Abstract
The kinetics of the oxidation of CoIILn complexes {where L = ethylenediaminetetraacetate (EDTA), diethylenetriaminepentaacetate (DTPA), or N-(2-hydroxyethyl)ethylenediaminetriacetate (HEDTA)} by CrVI were studied under pseudo-first-order conditions with [CoIILn] ≫ [CrVI]. The kinetics showed first-order dependence on [CrVI]. The rate constant, k obs, decreases with increasing concentration of [CrVI]. At constant [H+], ionic strength, and temperature, the rate law is described by Eq. (i)
Both k 2 and k 3 showed acid-dependent and acid-independent pathways. The direct conversion CoIILn to CoIIILm is ruled out by spectrophotometric and ESR spectroscopic measurements that showed the formation of initial reaction intermediate(s). The rate law is consistent with one-electron and concurrent two-electron transfers leading to the formation of CrV and CrIV, respectively. An inner-sphere process, at least for the first term, leading to the formation of a relatively stable CrV species is almost certain. The kinetic term showing second-order dependence on [CoIILn], most likely, involves concurrent two-electron transfer leading to the formation of CrIV. The type of rate law and the proposed mechanism, reported here, depart from the well-established rate laws observed and mechanisms proposed for the oxidation of one-electron reductants by CrVI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxidation of organic as well as inorganic compounds by CrVI has been a subject of long-standing interest. The mechanisms of these oxidations have been reviewed in detail by a number of investigators [1–5]. A general mechanism for the oxidation of one-electron reductants by CrVI has been proposed by King and co-workers [6, 7]. In this mechanism, a sequence of three one-electron transfer steps is involved in the reduction of CrVI to CrIII as shown in Eqs. (1–3).
The general rate law is complicated and has been simplified by making assumptions: (i) A steady-state concentration of CrV intermediate exists and (ii) the rate of oxidation of CrIV–CrV is negligible.
With these assumptions, the general rate law is described by Eq. (4)
The complete form of the rate law has been observed in the oxidation of NpIV–NpV and in the oxidation of FeII–FeIII [8, 9]. Limiting forms of the rate law have been observed in other studies. In the oxidation of VIV–VV, the rate law is given by Eq. (5) where inhibition by the product VV was observed [10, 11]. This indicates that k 56 > k 54.
The simple second-order rate of Eq. (6) has been observed with a number of reductants. These include [Fe(phen)3]2+ [7], VIII [12], [Fe(bipy)3]2+, Fe(bipy)2(CN)2, [Fe(bipy)(CN)4]2−, [Fe(CN)6]4− [13], [Ta6Br12]2+, and [Ta6Cl12]2+ [14]. The kinetics of oxidation of [Fe(CN)6]4− by CrVI are also reported to be retarded by the accumulation of the reaction product[Fe(CN)6]3− and by its deliberate addition [15].
In a previous report on the reaction between CoIIL1 (L1 = EDTA) and CrVI, it was claimed that both CrV and CrIV are formed. The formation of CrV was ascertained by ESR spectroscopy, but no direct or indirect evidence for the formation of CrIV was presented. It is claimed that CrV and CrIV are stabilized by L1 [16]. There is no report in the literature on the isolation of CrV or CrIV by polyaminocarboxylates. In the oxidation of CrIIIL1 by N-bromosuccinimide, however, the first product in the biphasic reaction is believed to be a CrIV L1 species [17].
In this work, we report the kinetics of oxidation of CoII complexes of the polyaminocarboxylate ligands, ethylenediaminetetraacetate (L1), diethylenetriaminepentaacetate (L2), and N-(2-hydroxyethyl)ethylenediaminetriacetate (L3). It is shown in this report that both one-electron and concurrent two-electron processes are operative.
Experimental
Materials
The chemicals potassium dichromate (99.5% s.d. Fine-Chem), cobalt(II) nitrate hexahydrate, Co(NO3)2·6H2O, acetic acid, sodium acetate trihydrate, and perchloric acid were reagent grade (BDH) and were used as received. Ethylenediaminetetraacetate (L1) (Sigma), diethylenetriaminepentaacetate (L2) (GES), and N-(2-hydroxyethyl)ethylenediaminetriacetate (L3) were used without further purification. Aqueous solutions of these chemicals were prepared by accurate weight. Fresh redistilled water was employed in all chemical preparations and experiments.
