Abstract
Two ternary mixed-ligand coordination polymers (CPs), namely [Ni(L)2(DCTP)] n (1) and [Co(L)(DCTP)] n (2), have been hydrothermally synthesized by reacting different transition metal salts with 2,5-dichloroterephthalic acid (H2DCTP) and 4,4′-[bis(imidazol-1-ylmethylene)]biphenyl (L) as ligands. Both CPs 1 and 2 were characterized by elemental analysis, IR spectroscopy, and single-crystal X-ray diffraction; CP 1 exhibits a non-interpenetrated six-connected α-Po (or pcu net) framework structure, with the point symbol {412·63}. CP 2 shows a fivefold-interpenetrating four-connected sqc6 topology with the point symbol {66}. The thermal stabilities, luminescence and catalytic properties of both CPs for the degradation of methyl orange in a Fenton-like process have been investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their interesting structures and potential applications in gas storage, molecular recognition, catalysis and magnetism, the design and synthesis of coordination polymers (CPs) are of considerable current interest [1–3]. The structural diversities of CPs may be tuned by a suitable choice of metal ions as well as ligands. With regard to the latter, variations in backbone flexibility, size, substituents, and symmetry of organic linkers can result in CPs with a wide range of structures and properties [4–6]. The most generally employed strategies for synthesis of CPs are the use of (a) neutral spacers to enhance the possibility of increasing the dimensionality of CPs, with charge neutrality maintained by non-coordinating anions; and (b) anionic bridging ligands, which can partially or fully counterbalance the charges of the metal centers. Therefore, rational selection of organic ligands based on their flexibility, functionality, shape, and symmetry plays a significant role in modulating structures and properties of CPs [7–9]. One of the most commonly effective routes for building such networks is to employ appropriate bridging ligands that can bind metal ions in different modes and so provide a possible route to novel CPs with intriguing structures and topological features [10].
In this field, polycarboxylates have been widely employed to manipulate coordination networks, in view of their versatile coordination modes and acidic nature [11]. In particular, terephthalic acid is one of the most popular rod-like building blocks that have been widely used to construct functional CPs [12]. However, research on the analogous halogenated terephthalic acids, with disparate steric and electronic effects, has been insufficiently unexplored so far [13–15]. In addition, CPs with open coordination sites have attracted much attention, and the incorporation of halogen atoms into carboxylate ligands may be an effective way to construct CPs with open coordination sites because of a diminished possibility for a priori structure prediction or crystal design [16]. Meanwhile, semi-flexible bis(imidazole) derivatives which can utilize diverse conformations to accommodate different metal centers have been extensively used as auxiliary N-donor ligands in the synthesis of mixed-ligand CPs [17, 18].
To explore the influence of different transition metal salts on the final structures and properties of such CPs, we have successfully synthesized two new CPs under the same reaction conditions, formulated as [Ni(L)2(DCTP)] n (1) and [Co(L)(DCTP)] n (2) [L = 4,4′-(bis(imidazol-1-ylmethylene))biphenyl, H2DCTP = 2,5-dichloroterephthalic acid]. These two CPs have totally different structures; CP 1 exhibits a 3D six-connected framework, while CP 2 reveals a 3D four-connected network.
Experimental
Materials and methods
The free ligand L was synthesized by the literature method [19]. The other reagents and solvents were purchased from commercial sources and used without further purification. Elemental analyses (C, N and H) were obtained on a PerkinElmer 240C elemental analyzer. A Varian FT-IR 640 spectrophotometer was employed for collection of IR spectra (using KBr pellets in the range of 4000–400 cm−1 region). The luminescence spectra of powdered solid samples were measured at room temperature, using a Hitachi F-7000 fluorescence spectrophotometer. Solid-state UV/Vis diffuse reflectance spectra were measured using a UV–Vis spectrophotometer (Puxi, UV 1901), and a BaSO4 plate was employed as the reflectance standard. X-ray powder diffraction (XRPD) patterns were obtained on a Rigaku D/Max-2500 diffractometer at 40 kV, 100 mA with a graphite monochromator and Cu-target tube (λ = 1.5406 nm). A NETZSCH TG 209 thermal analyzer was applied to record thermal curves under N2 atmosphere from room temperature to 800 °C with a heating rate of 10 °C/min. The absorptivity value of methyl orange (MO) was measured at the maximum wavelength of 506 nm with a Shanghai Jingke 722N spectrophotometer.
