constant potential of ca. 0.35 V (Ag/AgCl) overnight. Copper and chloride solutions (0.01 M) are made with DI water and, after obtaining the results in “impurity-free” electrolytes, small volumes (ranging from 10−7 to 10−6 M) are added to the supporting electrolytes. All gases (Argon, Oxygen, Hydrogen) were of 5N5 quality purchased from Airgas Inc.
2.3 Electrochemical Measurements
All electrodes were embedded into the rotating disk electrode (RDE) and transferred into a standard three-compartment electrochemical cell containing 0.1 M electrolytes. In each experiment, the electrode was immersed at 0.05 V in a solution saturated with Ar. After obtaining a stable cyclic voltammetry (CV) by potential cycling (50 mV/s) between 0.05 and 0.7 V the electrolyte was saturated with gasses following with recording the polarization curves (50 mV/s) at 1600 rpm. All polarization curves were corrected for the iR contribution within the cell. A typical three electrode FEP cell was used to avoid contamination from glass components. Experiments were controlled using an Autolab PGSTAT 302 N potentiostat with built-in positive feedback for iR compensation. In all experiments a Pt wire was used as counter electrode. A Ag/AgCl reference electrode was used in all measurements, however, all potentials are given on RHE scale.
2.4 Scanning Tunneling Microscopy (STM) Measurements
For the as-prepared and the modified surfaces, the STM images were acquired with a Digital Instruments Multi-Mode Dimension STM controlled by a Nanoscope III control station. During the measurement, the microscope with the sample was enclosed in a pressurized cylinder with a CO atmosphere. For further details see [40].
3 Results and Discussion
3.1 The Role of Cl−/\( {\text{NO}}_{ 3}^{ - } \) Anions: the ORR Case
We begin with a brief description of the role of trace levels of anions—in particular Cl−—on the ORR, a cathodic half-cell reaction in polymer electrolyte membrane (PEM) fuel cells. We chose Cl− anions because they are commonly present as an impurity in commercially-available HClO4 at concentrations of at least 10−7 M [23]. We also note that in addition to Cl−, \( {\text{NO}}_{ 3}^{ - } \) ions are also present as a common impurity in perchloric acid solutions [33]. Two types of electrolytes are tested; 0.1 M HClO4 prepared from commercially-available acid (denoted hereafter as “standard acid”) and 0.1 M HClO4 prepared by the purification method described in the experimental section (hereafter “purified acid”). It is well-established that the best method for probing the effect of trace level of electrolyte impurities is to study anion-induced changes in adsorption and catalytic properties on single crystal electrodes. To enhance the mass transport of anions present in the supporting electrolyte, we utilize the RDE technique—a method that is known to control mass transport to electrochemical interfaces [41]. We first compare CVs of Pt(111) in “standard” and “purified” 0.1 M HClO4, then we use the RDE to record the polarization curves for the ORR and finally we present chronoamperometric measurements in which temporal variations (up to 1000 s) in anion-induced changes of the activity (current density) were monitored at a constant electrode potential (E = 0.92 V).