Chromium(VI) solution was prepared by dissolving a weighed amount of potassium dichromate in distilled water. Solutions of the complexes CoIIL1, CoIIL2, and CoIIL3 were prepared by mixing solutions of L1, L2, and L3 with a Co(NO3)2.6H2O solution of known concentration. For all three complexes, [ligand] was 1.2[CoII] to ensure that virtually all CoII was complexed by the ligands. Buffer solutions were prepared using acetic acid and sodium acetate solutions of known concentrations. A solution of NaClO4 of known concentration was used to adjust ionic strength.
Instruments
UV–visible and pH measurements
The UV–visible absorption spectra of the reactions were measured using a Shimadzu spectrophotometer model 1800. The spectrophotometer was equipped with a thermostated cell holder. The pH of the reaction solution was measured using a Hanna pH meter model 211.
Kinetic procedures
The oxidations were carried out in an aqueous acidic medium. The pH was kept constant during the reaction using sodium acetate/acetic acid solutions of known concentrations. The ionic strength was maintained by adding sodium perchlorate solution of known concentration. The kinetic measurements for the oxidation of [CoIILn] (L = EDTA2−, DTPA3−, or HEDTA−) by CrVI were conducted under pseudo-first-order conditions, where [CoIILn] was present in a large excess over [CrVI] (more than tenfold). The course of the reaction was followed spectrophotometrically by recording the increase in absorbance of initial CoIII product at 550 nm as a function of time. The effects of various concentrations of the oxidant and complex, pH, and ionic strength on the rate of the reaction were investigated. At the completion of the kinetic runs, the solutions were examined spectrophotometrically. The values of the wavelength for the maximum absorbance and the molar absorption coefficient (ε) at the maxima were determined. By comparison with literature values, the ratio of ε at these maxima was used for the characterization of the products.
ESR measurements
ESR spectra were measured with an X-band ESR spectrometer (Bruker, EMX) at liquid nitrogen temperature (−60 °C) using a high sensitivity standard cylindrical resonator (ER 4119 HS) operating at 9.7 GHz. The ESR parameters were chosen to provide the maximum signal-to-noise ratio for non-distorted signals. Operating parameters for X-band ESR were as follows: power, 19.97 mW; modulation frequency, 100 kHz; modulation amplitude, 4.0 G; receiver gain, 5.02 × 104; conversion time, 81.92 ms; time constant, 20.48 ms; sweep time, 83.886 s; center field, 3450 G; sweep width, 500 G; and number of scans, 4. All ESR measurements were taken using a reaction mixture of 6.0 × 10−3 M CoIILn and 4.5 × 10−4 M of CrVI.
Results and discussion
The UV–visible absorption spectra of the initial products of the oxidation of [CoIILn]
The UV–Vis absorption spectra (over the 350–800 nm range) for the formation of the initial product of the oxidation were recorded as a function of time and are shown in Figs. 1, S1, and S2 (S indicates supplementary material).
The present study shows that the oxidation of CoIILn by CrVI does not lead directly to the formation of CoIIILn. In an earlier report of the oxidation of CoII L1 by CrVI, formation of Cr(V) and Cr(IV) has been proposed [16]. The ratio of the molar absorption coefficients (ε) at two maxima at the end of the oxidation reaction suggests that the final CoIII is not formed. In the literature, the ratio (ε535/ε382 = 322/230 M−1 cm−1) = 1.47 [18] is used to quantify the formation of CoIIIL1. However, the value of the ratio at these wavelengths after 10 days was 1.65. This shows that even after this long period of time the final product CoIIIL1 is not formed. In one experiment where [CoIIL1] = 2.4 × 10−3 M and [CrVI] = 9.95 × 10−4 M at pH 3.40, the final absorbance at 534 nm was determined as 0.82 [16]. This value is higher than the calculated value of 0.77 calculated using the molar absorbance at this wavelength.
A similar behavior is shown in Figs. S1 and S2 where an increased reaction time caused a shift in the peak position and a decrease in the peak amplitude (below 400 nm) accompanied by an increase in peak amplitude (above 500 nm). This is consistent with the formation of an initial CoIII product which is very slowly converted to a final product.
The observation of isosbestic points (Figs. 1, S1, and S2) indicates the existence of species at equilibrium during the oxidation of CoIILn by CrVI. For comparison, in one experiment H2O2 was used as an oxidant instead of CrVI, and no isosbestic point was observed, indicating the absence of species at equilibrium in this case. The final spectrum (curve 6) in each of the three figures does not pass through the isosbestic points.
ESR of CrV species
Figure 2 shows the ESR spectra of the intermediates formed immediately after the initiation of the oxidation of CoIILn by CrVI. The spectra are dominated by sharp single signals with the g-values 1.985, 1.982, and 1.987.