Synthesis of [Ni(L)2(DCTP)] n (1)
A mixture of Ni(OAc)2·4H2O (24.9 mg, 0.1 mmol), the free ligand L (31.4 mg, 0.1 mmol), H2DCTP (23.5 mg, 0.1 mmol), and distilled water (10 mL) was charged into a Teflon-lined stainless vessel and heated from room temperature to 140 °C for 72 h and then cooled to room temperature at a rate of 5 °C/h. The resulting crystals were filtered out, washed with small quantities of cold distilled water and dried overnight, giving green block crystals. Yield, 47.7% referred to Ni(OAc)2·4H2O. Anal. Calc. for C48H38NiCl2N8O4 (M r = 920.47): C, 62.6; H, 4.2; N, 12.2%. Found: C, 62.6; H, 4.2; N, 12.2%. IR (KBr, cm−1): 3033w, 2966m, 1639m, 1578s, 1352w, 1233w, 1034s, 773w, 659m, 568s, 498w.
Synthesis of [Co(L)(DCTP)] n (2)
The method of synthesis for CP 2 was similar to that for CP 1, except that Co(OAc)2·4H2O (24.9 mg, 0.1 mmol) was used instead of Ni(OAc)2·4H2O. Red block crystals of 2 were obtained with a yield of 42.6% [based on Co(OAc)2·4H2O]. Anal. Calc. for C28H20CoCl2N4O4 (M r = 606.31): C, 55.47; H, 3.32; N, 9.24%. Found: C, 55.5; H, 3.3; N, 9.3%. IR (KBr, cm−1): 2924m, 1592s, 1520s, 1443w, 1369s, 1233s, 1105w, 823m, 741s, 658m, 578w, 496m.
X-ray crystallography
Suitable single crystals of CPs 1 and 2 were selected for single-crystal X-ray diffraction analyses. The intensity data for both CPs were collected with a Bruker Smart CCD diffractometer with Mo–K α radiation (λ = 0.71073 Å) using ω scan mode at 296(2) K. The multiscan program [20] was employed to collect the absorption corrections. The structures were solved by direct methods applying the SHELXS-2014 program and refined through full-matrix least-squares on F 2 using the SHELXTL program package [21]. Hydrogen atoms of the organic parts were located from difference maps and refined with isotropic temperature factors, and the anisotropic thermal parameters were refined for non-hydrogen atoms. Metal atoms in both CPs were located in the E-maps. The detailed crystallographic data and structure refinement parameters for both CPs are given in Table 1.
Catalysis experiments
In order to assess the potential of these CPs as catalysts for the oxidation of MO, the following procedure was followed: briefly Na2S2O8 (25.0 mg) was placed in a 150-mL round-bottom flask and dissolved in 100.0 mL of an aqueous solution of MO (10 mg/L). The pH was adjusted to 3.0 using 0.1 mol/L H2SO4, and finally a powdered sample of the required CP (30.0 mg) was added. The mixture was stirred magnetically at a constant temperature of 30 °C. Aliquots (5.0 mL) of the reaction solution were removed at given time intervals and centrifuged to remove the residual catalyst and then analyzed with a Shanghai Jingke 722N visible spectrophotometer at 506 nm. A control experiment was carried out in the absence of either CP under the same conditions. The degradation efficiency of MO was evaluated based on the following formula [22]:
where A 0 is the initial absorbance of MO aqueous solution and A t (mg/L) is the absorbance at reaction time t. The MO concentration was below 10 mg/L and was linearly proportional to the intensity of the measured peak.
Results and discussion
General characterization of the CPs
The hydrothermal approach plays a significant role in the self-assembly of CPs. By varying the metal salt, single crystals of CPs 1 and 2 were successfully obtained. Both CPs are stable in the solid state and insoluble in water and common organic solvents, such as dichloromethane, alcohol, benzene and acetonitrile. In addition, both CPs were structurally characterized by X-ray single-crystal diffraction analysis. Selected bond distances and angles for the CPs are listed in Table 2.