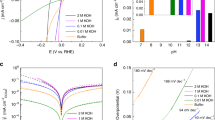
Figure 1a shows cyclic voltammograms of Pt(111) in 0.1 M HClO4, along with polarization curves for the ORR in both “standard” and “purified” acid electrolytes. A characteristic STM image of the as-prepared Pt(111) surface is given in Fig. 1b. As well-established in previous studies [33], the CVs of Pt(111) show three characteristic potential regions: adsorption of hydrogen (H+ + e− = Hupd) between 0.05 and 0.4 V, followed first by double layer charging and then by reversible adsorption of OHad species (OH− = OH ad + e−) in the potential range 0.65–0.85 V. Traditionally, the latter pseudocapacitive feature is termed as the “butterfly” feature [33]. Previous work has shown that at least part of this butterfly feature is associated with Cl− adsorption, which is present as an impurity in even the most meticulously prepared 0.1 M HClO4 electrolytes [23]. Figure 1a shows that under our experimental conditions the CVs and polarization curves recorded in the “standard” and “purified” electrolytes are almost identical; implying that commercially-available perchloric acid is impurity free. The small differences observed in the CV under the hydrogen adsorption peaks recorded under the negative sweep directions are mainly due to trace levels of NO -3 impurities [33]. However, the current–time curves summarized in Fig. 1c reveal very important differences between these two electrolytes. For example, in contrast to purified solutions, significant deactivation is observed in the “standard” electrolyte; e.g., the initial current density of ~0.35 mA/cm2 monotonically decreases so that after 900 s the activity approaches zero. Therefore, from results in Fig. 1 we conclude that: (i) neither the voltammetry nor polarization curves for the ORR recorded with fast sweep rates are capable of detecting small levels of anion impurities in the supporting electrolytes; (ii) even the purest commercially-available HClO4 contains sufficient levels of anion impurities (e.g., Cl− and \( {\text{NO}}_{ 3}^{ - } \)) that inevitably affect the ORR activity; and (iii) as a consequence, at least one part of the observed deactivation of catalysts in the PEMFC is due to the slow but persistent adsorption of trace levels of anions (and other impurities) that affect the number of available sites for the adsorption of O2 and reaction intermediates.
a Comparison of cyclic voltammograms and polarization curves (recorded at 50 mV/s) for the ORR on Pt(111) in “standard” (red curves) and “purified” (black curves) 0.1 M HClO4 electrolytes; b representative STM image of as-prepared Pt(111) electrode; c chronoamperometric measurements of the ORR in “standard” (red curves) and “purified” (black curves) 0.1 M HClO4 electrolytes; d comparison of cyclic voltammograms and polarization curves (recorded at 50 mV/s) for the ORR on Pt nanoparticles supported on glassy carbon disk electrode in “standard” (red curves) and “purified” (black curves) 0.1 M HClO4 electrolytes; e TEM image of carbon supported Pt nanoparticles; f chronoamperometric measurements of the ORR in “purified” 0.1 M HClO4 electrolytes without and with various concentration of Cl− anions
We sought to confirm the possible role of anion impurities in the ORR on high surface area Pt materials by means of chronoamperometry measurements, first in “purified” electrolytes and then in electrolytes with various amounts of Cl- added intentionally. Figure 1d shows both a characteristic CV for Pt nanoparticles in the “purified” perchloric acid solutions as well as the polarization curves for the ORR. Figure 1e depicts a representative TEM image of Pt nanoparticles with diameters ~3–6 nm, which are size scales relevant to commercial fuel cell materials. The recorded CVs and polarization curves are found to be stable during potential cycling, confirming that the role of anions is rather small under these experimental conditions. As in Fig. 1c, however, the transient current recorded in the “purified” solution shows small, yet clearly discernable, deactivation after 200 s. As expected, the rate of deactivation is strongly enhanced when Cl− is added to the supporting electrolyte in concentrations of 10−7 to 10−6 M, shown in Fig. 1f, again confirming that trace levels of Cl− impurities indeed have a large effect on the ORR activity. The significance of these results goes well beyond fundamental curiosity, in fact, they serve as persuasive motivation to explore and eventually understand differences observed between the ORR recorded in the rotating disk configuration and those established during testing of the very same catalysts in real PEMFCs.
3.2 The Role of Cu Cations in Alkaline Solutions: The CO Oxidation Reaction Case
Having established that a small level of anion impurities present in the supporting electrolytes may have a big impact on the ORR, we turn our attention towards the role of trace levels of cation impurities. Here, we present the effect of Cu UPD (Cuupd) on the oxidation of CO in alkaline solutions. In general, it has been found that Cuupd has a strong inhibiting effect on the HOR, ORR and oxidation of methanol in acidic media [30–32, 34, 42]. It was suggested that the inhibiting effect of Cuupd arises due to the Cuupd-induced adsorption of anions (Cl−, \( {\text{HSO}}_{ 4}^{ - } \), \( {\text{NO}}_{ 3}^{ - } \)) on platinum sites that are adjacent to the Cu ad-atoms, which results in a decrease in the availability of metal sites that are required for the adsorption of reactants and intermediates. Very recently, however, it has been found that Cuupd on Pt may actually serve as a promoter in alkaline solutions, improving the CO oxidation reaction [24]. Previous reports have established that the introduction of more oxophilic sites can improve the CO oxidation reaction, by making it possible for the adsorption of OH− to compete with the CO adsorption from the bulk of electrolytes [33, 38, 43–45]. Such behavior may explain why Cuupd can serve as a promoter, rather than an inhibitor, in alkaline solutions.