An ESR signal (g = 1.985) assigned to CrV species has already been reported [19]. The electronic configuration of CrV (d1) makes it convenient for detection by ESR spectroscopy. Stabilization of CrV complexes is enhanced if a chelate ring is bonded to CrV [20]. Since in the present work the oxidation is not carried out in the presence of excess EDTA, the CrV species formed is not likely to be CrV L1, but rather a CrV species coordinated to CoIII via an oxygen atom (L1CoIII–O–CrV). In an earlier study, however, CrVL1 is assumed to be formed in the oxidation of CoIIL1 by CrVI [16]. The observation of a peak at 728 nm in the present study further supports the formation of a CrV species as it is the only absorbing species at 725 nm [21].
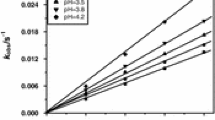
Variation of reaction rate with [CrVI]
Under pseudo-first-order conditions ([CoIILn] ≫ [CrVI]), plots of −ln(A∞ − At) versus time were found to be linear up to 90% of the reactions where A∞ and At are absorbance values at infinity and time t, respectively. The values of pseudo-first-order constants (k obs) were determined in each case from the slopes of the linear plots. It was found that the rate at which CrVI disappeared follows a first-order rate law. It was also observed (Tables 1, S1, and S2) that the values of k obs decrease with increasing [CrVI]T, i.e., initial gross CrVI concentration. This suggests that the acid chromate ion (HCrO4 −) is probably the active oxidizing species. Such an observation in CrVI oxidation, which was first observed by Novick [22], has been reported by several other authors [23–25], and it was well explained by Wiberg [2]. The concentration of hydrogen chromate ion (HCrO4 −) may be calculated from Eq. (7) using [CrVI]T and the reported K d value.
From Eq. (7), the ratio [HCrO4 −]/[CrVI]T can be given by Eq. (8)
It is clear from Eq. (8) that as [CrVI]T increases a progressively smaller portion of the total CrVI would be in HCrO4 − form. This explains the decreases in k obs with increasing [CrVI]T.
The plots of log (k obs[CrVI]T) versus log [HCrO4 −] (Figs. 3, S3, and S4) gave straight lines with slopes equal to 0.42, 0.88, and 0.75 for each of the three complexes, respectively.
The rate of the reaction is given by Eq. (9), where [CrVI]T = [HCrO4 –] + [Cr2O 27 ].
Data in Tables 1, S1, and S2 show that k obs[CrVI]T/[HCrO4 −]0.88, k obs[CrVI]T/[HCrO4 −]0.42, and k obs [CrVI]T/[HCrO4 −]0.75 are constants (=k corrected). From these relations, k obs[CrVI]T is equal to k corr [HCrO4 –]0.88, k corr [HCrO4 –]0.42, and k corr [HCrO4 –]0.75, respectively. By substituting for k obs [CrVI]T in Eq. (9), the rate is given by Eq. (10), which shows that the rate is proportional to [HCrO4 –]m, where m = 0.88, 0.42, or 0.75 for the oxidation of CoIIL1, CoIIL2, and CoIIL3, respectively.
In the literature, the rate of oxidation of certain organic compounds by CrVI is reported to be proportional to [HCrO4 −]X. Thus, in the oxidation of α-hydroxy-iso-butyric acid by CrVI the rate was found to be proportional to [HCrO4 −]0.33 and varies as [HCrO4−]0.65 in the oxidation of oxalic acid [23, 26]. It is not obvious why the exponents vary with variation of the reductant. The fraction does not represent the order with respect to [HCrO4 −]. In the present study, Eq. (10) shows that the rates of oxidation of CoIILn complexes by CrVI are proportional to [HCrO4 −]. It is well known that at very low concentration of dichromate solution, [CrVI]T ≤ 4.0 × 10−3 M, CrVI exists almost exclusively as [HCrO4 −] [27]. For this reason, in many studies of CrVI oxidations, its concentration was kept purposely very low to avoid the possibility of more complex rate laws. It should be mentioned, however, that the fractional exponents to which the [HCrO4 −] is raised are not the order with respect to this species.
An alternative approach to the issue of the variation of k obs with the initial [CrVI] is to plot 1/k obs versus [CrVI]. The results in Tables 1, S1, and S2 show that an inverse proportionality exists between k obs and [CrVI]. These plots are linear with the intercept of each of the plots which gives the value of 1/k obs at infinite dilution, namely when all the CrVI exists as the monomer HCrO4 −. The reciprocal of the intercepts is in agreement with the values of k obs at the lowest [CrVI] used.
