Abstract
Two 1D coordination polymers based on transition metals, namely [Co(4,4′-bpy)2(H2bptc)] (1) and [Ni(4,4′-bpy)(H2O)4](H2bptc)·2H2O (2) (4,4′-bpy = 4,4′-bipyridine; H4bptc = 3,3′,4,4′-biphenyltetracarboxylic acid), had been synthesized under hydrothermal conditions and characterized by single-crystal X-ray diffraction, FTIR spectroscopy, elemental analysis, powder X-ray diffraction, and UV–Vis diffuse reflection spectroscopy. The photocatalytic degradation of methylene blue, methyl orange, and rhodamine B by these complexes under UV light irradiation was investigated and shows that both complexes can catalyze each of these reactions. A possible catalytic mechanism is proposed in terms of the HOMO–LUMO gap and the associated light-induced transitions in these complexes. This was further supported by ·OH radicals trapping experiments using isopropanol as radical scavenger. Complex 1 showed no obvious decay of photocatalytic efficiency even after recycling five times, implying excellent stability and recyclability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid development of coordination polymers (CPs) as functional inorganic–organic hybrid porous materials is associated with their diverse and easily tailored structures [1–3] and their various potential applications, including catalysis [4, 5], separation [6], gas storage [7], and carbon dioxide capture [1, 8, 9]. Recently, CPs and other metal–organic frameworks (MOFs) have also shown prospective opportunities for heterogeneous photocatalysis due to the presence of catalytically active metals and/or functional organic linkers as well as their easily tailorable physical, chemical, and catalytic properties [10]. Consequently, much effort has been devoted to developing new CPs and MOFs with photocatalytic potential, in particular with respect to the development of photocatalysts for organic pollutant degradation [11, 12], CO2 reduction [13, 14], and water splitting [15–17].
In general, the construction of CPs and MOFs is influenced by a wide range of considerations, including the organic ligands, solvents, metal atoms, and counterions [1, 12, 18]. Polycarboxylate ligands, as good candidates for the construction of CPs and MOFs, have attracted much interest from chemists. For example, 3,3′,4,4′-biphenyltetracarboxylic acid (H4bptc) has been employed as exo-multidentate ligand for the design and construction of novel compounds with high thermal stability and symmetry [19].
In this paper, we present two transition metal-based CPs, namely [Co(4,4′-bpy)2(H2bptc)] (1) and [Ni(4,4′-bpy)(H2O)4](H2bptc)·2H2O (2), constructed from 4,4′-bpy and H4bptc (define abbreviation). The optical band gaps, photocatalytic activities, and possible photocatalytic degradation mechanisms toward methylene blue (MB), methyl orange (MO), and rhodamine B (RhB) have been investigated.
Experimental
Materials and method
All chemicals were commercially available reagent grade and used without further purification. Elemental analyses were obtained using an Elementar Vario EL-III instrument. Powder X-ray diffraction (PXRD) patterns of the samples were determined with a Dandonghaoyuan DX-2700B diffractometer in the range of 2θ = 5°–40° with Cu Kα radiation. The Fourier transform infrared (FTIR) spectra, in the region (400–4000 cm−1), were recorded on a Nicolet 6700 FTIR spectrophotometer. UV–Vis diffuse reflectance spectrum (DRS) of solid samples were measured from 200 to 1200 nm with a PerkinElmer Lamda 650S spectrophotometer, in which BaSO4 was used as the standard with 100 % reflectance.
Synthesis of complex 1
A mixture of CoCl2·6H2O (0.3 mmol, 0.0714 g), H4bptc (0.3 mmol, 0.0991 g), and 4,4′-bpy (0.6, 0.0937 mmol) with a molar ratio of 1:1:2 was sealed in a 25-mL Teflon-lined stainless steel Parr bomb containing deionized H2O (20 mL). The mixture was heated at 160 °C for 72 h and then cooled down to room temperature. Purple block-like crystals were isolated and washed with deionized water and ethanol (yield 86 % based on CoCl2·6H2O). Anal. Calcd. for 1, C36H24CoN4O8: C, 61.8; N, 8.0; H, 3.4. Found: C, 62.0; N, 7.9; H, 3.5 %. IR (KBr)/cm−1: 3445.0(s), 3042.7(s), 2450.75(s), 1867.8(m), 1698.6(s), 1600.0(s), 1549.9(s), 1488.1(s), 1429.4(s), 1396.9(s), 1372.1(s), 1275.6(s), 1248.7(s), 1219.6(s), 1209.6(s), 1155.7(s), 1066.3(s), 1020.6(s), 1005.3(s), 892.4(m), 863.5(m), 808.7(s), 777.4(s), 750.2(s), 731.3(m), 708.9(m), 692.5(m), 676.6(m), 656.6(m), 627.9(s), 575.8(m), 539.4(m), 487.7(m), 437.1(m), 421.1(m).