We start to explore the effect of Cuupd on the CO oxidation reaction by a brief description of CVs recorded on Pt(111) and Pt(111) in the presence of 1 × 10−6 M Cu2+ in an alkaline supporting electrolyte. In Fig. 2a two characteristics that represent the effect of Cuupd on the CV are noteworthy. First is that the deposition and stripping of Cu occurs as sharp peaks centered at ~0.8 V, which overlaps with the adsorption/desorption of OHad in the butterfly region. As a result, OHad serves as a promoter for the formation of Cu ad-atoms, an effect that was clearly observed for the UPD of Cu in acid solutions but only in the presence of halide ions [32, 34]. The second characteristic we note is that the adsorption of hydrogen is attenuated on surfaces covered by Cuupd, indicating that Pt(111) is modified by Cuupd (ca. 0.15–0.2 ML) in the hydrogen adsorption region. This is confirmed by analyzing such Pt surfaces with STM (Fig. 2b), where we observe a small number of Cu ad-islands with monoatomic heights. Based on these results, we conclude that the pseudo-capacitance under the sharp peaks at ca. 0.8 V is due to an attractive interaction between Cu and OHad, suggesting that Cuupd acts as an effective OHad source even below 0.6 V. As pointed out earlier, if OHad is an active intermediate, as in the case of CO oxidation reaction, then one should expect to see an improvement in the CO oxidation on the Pt(111)-Cuupd surface in alkaline solutions, an effect we discuss below.
a Comparison of cyclic voltammograms (recorded at 50 mV/s) for Pt(111) in 0.1 M KOH without and with 1 × 10−6 M Cu2+ (dashed curve); b STM image of Pt(111) covered by Cuupd adatoms; c comparison of polarization curves for CO oxidation reaction on Pt(111), Pt(100), Pt(111)-Cuupd and Pt(100)-Cuupd electrodes in 0.1 M KOH (sweep rates 50 mV/s)
Indeed Fig. 2c shows a substantial improvement in the CO oxidation reaction on Pt(111) and Pt(100) covered by Cu ad-atoms. We note that the onset of CO oxidation on Cuupd-free Pt electrodes coincides with OHad formation at ~0.6 V. After reaching a maximum activity at around 0.9 V, CO oxidation sharply decreases, presumably due to the formation of Pt oxide which is known to be inactive in the CO oxidation reaction [24, 33, 43, 45]. Importantly, the onset of CO oxidation on Pt(111) and Pt(100) covered by Cuupd is observed as low as 0 V, highlighting that the Pt(hkl)–Cuupd system is one of the best CO oxidation catalysts in alkaline environments. Figure 2c also shows that the CO oxidation reaction on Pt(100) modified by Cuupd is more active than the corresponding Pt(111) surface. In particular, on Pt(100) the reaction gradually increases above 0.3 V, reaching a diffusion-limited plateau at 0.5 V, which is followed by a sharp peak that coincides with the stripping of Cuupd. At more positive potentials the currents decreases sharply because the bulk oxide formation above 0.8 V blocks the sites required for CO adsorption. Therefore, the catalytic role of Cuupd for CO oxidation reaction in alkaline solutions is anticipated to arise through enhancing the adsorption of OHad species below 0.6 V, presumably by lowering the work function of neighboring Pt atoms [24]. Overall, the effect of Cuupd on CO oxidation reaction in alkaline solutions is significantly different than that observed in acidic media [33]. However, it is important to point out that by increasing the surface coverage by Cuupd beyond 0.3 ML, the Pt–Cuupd electrodes behave rather as an inhibitor than catalyst for the CO oxidation reaction as is observed in acidic media. This behavior further emphasizes our assertion that small levels of foreign metal “impurities” can have a big effect in the activity of electrocatalytic processes.