Crystal structure of CP 1
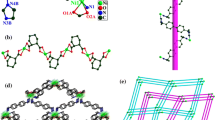
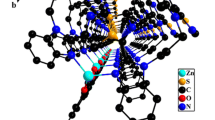
X-ray single-crystal diffraction analysis reveals that CP 1 formulated as [Ni(L)2(DCTP)] n crystallizes in the monoclinic P2(1)/c space group. The asymmetric unit contains half of an Ni(II) atom, one L ligand, and half of a DCTP2− ligand. As illustrated in Fig. 1a, the nickel center has an ideal [NiO2N4] octahedral geometry, provided by two oxygen atoms (O1, O1A, symmetry code: A = −x + 2, −y + 1, −z + 1) from carboxylate groups belonging to two distinct DCTP2− ligands and four nitrogen atoms (N1B, N1C, N4, N4A, symmetry codes: B = x + 1, −y + 1/2, z + 1/2; C = −x + 1, y + 1/2, −z + 1/2) from four distinct L ligands. The values of the Ni–O bond distances are 2.097(2) Å, and the two pairs of Ni–N bond distances are 2.102(3) and 2.103(3) Å, respectively. These values are all in the normal ranges according to the literature [23].
a Coordination environment around the Ni(II) centers in CP 1. Hydrogen atoms are omitted for clarity (symmetry codes: A: −x + 2, −y + 1, −z + 1; B: x + 1, −y + 1/2, z + 1/2; C: −x + 1, y + 1/2, −z + 1/2; D: −x + 1, −y + 1/2, −z + 1/2). b The 3D network of CP 1 constructed by Ni(II) centers, L ligands, and DCTP2− ligands
In CP 1, each anionic DCTP2− ligand adopts a (κ1–κ0)–(κ1–κ0)–µ2 coordination mode to link adjacent Ni(II) centers into a 1D linear chain, with an Ni···Ni distance of 11.530(5) Å. The ligand L adopts a trans-conformation to connect the Ni atoms into a 2D reticular layer (Fig. S1). The dihedral angle between two imidazole arms of each entire ligand L is 65.78°, while the Ni···Ni distance is 15.677(5) Å. The reticular 2D layers and 1D linear chains are interlaced with each other to generate an intricate 3D network (as shown in Fig. 1b). In order to better understand the structure of CP 1, topological analysis of the intricate network was performed using TOPOS 4.0 software [24]. The Ni(II) center can be simplified as six-connected, and the anionic DCTP2− ligands and L ligands as linkers. The structure of the CP 1 can then be represented as a six-connected α-Po (or pcu net) primitive cubic net with the point symbol {412·63}. The cubic pore dimensions are approximately 15.677 × 15.677 × 11.530 Å3 (as shown in Fig. S2).
Crystal structure of CP 2
X-ray single-crystal diffraction analysis shows that CP 2 formulated as [Co(L)(DCTP)] n crystallizes in the monoclinic C2/c space group. The asymmetric unit is composed of half of a Co(II) center, half of a L ligand, and half of a DCTP2− ligand. As shown in Fig. 2a, the Co(II) centers are four-coordinated in a distorted [NiO2N2] tetrahedral geometry provided by carboxyl oxygen atoms from two different DCTP2− ligands [Co(1)–O(1) = 1.896(4) Å, Co(1)–O(1)A = 1.896(4) Å, symmetry code: A = –x + 1, y, –z + 3/2; B = −x + 1, −y, −z + 1] plus two nitrogen atoms from two disparate L ligands [Co(1)–N(1) = 1.948(10) Å, Co(1)–N(1)A = 1.948(10) Å]. These values are all in the normal ranges according to the previous reports [25].