Synthesis of complex 2
Green block-like crystal of complex 2 (yield 88 % based on NiCl2·6H2O) was synthesized from a mixture of NiCl·6H2O (0.3 mmol, 0.0713 g), H4bptc (0.3 mmol, 0.0991 g), and 4,4′-bpy (0.6, 0.0937 mmol) with a molar ratio of 1:1:2 M under the same conditions as 1. Anal. Calcd. for 2, C26H28N2NiO14: C, 47.9; N, 4.3; H, 4.3. Found: C, 48.1; N, 4.2; H, 4.5 %. IR (KBr)/cm−1: 3283.9(s), 2952.8(s), 1611.2(m), 1536.0(m), 1482.5(s), 1356.5(m), 1224.3(s), 1170.6(s), 1094.5(s), 1070.6(s), 1013.9(s), 889.4(m), 861.0(s), 838.1(s), 823.9(s), 767.6(s), 730.7(s), 706.5(s), 686.2(s), 637.0(s), 570.8(s), 519.5(s), 469.7(s), 410.0(m).
X-ray crystallography
X-ray single-crystal data collection for complex 1 and complex 2 was performed with a Bruker Smart 1000 CCD area detector diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) using φ − ω mode at 293(2) K. The SMART program [20] was used for data collection and the SAINT software [21] for data extraction. Empirical absorption corrections were performed with the SADABS program [22]. The structures were solved by direct methods (SHELXS-97) [23] and refined by full-matrix least-squares techniques on F 2 with anisotropic thermal parameters for all of the non-hydrogen atoms (SHELXL-97) [23]. The hydrogen atoms of the organic ligands were added according to theoretical models, and those of water molecules were found by difference Fourier maps. All structural calculations were carried out using the SHELX-97 program package [23]. Crystallographic data and structural refinements for the complexes are summarized in Table 1. Selected bond lengths and angles for both compounds are listed in Table 2.
Photocatalytic degradation experiments
The potential of these complexes as photocatalysts was evaluated via degradation of MB, MO, and RhB dyes at room temperature and under 500-W Hg lamp irradiation in a photocatalytic assessment system (Beijing Aulight Co. Ltd). The distance between the light source and the beaker containing the reaction mixture was fixed at 5 cm. Fifty micrograms of the required CP was added to 200 mL of MB (10 mg/L), MO (10 mg/L) or RhB (10 mg/L) aqueous solution in a 300-mL beaker. Prior to irradiation, the suspension was magnetically stirred in the dark for 120 min to ensure the establishment of an adsorption/desorption equilibrium. During the photocatalytic degradation experiment, stirring was maintained to keep the mixture in complete suspension. Aliquots of 1 mL volume were extracted at regular intervals using a 0.45-μm syringe filter (Shanghai Troody) for analysis. A Laspec Alpha-1860 spectrometer was used to monitor the changes in the dye absorbance in the range of 400–800 nm in a spectrometric quartz cell with 1-cm path length. The MB, MO, and RhB concentration was determined by the maximum absorbance at 664, 463, and 554 nm, respectively.