3.3 The Role of Surface Defects: The HER/HOR Case Effects
An interesting aspect of relationships between trace levels of impurities and electrocatalysis concerns the role of various levels of surface defects that, as demonstrated recently, can be controlled either by removing inherently present ad-islands on platinum single crystal surfaces by the CO-annealing protocol [40], or to intentionally deposit Pt ad-islands on Pt substrates [39]. To visualize the appearance or disappearance of Pt surface imperfections on Pt(111), we use STM to compare the surface atomic structures of CO-annealed surfaces (Fig. 3a) and Pt decorated with 1–2 nm diameter, 2D islands with monatomic height (Fig. 3b). Such well-defined surfaces offer a unique opportunity to correlate the kinetic rates of the HER/HOR with truly atomically-resolved densities of Pt ad-atom “impurities”. In alkaline solutions, the HER is usually assumed to proceed by an initial discharge of water and the formation of Had in the so-called Volmer step (H2O + e− + M ⇆ M–Had + OH−); followed by either the electrochemical Heyrovsky step (H2O + M–Had + e− ⇆ M + H2 + OH−) or the chemical Tafel recombination step (2Had ⇆ H2). The reaction pathway in acidic solutions is similar except that Had is formed by discharge of the hydronium ion (H3O+ + M + e− ⇆ Had + H2O). As shown in Fig. 3c, there are characteristic trends in the rate of the HER/HOR on Pt(111) pretreated by the CO-annealing and the deposition protocol: the highest activity is observed on Pt(111) modified with Pt islands, followed by the as-prepared Pt(111) and finally with the CO-annealed Pt(111). Clearly, Fig. 3c shows that the order in activity correlates nicely with the density of surface defects, confirming that small levels of surface defects (impurities) play a pivotal role in hydrogen production and its utilization. Although the thermodynamics of the HER and HOR are the same, the role of surface defects is different for the HER than for the HOR. In particular, because the hydrogen is discharged from water in the Volmer step in alkaline solution, the large promoting effect of low-coordinated atoms is due to more facile dissociative adsorption of water molecules at edge-step sites. On the other hand, in the case of the HOR it has been suggested that Pt ad-islands assist in the facile adsorption of OH− and the formation of OHad. This behavior is important as these OHad species both fulfil the stoichiometry of the reaction, as well as play an active catalytic role in the HOR (for more details see Ref. [8]). Unfortunately, given that the current experimental methods are not capable of “seeing” OHad, it is impossible to unambiguously confirm the existence of this species on any electrode surface. In order to overcome these limitations, in our recent work we probed the role of surface coverage and energetics of OHad in the HOR by establishing adsorption/catalytic trends that are known to depend on the oxophilicity of the surface atoms, which is a thermodynamic driving force to form an oxide. It is found that the most active monometallic material for the HOR is Ir—not Pt—simply because OHad is neither too strongly nor too weakly adsorbed on this electrode. Modifying Pt(111) with a small number of surface defects (Fig. 3) greatly increases the rate of HOR so that the activity of Pt(111) decorated with Pt islands approaches the activity of Ir [8]. However, removing the very same islands by the CO annealing, the Pt(111) activity decreases significantly, confirming that small number of surface defects can indeed play a big role in electrocatalysis.
a STM image of Pt(111) after CO-annealing pretreatment; b STM image for Pt(111) decorated with Pt-ad-islands; c comparison of polarization curves for the HER/HOR on as-prepared Pt(111), CO-annealed Pt(111) and Pt(111) modified by Pt ad-islands. HER/HOR potential regions are shown for clarity. At potentials above 0.9 V HOR deactivation occurs by surface oxide formation
4 Conclusions
We conclude by positing that in addition to controlling energetic parameters such as covalent and non-covalent forces, electrocatalysis will only continue to expand if we understand how trace levels of impurities may affect chemical transformations at electrochemical interfaces. The examples we used in this report are chosen to demonstrate that trace levels of impurities play a tremendous role in governing electrocatalysis of the reactions that determine the efficiency of electrochemical devices. Such studies, especially in conjunction with other information such as the structure and the chemical nature of electrochemical interfaces, should lead to a deeper understanding of the still mysterious role of impurities in electrocatalytic processes.