In CP 2, the anionic DCTP2− ligands employ a (κ1–κ0)–(κ1–κ0)–µ2 coordination mode to connect the neighboring Co atoms into a 1D wave-like chain with a Co···Co distance of 11.013(2) Å and Co···Co···Co angle of 136.3(3)°. In addition, the ligand L acts as an µ2 liker which adopts trans-conformation to link the contiguous Co atoms into a 1D “W”-like chain with a somewhat longer Co···Co separation of 18.230(3) (Fig. S3). Furthermore, the 1D wave-like chains and “W”-like chains interact with each other to construct a 3D circularly heart-shaped framework (as shown in Fig. 2b). The topological method was used to study the nature of the resulting 3D structure. Thus, the Co(II) atoms can be simplified as four-connected, and the L and DCTP2− ligands as linkers. In this way, the intricate 3D structure can be represented as a uninodal four-connected sqc6 topological network with the point symbol {66}. The potential spaces are large enough to be filled via mutual interpenetration of five independent 3D networks, generating a fivefold-interpenetrating 3D structure (as shown in Fig. S4). The interpenetration can be classified as type Class Ia, Z = 5 × 1 = 5 (Zt = 5; Zn = 1), where Zt and Zn are the number of interpenetrated nets related by translation and crystallographic symmetry, respectively [26]. There is no residual solvent accessible void in CP 2 based on a PLATON calculation [27].
To summarize, for CPs 1 and 2, obvious structural differences are observed in their discrete mononuclear motifs to 3D coordination frameworks, which can be explained by the different metals involved. Thus, the metal centers in these two CPs have different coordination numbers and geometries. For CP 1, the Ni(II) centers are six-coordinate with an ideal [NiO2N4] octahedral geometry, whereas, in CP 2, the Co(II) centers are four-coordinate with a distorted [NiO2N2] tetrahedral geometry. The DCTP2− ligands in both CPs are all unidentate, acting as the spacers to bridge pairs of metal atoms. The DCTP2− ligands are almost perpendicular to the Ni(II) and Co(II) centers, due to the significant steric effects of the chloro substituents. As for ligand L, it serves as either a bridging spacer or as a unidentate terminal ligand in these CPs. Hence, the metal–ligand synergistic effect is essential to modulate the resulting structures.
Spectroscopic powder diffraction studies
The IR spectra for free H2DCTP, L ligands and the two CPs 1–2 are summarized in Table 3. In the IR spectra of both CPs, a band around 1578 cm−1 can be attributed to the ν C=N stretching vibration of the L ligand, while a peak around 660 cm−1 can be assigned to the ν C–Cl stretching vibration from the H2DCTP ligand. There are no strong absorption peaks around 1700 cm−1, suggesting that the carboxylic groups of the H2DCTP ligands are completely deprotonated in both CPs. The peaks for the carboxylate stretching are at 1352 cm−1 for the symmetric mode (ν s), 1578 cm−1 for the asymmetric mode (ν as) for CP 1; and at 1369 cm−1 for ν s and 1592 cm−1 for ν as for CP 2. The values of ∆ν[νas(COO)–νs(COO)], 226 and 223 cm−1 for 1 and 2, respectively, imply the monodentate coordination of the carboxyl groups from H2DCTP in both of these CPs [28].
XRPD measurements were performed in order to examine the phase purity of bulk samples of CPs 1 and 2. The obtained patterns are consistent with the simulated patterns obtained from their single-crystal structures (Fig. S5), confirming the phase purities of the CPs. XRPD patterns of the CPs 2 months later were still in good agreement with the simulated patterns, indicating that these CPs are stable at room temperature.
Thermal properties
Thermogravimetric (TGA) measurements were performed under an N2 atmosphere on polycrystalline samples of CPs 1 and 2, and the resulting TGA curves are shown in Fig. 3. The frameworks show good thermal stabilities, being stable up to 278 °C for CP 1 and 239 °C for CP 2. The TGA curves reveal a weight loss of 23.7% (calcd. 23.6%) for CP 1 at 278–390 °C, and a similar loss of 35.7% (calcd. 35.8%) for CP 2 from 239 to 400 °C, corresponding to the loss of H2DCTP ligands in both cases. A second weight loss of 68.1% (calcd. 68.3%) for CP 1 took place from 390 to 588 °C, which was ascribed to decomposition of the L ligands. For CP 2, the corresponding weight loss of 51.7% (calcd. 51.8%) in the range of 400–580 °C was also ascribed to decomposition of L ligands. The remaining residual masses of 8.2% (calcd. 8.1%) for CP 1 are ascribed to NiO, and 12.3% (calcd. 12.4%) for CP 2 corresponds to CoO.