Results and discussion
Structures of complex 1 and complex 2
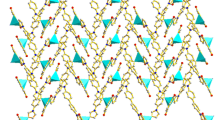
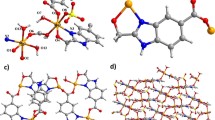
The complex [Co(4,4′-bpy)2(H2bptc)] (1) was synthesized under hydrothermal conditions, and is insoluble in common solvents including but not limited to water, methanol, ethanol, and ether. The crystal structure analysis reveals that complex 1 is built up of 1D neutral [Co(4,4′-bpy)2(H2bptc)] chains, as illustrated in Fig. 1d. The Co(II) center, in an octahedral geometry, is six-coordinated by two nitrogen atoms from two different 4,4′-bpy ligands, two oxygen atoms from two different monodentate H2bptc2− ligands, and two oxygen atoms from one H2bptc2− ligand in a chelating mode, such that two nitrogen atoms (N1 and N3) occupy the axial positions and the remaining four carboxylic oxygen atoms (O3, O4, O7, O8) lie in the four sites of the equatorial plane, as depicted in Fig. 1a. The Co–O and Co–N bond distances are comparable with the normal values for these bonds found in similar CPs [18, 24]. In the equatorial plane, the O4#1–Co1–O8, O3–Co1–O4#1, O3–Co1–O7, and O7–Co1–O8 bond angles are 88.05(15)°, 106.40(17)°, 104.73(16)°, and 59.67(14)°, respectively, while the N1–Co1–N3 bond angle is 167.32(18)°, revealing that the co-centered coordination octahedron is slightly distorted.
The H4bptc ligand has often been adopted as a component for building CPs with or without the auxiliary ligands. This polydentate ligand which may act as a linker with different geometric effects to connect metal centers into multidimensional structures via various coordination modes is shown in Scheme 1a–h [19, 25, 26]. In complex 1, the partly deprotonated H2bptc2− acts as a tridentate ligand, joining the Co(II) centers via both chelating and monodentate modes to form one-dimensional [Co(H2bptc)] chain, as illustrated in Scheme 1a, Fig. 1b, c. Again, in complex 1, only one nitrogen atom of the 4,4′-bpy ligands is coordinated to the metal, with the other nitrogen being terminal without coordinating to any metal ions, hence the 4,4′-bpy acted as a monodentate ligand to complete the Co centers’ coordination environment, much different from its role as typical bidentate linker in other reported CPs [27–29].
The complex [Ni(4,4′-bpy)(H2O)4](H2bptc)·2H2O (2) was synthesized under identical conditions to those used for complex 1, and is also insoluble in common solvents including but not limited to water, methanol, ethanol, and ether. In complex 2, the Ni(II) center is octahedrally coordinated by O9, O10, O11, and O12 from four water ligands, N1 and N2 from two different 4,4′-bpy ligands (Fig. 2a). In the equatorial plane, the N2–Ni1–N2, O11–Ni1–N2, O11–Ni1–O9, O9–Ni1–O10, and O10–Ni1–O12 bond angles are 88.01(19)°, 88.76(18)°, 86.54(16)°, 89.24(17)°, and 174.52(16)°, respectively, showing that the coordination geometry is a slightly distorted octahedron. In contrast to the monodentate mode in 1, the 4,4′-bpy ligands in complex 2 act as a typical bridging linker to join Ni2+ centers into a cationic [Ni(4,4′-bpy)(H2O)4]2+ zigzag chain (Fig. 2b). The adjacent [Ni(4,4′-bpy)(H2O)4]2+ chains and partly deprotonated H2bptc2− moieties are linked into a three-dimensional framework with the aid of electrostatic interactions and abundant hydrogen-bonding interactions, as illustrated in Fig. 2b–d and Table 3.
Optical energy gap
In order to explore the conductivities of the title compounds, their DRS were measured for powder samples in order to obtain the band gap E g [30, 31]. This parameter was determined as the intersection point between the energy axis and the line extrapolated from the linear portion of the absorption edge in a plot of Kubelka–Munk function F against energy E. The Kubelka–Munk function, F = (1 − R)2/2R, was obtained from the UV–Vis DRS data, where R is the reflectance of an infinitely thick layer at a given wavelength. Plots of F versus E for complexes 1 and 2 are shown in Fig. 3, where steep absorption edges are displayed, and the E g values of complexes 1 and 2 were obtained as 2.8 and 3.4 eV, respectively, indicating selective absorption in the visible and ultraviolet spectrum region for both complexes [32, 33].