Dependence of reaction rate on [CoIILn]
In the oxidation of CoIILn by CrVI, the variation in the rate constant with increasing complex concentration was investigated at constant reaction conditions. The results in Tables 2, 3S, and 4S show that the value of the observed rate constant, k obs, increases nonlinearly with increasing concentration of [CoIILn].
At constant reaction conditions, the dependence of k obs on [CoII L1] was found to fit a plot of k obs versus [CoIILn] using a polynomial fit of the second degree as shown in Figs. 4, S5, and S6.
The dependence of k obs on [CoIILn] is thus described by Eq. (11)
The rate law shows first- and second-order dependence on [CoIILn]. The plot of k obs/[CoIIL1] versus [CoIIL1] was found to be linear with both intercept and slope (Fig. 5).
Effect of [H+] on reaction rate
The results in Tables 2, 3S, and 4S show that for the oxidation of CoIILn by CrVI the value of k obs increased with increasing [H+]. The effect of pH on the values of k 2 and k 3 was investigated by increasing [CoIILn] and keeping all other variables constant at various pH values.
The results in Tables 3, 5S, and 6S show that both k 2 and k 3 increased with increasing [H+].
Plots of both k 2 and k 3 versus [H+] were found to be linear, each with an intercept (I1 and I2) and a slope (S1 and S2), respectively, as shown in Figs. 5 and 6 for the oxidation of CoIIL1. Similar plots were found in the variation of k 2 and k 3 in oxidation of CoIIL2 and CoIIL3 and are shown in Figs. S7, S8, S9, and S10.
The dependences of k 1 and k 2 on [H+] are described by Eqs. (12) and (13), respectively.
By substituting for the values of k 2 and k 3 in Eq. (11), k obs is given by Eq. (14)
From Eqs. (11) and (14), the experimental rate law for the oxidation of each of the three complexes [CoIILn] by CrVI is given by Eq. (15)
Generally, oxidations by CrVI are acid dependent and are accompanied by the transformation of HCrO4 − to [Cr(H2O)6]3+, and this process consumes hydrogen ions. This can partially explain the increase in the rate with increasing [H+]. It is also well documented that the CrVI reduction potential is relatively high in acidic media compared with neutral and basic media. Edwards has summarized observations on a large number of oxoanion reactions, noting that the rates of such reactions are usually accelerated by increasing [H+] [28].
The effect of ionic strength
The oxidation of CoIILn by CrVI was investigated as a function of ionic strength (µ) at constant reaction conditions. The results in Table 4 show that k obs increases with increasing the ionic strength.
Plots of log k obs versus µ1/2/(1 + µ1/2) were found to be linear as shown in Fig. 7.
The increase in k obs with increasing ionic strength is consistent with positive Bronsted–Debye salt effect, implying that the activated complex is composed of reactants of like charges (Fig. 8). Disagreement between the value of the slope and the product of charges (ZAZB) was observed in all three cases. The failure to obtain the expected slope according to this law can be explained partially by considering the dimerization constant (K d), which increases with µ. Increasing µ will favor the formation of Cr2O7 2−, and this will cause the equilibrium (2HCrO4 − ⇌ Cr2O7 2− + H2O) to shift toward Cr2O7 2−. Thus, an increase in µ decreases the rate of the reaction [29]. For this reason, it is recommended that oxidations by CrVI should always be performed at constant µ, to avoid such a conflict.
A mechanism for the oxidation of CoIILn by CrVI consistent with the rate law may be described by Scheme 1.
From Scheme 1, the rate law in Eq. (24) can be derived
From Eqs. (15) and (24), it can be seen that I1 = k5K1, S1 = k6K1k2, I2 = k7K1K3, S2 = k8K3K4. The derived rate law has the same form as the experimental one shown in Eq. (15).
In Eq. (16) of Scheme 1, it is proposed that HCrO4 − bonds to CoII by an oxo group. The CoII–CrVI species seems to undergo inner-sphere electron transfer which is either proton assisted [Eq. (21)] or not [Eq. (20)]. The existence of a pathway showing a second-order dependence on [CoIIL]n necessitates the formation of CoII–CrVI prior to attack by a second CoIILn species. It is well documented that termolecular reactions are very slow, and thus, it is very unlikely that two CoIILn species would simultaneously react with CrVI. Inner-sphere electron transfer may operate, following the bridging of a second CoIIL2− ion, with concurrent two-electron transfer and the formation of a stabilized CrIV species. This electron transfer is also either proton assisted [Eq. (23)] or not [Eq. (22)]. The ESR detection of CrV species lends support to this mechanism. It has been noticed earlier that reactions of CrVI proceeding through CrV are those in which the primary reducing agent is an ion that can provide one electron such as VIII and FeII [27]. It is unfortunate that CrIV is ESR silent and its formation is not certain. An outer-sphere mechanism in the step involving the oxidation of two CoII cannot be ruled out. This is shown in Scheme 2. A reaction pathway in which both inner- and outer-sphere electron transfers occur simultaneously is depicted in Eqs. (16)–(21) and Eqs. (25) and (26).