References
Markovic NM (2013) Electrocatalysis: interfacing electrochemistry. Nat Mater 12:101–102
Wieckovski A (2009) Fuell cell catalysis; a surface science approach. In: Koper MTM (ed) The wiley series on electrocatalysis and electrochemistry. Wiley, New York
Wieckowski A, Savinova E, Vayenas CG (2003) Catalysis and electrocatalysis at nanoparticle surfaces. Marcel Dekker Inc, New York
Markovic NM, Ross PN (2000) New electrocatalysts for fuel cells. Cattech 4:110–126
Greeley J, Markovic NM (2012) The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy Environ Sci 5:9246–9256
Breiter MW, Lipkowski J, Ross PN (eds) (1998) Electrocatalysis. Wiley, New York 75
Conway BE, Jerkiewicz G (2000) Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’ for cathodic H2 evolution kinetics. Electrochim Acta 45:4075–4083
Strmcnik D, Uchimura M, Wang C et al (2013) Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat Chem 5:300–306
Strmcnik D, Kodama K, Van der Vliet D et al (2009) The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat Chem 1:466–472
Schmickler W, Santos E (2010) Interfacial electrochemistry. Springer, Berlin, p 163
Tarasevich MT, Sadkowski A, Yeager E (1983) In: Bockris JOM, Conway BE, Yeager E, Khan SUM, White RE (eds) Comprehensive treates in electrocehmistry. Plenum Press, New York, p 301
Chang SH, Danilovic N, Chang K-C et al (2014) Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat Commun 5:4191
Chang SH, Connell JG, Danilovic N et al (2014) Activity-stability relationship in the surface electrochemistry of the oxygen evolution reaction. Faraday Discuss 176:125–133
Parsons R, VanderNoot T (1988) the oxidation of small orgnaic molecules: a survey of recent fuel cell related research. J Electroanal Chem 257:9–45
Jarvi TD, Stuve E (1998) In: Lipkowski J, Ross PN (eds) Electrocatalysis. Wiley, New York, p 75
Adzic RR (1990) In: White RE, Bockris JOM, Conway BE (eds) Modern aspects of electrochemistry. Plenum Press, New York, p 163
Farias MJS, Camara GA, Tanaka AA, Iwasita T (2007) Acetaldehyde electrooxidation: the influence of concentration on the yields of parallel pathways. J Electroanal Chem 600:236–242
Iwasita T (2002) Electrocatalysis of methanol oxidation. Electrochim Acta 47:3663–3674
Adziv RR, Tripkovic A, Markovic NM (1983) Structural effects in electrocatalysis: oxidation of formic acid and oxygen reduction on single-crystal electrodes and the effects of foreign metal adatoms. J Electroanal Chem 150:79
Iwasita T, Pastor E (1994) A dems and FTir spectroscopic investigation of adsorbed ethanol on polycrystalline platinum. Electrochim Acta 39:531–537
Leung LH, Weaver MJ (1988) Real-time FTIR spectroscopy as a quantitative kinetic probe of competing electrooxidation pathways for small organic molecules. J Phys Chem 92:4019–4022
Knözinger H, Kochloefl K (2000) Heterogeneous catalysis and solid catalysts. Ullmann’s encyclopedia of industrial chemistry. Wiley, New York, p 8
Subbaraman R, Tripkovic D, Strmcnik D et al (2011) Enhancing Tailoring Li+-Ni (OH) 2-Pt interfaces. Science 334:1256–1260
Kodama K, Morimoto Y, Strmcnik DS, Markovic NM (2015) The role of non-covalent interactions on CO bulk oxidation on Pt single crystal electrodes in alkaline electrolytes. Electrochim Acta 152:38–43
Strmcnik D, Escudero-Escribano M, Kodama K, Stamenkovic V, Markovic NM (2010) Enhanced electrocatalysis of the oxygen reduction reaction based on patterning of platinum surfaces with cyanide. Nat Chem 2:880–885
Strmcnik D, van der Vliet DF, Chang K-C et al (2011) Effects of Li+, K+, and Ba2+ cations on the ORR at model and high surface area Pt and Au surfaces in alkaline solutions. J Phys Chem Lett 2:2733–2736