Photocatalytic performance studies
The photocatalytic performances of complexes 1 and 2 for the degradation of MB, MO, and RhB under UV irradiation were assayed. In addition, control experiments on degradation of MB, MO, and RhB in the absence of any photocatalysts under UV light were performed. PXRD measurements were used to confirm the phase purities of the complexes. Taking complex 1 as example, the measured PXRD patterns matched well with the corresponding simulated patterns from the single X-ray crystal structure data, demonstrating the phase purity of the complex 1, as illustrated in Fig. 4. The slight differences in intensities may be attributed to the preferred orientation of the crystalline powder samples. The photocatalytic performances of the complexes were monitored by measuring the maximum absorbance intensity at λ = 664, 463, and 554 nm to determine the residual concentrations of MB, MO, and RhB, respectively.
The efficiencies of complexes 1 and 2 for photodegradation of MB, MO, and RhB are shown in Fig. 5a–c. All data are the averages of three parallel experiments. The degradation of MB increased from 25.9 % (control experiment without any photocatalyst) to 83.4 and 65.7 % for complexes 1 and 2, respectively, after 120 min. The decomposition efficiency for MO increased from 20.9 % (without any photocatalyst) to 95.6 % after 60 min and 48.3 % after 120 min for complexes 1 and 2, respectively. Furthermore, degradation of MO was nearly complete (ca. 95.6 %) after 40 min of UV irradiation in the presence of complexes 1. For RhB, after 120 min of UV irradiation, 80.6 and 81.7 % degradation efficiencies were achieved in the presence of complexes 1 and 2, respectively, whereas only 21.5 % RhB was decomposed in the control experiment. All of these photocatalytic degradation reactions followed pseudo-first-order kinetics, evidenced by the linear plots of ln(C/C 0) versus reaction time t, except for the RhB degradation with complex 1. In this case, the reaction was very rapid during the first 20 min, but slow in the next 100 min. The pseudo-first-order rate constants (k) and the corresponding square of the correlation coefficients (R 2) for the photocatalytic degradation reactions are listed in Table 4.
As seen from Fig. 5d–f, when solutions of MB, MO, and RhB were irradiated under UV light in the presence of complex 1 as photocatalyst, the maximum absorption peaks of all three dyes decreased with the reaction time showing that this complex has good photocatalytic performance.
In the past few years, several studies of the semiconducting properties of CPs have been reported due to their optical transition properties, electrochemical, and photochemical activities [34, 35]. Recently, Gascon et al. have pointed out that such semiconducting behavior only occurs in a very limited subset of MOFs, like MOF-5 due to the presence of ZnO [35]. In terms of their photocatalytic properties, MOFs can be considered as molecular catalysts rather than as typical semiconductors, and the HOMO–LUMO gap terminology has been used to describe the discrete character of the light-induced transitions in CPs and MOFs. Our previous research showed that a range of [M(phen)3(H3bptc)2] CPs possessed nearly identical optical energy gaps, with E g values for the complexes of 3.0, 3.3, 3.4, 3.4, and 3.3 eV for M = Co, Ni, Zn, Cd, respectively, while the values for [Mn(phen)2(Hbptc)]·5H2O were 3.3 eV [18]), but only [Ni(phen)3(H3bptc)2] and [Mn(phen)2(Hbptc)]·5H2O exhibited good photocatalytic performance for dye degradation. A possible mechanism for the photocatalytic behavior of complex 1 is shown in Scheme 2.
a Asymmetric unit of [Ni(4,4′-bpy)(H2O)4](H2bptc)·2H2O (2) and coordination environments around the Ni(II) atoms. b 2D sheet constructed from [Ni(4,4′-bpy)(H2O)4](H2bptc)·2H2O chains. c Packing view of 3D framework built from [Ni(4,4′-bpy)(H2O)4](H2bptc)·2H2O and lattice water molecules via hydrogen-bonding interactions for compound 2. d The hydrogen-bonding interactions in complex 2
In the presence of UV light, charge transfer presumably takes place from the HOMO, involving O and/or N atoms, to the LUMO, involving the metal atom [34–37]. The excited-state electron in the LUMO is usually very easily lost, while the HOMO of the excited species can accept one electron [34]. Therefore, electrons are captured from water molecules to produce ·OH active species, which can destroy the organic dye efficiently to complete the photocatalysis process [37, 38].