A rate law having the same form as that given by Eq. (16) can be derived from Scheme 2.
The rate laws derived from Schemes 1 and 2 are similar to that obtained experimentally in Eq. (15). In the literature, a rate law with a term independent of [H+] in oxidations by CrVI is very rare. It was observed in the oxidation of 2-deoxy-D-ribose [30], D- and L-rhamnose [31], and VIV [27]. The path independent of [H+] arises because of the low redox potential of CoIII/CoII couple of the polyaminocarboxlate complexes. The formation of relatively stable CrIV and CrV complexes during reduction of CrVI has been observed earlier in a number of investigations [17, 19].
Conclusion
The oxidation of CoIILn complexes by CrVI does not lead directly to the formation of CoIIILn. This is confirmed by the absorption spectra of the initial products as a function of time. The formation of CrV species is confirmed by ESR spectroscopy. The rate law of oxidation of the three CoIILn complexes by CrVI showed the same kinetic behavior. Evidence that supports the proposed mechanism included kinetic results, the ratio of the molar absorbance at the two maxima of CoIIILn, the presence of isosbestic points, the existence of CrV species, and the observation of a peak at 728 nm.
References
Westheimer FH (1949) Chem Rev 45:419
Wiberg KB (1965) Oxidation in organic chemistry, Part A. Academic Press, New York
Waters WA (1958) Quart Rev 12:277
Espenson JH (1970) Acc Chem Res. 3 347 and references therein
Beattie JK, Haight GP (1972) Prog Inorg Chem 17:93
Tong JY, King EL (1960) J Amer Chem Soc 82:3805
Espenson JH, King EL (1963) J Amer Chem Soc 85:3328
Sullivan JC (1965) JAmer Chem Soc 87:1495
Espenson JH (1970) J Amer Chem Soc 92:1880
Espenson JH (1964) J Amer Chem Soc 86(1883):5101
Rosseinsky DR, Nicol MJ (1970) J Chem Soc A 1196
Davies KM, Espenson JH (1970) J Amer Chem Soc 92:1884
Birk JP (1969) J Amer Chem Soc 91:3189
Espenson JH, Kinney RJ (1971) Inorg Chem 10:376
Howelet KE, Sulfab Y (1976) Inorg Chim Acta 17:129
Ohashi K, Aramaki M, Kaise M, Yamamoto K (1989) Anal Sci 5:73
Eljack ND, Sulfab Y (2012) Polyhedron 44:28
Rosenheim L, Speiser D, Haim A (1974) Inorg Chem 13:1571
Headlam HA, Lay PA (2001) Inorg Chem 40:78
Mitewa M, Bontchev PR (1985) Coord Chem Rev 61:241
Krumpolc M, Roček J (1979) J Amer Chem Soc. 101:3206
Westheimer FH, Novick A (1943) J Chem Phys 11:500
Bakore GV, Jain CL (1968) J Inorg Nuc Chem 31:2527
Sen Gupta KK (1975) Tetrahedron 31:123
Babu PSS, Khan Z, Kabir-ud-Din (2004) Transition Met Chem 29:885
Kemp TJ, Water WA (1964) J Chem Soc 3193
Espenson JH (1964) J Amer Chem Soc 86:5101
Edwards JO (1952) Chem Rev 50:455
Wiberg KB, Mill T (1958) J Amer Chem Soc 80:3022
Daier V, Singorella RS, Rizzotto M, Frascaroli MI, Palopoli C, Brondino C, Salas-Peregrin JM, Sala LF (1999) Can J Chem 77:57
Sala LF, Signorella RS, Rizzotto M, Frascaroli MI (1992) Gandolfo F Can J Chem 70:2046
Acknowledgments
I. M. A. Salih would like to thank Professor Gamal Mohamed Hassan, Head of the Department of Ionizing Radiation Metrology, National Institute for Standards, Ministry of Scientific Research, El-Haram, El-Giza, Egypt, for conducting ESR measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstracted from the Ph. D. thesis of I. M. A. Salih.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salih, I.M.A., Sulfab, Y. One- and two-electron reduction of chromium(VI) by polyaminocarboxylatocobaltate(II) complexes and the formation of chromium(V) and chromium(IV). Transition Met Chem 41, 315–323 (2016). https://doi.org/10.1007/s11243-016-0024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0024-9