Luminescence properties
CPs with transition metal centers have been investigated for potential luminescence applications [29]. In the present work, the luminescence properties of CPs 1 and 2 were determined in the solid state at room temperature. The emission spectrum of the free ligand L was also investigated under similar experimental conditions, in order to clarify the nature of the luminescence. As shown in Fig. 4a, upon excitation at ca. 250 nm for the free ligand L, an intense emission was observed at ca. 335 nm, which could be assigned to π → π* or π* → n charge transfer [30]. Emission peaks for CPs 1 and 2 were observed at ca. 341 and 355 nm, respectively, with excitations at ca. 260 and 280 nm. Hence, in comparison with the free ligand L, the emission spectra of both CPs are red-shifted, by 6 nm for CP 1, and 20 nm for CP 2. The various interactions within CPs networks often influence their emission spectra [31].
UV/Vis diffuse reflectance spectroscopy
The UV–Vis absorption spectra of free L and the two CPs were recorded in the crystalline state at room temperature. As illustrated in Fig. 4b, the free ligand L displays strong absorption bands at 272 nm, which can be attributed to π* → π transitions. The lower energy bands in the visible region at 547 nm for 1, and 550 nm (605 nm) for 2 can be ascribed to spin-allowed d–d transitions of the d 8 (Ni2+) and d 7 (Co2+) ions, respectively. Strong absorptions around 269 nm for 1, and 255 nm for 2 can be attributed to LMCT transitions [32] (Fig. 5).
Catalytic degradation properties
Wastewater produced in industrial processes often contains organic compounds which are toxic and not amenable to direct biological treatment [33]. These can include organic dyes, phenols, pesticides, biphenyls, detergents, hydrocarbons, plasticizers, pharmaceuticals and so on. Thus, economical and effective methods are urgently required to degrade organic pollutants in wastewater streams. In this context, advanced oxidation processes (AOPs), especially the Fenton reaction, are attractive on account of their high simplicity, efficiency, and reproducibility [34]. The homogenous Fenton reaction is one of the most environmentally friendly and economical systems. Both Ni(II) and Co(II) centers are able to activate persulfate to produce highly reactive species such as sulfate radicals \(({\text{SO}}_{4 \cdot }^{ - } )\) and hydroxyl radicals (HO·). These radicals are able to oxidize organic pollutants to CO2, H2O and inorganic species [35].
In this work, we chose MO as a model contaminant to assess the efficiency of our CPs as heterogeneous catalysts, with Na2S2O8 as the primary oxidant. As illustrated in Fig. 6a, in a control experiment in which only Na2S2O8 was added to a solution of MO, the color of MO showed no obvious change after 120 min. The MO degradation in this control experiment reached only 12.43%. On the other hand, when powdered samples of CPs 1 and 2 were added to the solution, the color of MO immediately faded. Thus, the degradation efficiencies reached 83.0% for CP 1, and 91.2% for CP 2. The degradation efficiency of CP 1 is lower than that of CP 2, suggesting that Co-based on CPs may have superior performance for degradation of organic contaminants [36]. In addition, both CPs show better catalytic performance than the previously reported Co(II)/NI(II) CPs [37, 38].
In order to evaluate the stabilities of the two CPs as catalysts for degradation of MO, the experiments were replicated three times under the same conditions. The results showed that both CPs are highly stable under the experimental conditions employed (Fig. S6).
Conclusions
In summary, two new CPs have been prepared and characterized. CP 1 has a 3D network structure with α-Po (or pcu net) primitive cubic topology. CP 2 has a 3D framework with the fivefold-interpenetrating sqc6 topology. The distinct three-dimensional architectures of the two CPs can be attributed to their different metal centers. CP 2 shows promise as a heterogeneous catalyst for degradation of organic pollutants in a Fenton-like process.