In order to confirm the proposed mechanism, radical trapping experiments were carried out to detect the main oxidative species in the photocatalytic process [39]. The addition of 1 mM isopropanol (IPA) as a radical scavenger significantly inhibited the degradation efficiency for MO [decrease from 95.6(2) % without IPA to 79.1(2) % with IPA), MB [decrease from 68.7(2) to 59.3(1) %], and RhB [decrease from 76.9(2) to 71.7(1) %] under UV light irradiation after 60 min, as illustrated in Fig. 6a. These results are consistent with the role of ·OH radicals as the main active species.
We also decided to test the recyclability and stability of complex 1 as a photocatalyst. Hence, a sample of complex 1 was used for five successive MO degradation experiments using identical reaction conditions. The results in Fig. 6b demonstrated that the photocatalytic performance remained almost unchanged after three runs and slowed only a small decrease after five runs. Furthermore, the PXRD diffraction pattern of the photocatalyst after these experiments gave a good match with the simulated ones of the as-prepared photocatalyst, as illustrated in Fig. 4, indicating that there was no noticeable change in the crystallographic structure of complex 1 during these experiments. Hence, complex 1 was stable under the experimental reaction conditions and could be used for several successive runs.
a Plots of concentration versus irradiation time for MB under irradiation with Hg lamp light using complexes 1 and 2 as photocatalysts. b Plots of concentration versus irradiation time for MO under irradiation with Hg lamp light with complexes 1 and 2 as photocatalysts. c Plots of concentration versus irradiation time for RhB under UV light irradiation with complexes 1 and 2 as photocatalysts. d UV–Vis absorption spectra of MB solution during the decomposition reaction under UV light irradiation in the presence of 1. e UV–Vis absorption spectra of MO solution during the decomposition reaction under UV light irradiation in the presence of 1. f UV–Vis absorption spectra of RhB solution during the decomposition reaction under UV light irradiation in the presence of 1
Conclusions
To summarize, two CPs were synthesized via hydrothermal methods, and structurally characterized. Complex 1 consists of 1D neutral [Co(4,4′-bpy)2(H2bptc)] chains, while complex 2 is built up from 1D zigzag [Ni(4,4′-bpy)(H2O)4]2+ chains, further linked into a 3D framework by electrostatic and hydrogen-bonding interactions. Both complexes are active photocatalysts for the degradation of a range of dyes under UV light. We are currently carrying out further research on such materials, in order to further explore their photocatalytic activities against other organic pollutants.
References
Wang C-C, Li H-Y, Guo G-L, Wang P (2013) Synthesis, characterization, and luminescent properties of a series of silver (I) complexes with organic carboxylic acid and 1, 3-bis (4-pyridyl) propane ligands. Transit Met Chem 38(3):275–282
Ming C-L, Hao Z-C, Yu B-Y, Van Hecke K, Cui G-H (2015) Synthesis, structures, and catalytic properties of three new metal–organic coordination polymers constructed from flexible benzimidazole-Based and cis-1,2-cyclohexanedicarboxylate synthons. J Inorg Organomet Polym Mater 25(3):559–568
Qin L, G-y Li, Zheng J, S-l Xiao, Cui G-H (2013) Two 3D supramolecular architectures from Ag (I) coordination polymers constructed by flexible Bis (benzimidazolyl) butane Ligand. J Inorg Organomet Polym Mater 23(6):1266–1273
Hasegawa S, Horike S, Matsuda R, Furukawa S, Mochizuki K, Kinoshita Y, Kitagawa S (2007) Three-dimensional porous coordination polymer functionalized with amide groups based on tridentate ligand: selective sorption and catalysis. J Am Chem Soc 129(9):2607–2614