Angelucci CA, Varela H, Tremiliosi-Filho G, Gomes JF (2013) The significance of non-covalent interactions on the electro-oxidation of alcohols on Pt and Au in alkaline media. Electrochem Commun 33:10–13
Sitta E, Batista BC, Varela H (2011) The impact of the alkali cation on the mechanism of the electro-oxidation of ethylene glycol on Pt. Chem Commun 47:3775–3777
Adzic RR (1984) In: Gerisger H, Tobias CW (eds) Advances in electrochemistry and electrocehmical engineering. Wiley, New York, p 159
Markovic NM, Gasteiger HA, Ross PNJ (1995) Copper Electrodeposition on Pt(111) in the presence of chloride and (Bi)sulfate: rotating ring-Pt(111) disk electrode studies. Langmuir 11:4098–4108
Marković NM, Lucas CA, Gasteiger HA, Ross PN Jr (1997) The structure of adsorbed bromide concurrent with the underpotential deposition (UPD) of Cu on Pt(111). Surf Sci 372:239–254
Markovic N, Ross PN (1993) Effect of anions on the underpotential deposition of copper on platinum(111) and platinum(100) surfaces. Langmuir 9:580–590
Marković NM, Ross PN Jr (2002) Surface science studies of model fuel cell electrocatalysts. Surf Sci Rep 45:117–229
Markovic N, Ross PN (1992) The effect of specific adsorption of ions and underpotential deposition of copper on the electro-oxidation of methanol on platinum single-crystal surfaces. J Electroanal Chem 330:499–520
Kokkinidis G (1986) Underpotential deposition and electrocatalysis. J Electroanal Chem 201:217–236
Stamenković V, Marković NM (2001) Oxygen reduction and hydrogen oxidation reactions on Pt(111) and Pt(100) in solutions containing copper ions. Langmuir 17:2388–2394
Danilovic N, Subbaraman R, Chang KC et al (2014) Using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angew Chem Int Ed 53:14016–14021
Danilovic N, Subbaraman R, Chang K-C et al (2014) Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J Phys Chem Lett 5:2474–2478
Subbaraman R, Tripkovic D, Chang K-C et al (2012) Trends in activity for the water electrolyser reactions on 3d M(Ni Co, Fe, Mn) hydr(oxy)oxide catalysts. Nat Mater 11:550–557
Strmcnik DS, Tripkovic DV, van der Vliet D et al (2008) Unique activity of platinum adislands in the CO electrooxidation reaction. J Am Chem Soc 130:15332–15339
Bard AJ, Faulkner LR (2000) Electrochemical methods: fundamentals and applications. Wiley, New York
Marković NM, Gasteiger HA, Lucas CA et al (1995) The effect of chloride on the underpotential deposition of copper on Pt(111): AES, LEED, RRDE, and X-ray scattering studies. Surf Sci 335:91–100
Lopes PP, Ticianelli EA (2010) The CO tolerance pathways on the Pt–Ru electrocatalytic system. J Electroanal Chem 644:110–116
Shibata M, Furuya N (1989) Catalysis by ad-atoms: part II. Enhancement of CO oxidation on Pt catalysts modified by Sn ad-atoms in the gaseous phase. J Electroanal Chem Interfacial Electrochem 269:217–221
Watanabe M, Shibata M, Motoo S (1985) Electrocatalysis by ad-atoms: part XII. Enhancement of carbon monoxide oxidation on platinum electrodes by oxygen adsorbing ad-atoms. J Electroanal Chem Interfacial Electrochem 187:161–174
Acknowledgments
This work is supported by the Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences, under contract DE-AC0206CH11357.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strmcnik, D., Li, D., Lopes, P.P. et al. When Small is Big: The Role of Impurities in Electrocatalysis. Top Catal 58, 1174–1180 (2015). https://doi.org/10.1007/s11244-015-0492-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-015-0492-8