Supplementary material
CCDC 1503823-1503824 contain the supplementary crystallographic data for the CPs 1 and 2. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
References
Bétard A, Fischer RA (2012) Chem Rev 112:1055–1083
Cui Y, Yue Y, Qian G, Chen B (2012) Chem Rev 112:1126–1162
Dhakshinamoorthy A, Garcia H (2014) Chem Soc Rev 43:5750
Xu C, Guo Q, Wang X, Hou H, Fan Y (2011) Cryst Growth Des 11:1869–1879
Cook TR, Zheng YR, Stang PJ (2013) Chem Rev 113:734–777
Yang L, Powell DR, Houser RP (2007) Dalton Trans 955–964
Cohen SM (2012) Chem Rev 112:970–1000
Hao SY, Liu YG, Hao ZC, Cui GH (2016) Z Anorg Allg Chem 642:618–625
Liu C, Cui GH, Zou KY, Zhao JL, Gou XF, Li ZX (2013) CrystEngComm 15:324
Liu D, Lang JP, Abrahams BF (2011) J Am Chem Soc 133:11042–11045
Wang XX, Yu B, Van Hecke K, Cui GH (2014) RSC Adv 4:61281–61289
Stepanow S, Strunskus T, Lingenfelder M, Dmitriev A, Spillmann H, Lin N, Barth JV, Wöll Ch, Kern K (2004) J Phys Chem B 108:19392–19397
Cui GH, He CH, Jiao CH, Geng JC, Blatov VA (2012) CrystEngComm 14:4210–4216
DeFuria MD, Zeller M, Genna DT (2016) Cryst Growth Des 16:3530–3534
Desmarets C, Poli F, Le Goff XF, Müller K, Amouri H (2009) Dalton Trans 10429–10432
Rather B, Zaworotko MJ (2003) Chem Commun 9:830–831
Wang C, Zhang T, Lin W (2012) Chem Rev 112:1084–1104
Zhang X, Zhao YQ, Wang FS, Dong GY (2016) Chin J Inorg Chem 35:765–773
Hao JM, Yu BY, Van Hecke K, Cui GH (2015) CrystEngComm 17:2279–2293
Sheldrick GM (1996) Program for empirical absorption correction of area detector data. University of Göttingen, Göttingen
Spek A (2009) Acta Cryst D 65:148–155
Zhang X, Liu YG, Yu B, Cui GH (2015) Transit Met Chem 41:213–223
Wang XL, Li J, Tian AX, Zhao D, Liu GC, Lin HY (2011) Cryst Growth Des 11:3456–3462
Blatov V, Shevchenko A (1999) TOPOS 4.0. Samara State University, Samara Oblast
Hu JM, Blatov VA, Yu B, Van Hecke K, Cui GH (2016) Dalton Trans 45:2426–2429
Shi Z, Pan Z, Jia H, Chen S, Qin L, Zheng H (2016) Cryst Growth Des 16:2747–2755
Sheldrick GM (2014) Program for X-ray crystal structure determination. Göttingen University, Göttingen
Yin HD, Hong M, Wang QB, Xue SC, Wang DQ (2005) J Organomet Chem 690:1669–1676
Hu Z, Deibert BJ, Li J (2014) Chem Soc Rev 43:5815
Kuritz N, Stein T, Baer R, Kronik L (2011) J Chem Theory Comput 7:2408–2415
Allendorf M, Bauer C, Bhakta R, Houk R (2009) Chem Soc Rev 38:1330–1352
Lampeka YD, Gavrish SP (2000) Polyhedron 19:2533–2538
Dai M, Li HX, Lang JP (2015) CrystEngComm 17:4741–4753
Wu XY, Qi HX, Ning JJ, Wang JF, Ren ZG, Lang JP (2015) Appl Catal B 168:98–104
Sun H, Wang S (2015) RSC Catal 27:209–247
Wang X, Huang J, Liu L, Liu G, Lin H, Zhang J, Chen N, Qu Y (2013) RSC Adv 3:13944
Jiao CH, He CH, Geng JC, Cui GH (2011) Transit Met Chem 37:17–23
Hao JM, Zhao YN, Yu BY, Van Hecke K, Cui GH (2014) Transit Met Chem 39:741–753
Acknowledgements
The project was supported by the National Natural Science Foundation of China (51474086), Natural Science Foundation-Steel and Iron Foundation of Hebei Province (B2015209299).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hao, S.Y., Hao, Z.C., Liu, C. et al. Three-dimensional nickel(II) and cobalt(II) coordination polymers constructed from 2,5-dichloroterephthalic acid and bis(imidazole) ligands. Transit Met Chem 42, 123–130 (2017). https://doi.org/10.1007/s11243-016-0114-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0114-8