Wen T, Zhang D-X, Liu J, Lin R, Zhang J (2013) A multifunctional helical Cu (I) coordination polymer with mechanochromic, sensing and photocatalytic properties. Chem Commun 49(50):5660–5662
Zhang Y-Q, Wang C-C, Zhu T, Wang P, Gao S-J (2015) Ultra-high uptake and selective adsorption of organic dyes with a novel polyoxomolybdate-based organic–inorganic hybrid compound. RSC Adv 5(57):45688–45692
Kuppler RJ, Timmons DJ, Fang Q-R, Li J-R, Makal TA, Young MD, Yuan D, Zhao D, Zhuang W, Zhou H-C (2009) Potential applications of metal-organic frameworks. Coord Chem Rev 253(23):3042–3066
Caskey SR, Wong-Foy AG, Matzger AJ (2008) Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J Am Chem Soc 130(33):10870–10871
Wang C-C, Guo G-L, Wang P (2013) Synthesis, structure, and luminescent properties of three silver (I) complexes with organic carboxylic acid and 4, 4′-bipyridine-like ligands. Transit Met Chem 38(4):455–462
Wang S, Wang X (2015) Multifunctional metal–organic frameworks for photocatalysis. Small 11(26):3097–3112
Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT (2009) Metal–organic framework materials as catalysts. Chem Soc Rev 38(5):1450–1459
Jing H-P, Wang C-C, Zhang Y-W, Wang P, Li R (2014) Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv 4(97):54454–54462
Wang C-C, Zhang Y-Q, Li J, Wang P (2015) Photocatalytic CO2 reduction in metal–organic frameworks: a mini review. J Mol Struct 1083:127–136
Fu Y, Sun D, Chen Y, Huang R, Ding Z, Fu X, Li Z (2012) An Amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew Chem 124(14):3420–3423
Lin H, Maggard PA (2008) Synthesis and structures of a new series of silver-vanadate hybrid solids and their optical and photocatalytic properties. Inorg Chem 47(18):8044–8052
Toyao T, Saito M, Horiuchi Y, Mochizuki K, Iwata M, Higashimura H, Matsuoka M (2013) Efficient hydrogen production and photocatalytic reduction of nitrobenzene over visible-light-responsive metal-organic framework photocatalyst. Catal Sci Technol 8:2092–2097
Liao Z-L, Li G-D, Bi M-H, Chen J-S (2008) Preparation, structures, and photocatalytic properties of three new uranyl—organic assembly compounds. Inorg Chem 47(11):4844–4853
Wang C-C, Zhang Y-Q, Zhu T, Zhang X-Y, Wang P, Gao S-J (2015) Four coordination compounds constructed from 1, 10-phenanthroline and semi-flexible and flexible carboxylic acids: hydrothermal synthesis, optical properties and photocatalytic performance. Polyhedron 90:58–68
Liu GX, Zhu K, Chen H, Huang RY, Ren XM (2009) New examples of metal coordination architectures of 3, 3′, 4, 4′-biphenyltetracarboxylic acid—syntheses, crystal structures, and physical properties. Z Anorg Allg Chem 635(1):156–164
Bruker AXS (2000) SMART, Version 5.611, Bruker AXS, Madison, WI, USA
Bruker AXS (2003) SAINT, Version 6.28, Bruker AXS, Madison, WI, USA
SADABS (2000) V2.03, Bruker AXS, Madison, WI
Sheldrick GM (1997) SHELX-97. Göttingen University, Germany
Pedro S, Brandão P, Shi F-N, Tedesco J, Reis M (2014) A new metal organic framework constructed of Co (II) ions six and seven-coordinated: synthesis, structure and magnetism. Polyhedron 81:210–215
Zhu S, Zhang H, Shao M, Zhao Y, Li M (2008) Monomeric and polymeric structures derived from 3, 3′, 4, 4′-biphenyltetracarboxylic acid, phenanthroline and metal ions. Transit Met Chem 33(6):669–680
Wang C-C, Jing H-P, Wang P, Gao S-J (2015) Series metal–organic frameworks constructed from 1, 10-phenanthroline and 3,3′,4,4′-biphenyltetracarboxylic acid: hydrothermal synthesis, luminescence and photocatalytic properties. J Mol Struct 1080:44–51
S-i Noro, Kitaura R, Kondo M, Kitagawa S, Ishii T, Matsuzaka H, Yamashita M (2002) Framework engineering by anions and porous functionalities of Cu (II)/4,4’-bpy coordination polymers. J Am Chem Soc 124(11):2568–2583
Khan MI, Yohannes E, Nome RC, Ayesh S, Golub VO, O’Connor CJ, Doedens RJ (2004) Inorganic-organic hybrid materials containing porous frameworks: synthesis, characterization, and magnetic properties of the open framework solids [{Co(4,4’-bipy)}V2O6] and [{Co2(4,4’-Bipy)3(H2 O)2}V4O12]·2H2O. Chem Mater 16(25):5273–5279
Wang Y, Feng L, Li Y, Hu C, Wang E, Hu N, Jia H (2002) Novel hydrogen-bonded three-dimensional networks encapsulating one-dimensional covalent chains: [M (4,4’-bipy)(H2O)4](4-abs)2·nH2O (4,4’-bipy = 4,4’-bipyridine; 4-abs= 4-aminobenzenesulfonate)(M= Co, n= 1; M= Mn, n= 2). Inorg Chem 41(24):6351–6357
Ji W-J, Zhai Q-G, Li S-N, Jiang Y-C, Hu M-C (2012) The ionothermal synthesis of a 3D indium metal–organic framework: crystal structure, photoluminescence property and photocatalytic activity. Inorg Chem Commun 24:209–211
Du P, Yang Y, Yang J, Liu B-K, Ma J-F (2013) Syntheses, structures, photoluminescence, photocatalysis, and photoelectronic effects of 3D mixed high-connected metal–organic frameworks based on octanuclear and dodecanuclear secondary building units. Dalton Trans 42(5):1567–1580
Stylianou KC, Heck R, Chong SY, Bacsa J, Jones JT, Khimyak YZ, Bradshaw D, Rosseinsky MJ (2010) A guest-responsive fluorescent 3D microporous metal-organic framework derived from a long-lifetime pyrene core. J Am Chem Soc 132(12):4119–4130
Laurier KG, Vermoortele F, Ameloot R, De Vos DE, Hofkens J, Roeffaers MB (2013) Iron (III)-based metal-organic frameworks as visible light photocatalysts. J Am Chem Soc 135(39):14488–14491
Wang C-C, Li J-R, Lv X-L, Zhang Y-Q, Guo G (2014) Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ Sci 7(9):2831–2867
Nasalevich M, Van der Veen M, Kapteijn F, Gascon J (2014) Metal–organic frameworks as heterogeneous photocatalysts: advantages and challenges. CrystEngComm 16(23):4919–4926
Lopez HA, Dhakshinamoorthy A, Ferrer B, Atienzar P, Alvaro M, Garcia H (2011) Photochemical response of commercial MOFs: Al2(BDC)3 and its use as active material in photovoltaic devices. J Phys Chem C 115(45):22200–22206
Mahata P, Madras G, Natarajan S (2006) Novel photocatalysts for the decomposition of organic dyes based on metal-organic framework compounds. J Phys Chem B 110(28):13759–13768
Yu ZT, Liao ZL, Jiang YS, Li GH, Chen JS (2005) Water-insoluble Ag–U–organic assemblies with photocatalytic activity. Chem A Eur J 11(9):2642–2650
Zhang H, Zong R, Zhao J, Zhu Y (2008) Dramatic visible photocatalytic degradation performances due to synergetic effect of TiO2 with PANI. Environ Sci Technol 42(10):3803–3807
Acknowledgments
We thank the financial support from National Natural Science Foundation of China (51578034), the Beijing Natural Science Foundation and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201410016018, KM201510016017), the Training Program Foundation for the Beijing Municipal Excellent Talents (2013D005017000004), the Importation & Development of High-Caliber Talents Project of Beijing Municipal Institutions (CIT&CD201404076), and 2011 Project for Cooperation & Innovation under the Jurisdiction of Beijing Municipality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, YQ., Wang, CC., Guo, XX. et al. Two 1D coordination polymers constructed from 3,3′,4,4′-biphenyltetracarboxylic acid and 4,4′-bipyridine: hydrothermal syntheses and photocatalytic performance. Transition Met Chem 41, 15–24 (2016). https://doi.org/10.1007/s11243-015-9992-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9992-4