Abstract
A series of transition metal(II) complexes of the type [M(PPQ)2] [PPQ = 2-pyridine-2-yl-3(pyridine-2-methylene-amino)quinazolin-4(3H)-one, M = Co(II), Cu(II), Ni(II), Zn(II), Cd(II)] have been prepared and characterized by IR spectroscopy, elemental analyses and X-ray crystal diffraction. The crystal structure studies revealed diverse coordination behavior of PPQ toward different metal ions, acting as an NNO donor in the cobalt(II) and zinc(II) complexes, but an NNN and NNO mixed donor in the copper(II) and nickel(II) complexes. The metal center generally has an octahedral coordination geometry with the tridentate PPQ ligand, except for the Cd(II) complex in which two PPQ ligands and a nitrate are coordinated (N4O4), forming a distorted triangular dodecahedron. The thermal stabilities, luminescence and magnetic properties of these complexes have been studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metal complexes of bidentate, tridentate and multidentate Schiff base ligands have been found to possess catalytic [1–3], magnetic [4, 5], luminescent [6, 7] and electrochemical properties [8, 9]. Since the properties of such complexes are heavily influenced by the nature of the ligands [10], much attention has been given to the design and synthesis of chelating ligands. Tridentate Schiff base ligands often exhibit versatile chelating modes, such as NNN, NNO, NNS and ONO [11–16]. Hence, the design and synthesis of such ligands remains an interesting area of current research.
Over the past few years, much work has been reported [17–19] on hydrazone Schiff base ligands obtained by the reaction of aldehydes with 2-aminobenzoylhydrazide [2-ABH]. Recently, it has been shown that the condensation of 2-ABH with aromatic and heterocyclic aldehydes yields ring-closed quinazolinone derivatives [20–24]. To date, five copper(II) and cadmium(II) complexes [25, 26] and six cobalt(II) complexes of the PPQ ligand have been reported [27]. In each case [25–27], the metal complexes were obtained in one pot by the reaction of the corresponding metal salts, 2-aminobenzoylhydrazide and pyridine-2-carboxaldehyde, giving complexes of 1:1 M:L stoichiometry. In the present work, we report some new coordination complexes of the PPQ ligand and of stoichiometry ML2. In addition, two coordination modes of the ligand, namely NNN and NNO, were observed, depending on the metal center. The X-ray crystal structures of these ML2 complexes [M = cobalt(II), nickel(II), copper(II), zinc(II) or cadmium(II)] and the related syntheses are reported herein. The complexes were also characterized by various physicochemical, magnetic and spectroscopic techniques (Scheme 1).
Experimental
Materials and methods
Ethyl anthranilate was purchased from Energy Chemical. 2-Picolinaldehyde and hydrazine hydrate were obtained from J&K Scientific Company. Dimethyl sulfoxide-d6 was purchased from Alfa Aesar. Other solvents and reagents were of analytical grade. 2-(Pyridin-2-yl)-3-((pyridin-2-ylmethylene)amino)-2,3-dihydroquinazolin-4(1H)-one, PPQ, was prepared through a modified literature procedure [28]. All the solvents were of analytical grade and distilled before use.
Physical methods
Elemental analyses (C, H and N) were obtained on a Perkin-Elmer 2400 analyzer. IR spectra were recorded on a Nicolet Magna-IR 750 spectrophotometer in the 4000–400 cm−1 region using KBr disks (w, weak; b, broad; m, medium; s, strong). 1H NMR spectra were recorded from DMSO-d6 solutions using a Bruker-400 spectrometer (s, singlet; d, doublet; t, triplet; m, multiplet; dd, doublet of doublets). Single-crystal structure analyses were performed on a Bruker Smart APEX II-CCD instrument. A FS5 fluorescence spectrofluorometer was used for fluorescence measurements. Thermogravimetric analysis (TGA) experiments were carried out in the temperature range of 25–650 °C on a NETZSCH TG 209 F3 thermal analyzer under N2 atmosphere at a heating rate of 10 °C min−1. Magnetic susceptibility measurements were performed using a Quantum Design MPMS XL-7 SQUID magnetometer, operating between 5 and 300 K at a sweeping rate of 1 K min−1 under an applied magnetic field of 2500 Oe. Pascal’s constants were used for the diamagnetic corrections.
Caution! Perchlorate salts are potentially explosive and should be treated with great caution. Only small amounts were used in the present work.
Preparation of complex 1
A solution of Co(ClO4)2·6H2O (18.1 mg, 0.05 mmol) in MeOH (6 mL) was added to a methanol solution (10 mL) of PPQ (32.9 mg, 0.1 mmol). The resulting brownish red mixture was stirred in a glove box under nitrogen for 20 min. The suspension was then filtered, and the filtrate was left to stand at room temperature. Light pink block-shaped single crystals of [Co(PPQ)2](ClO4)2·H2O, complex 1, suitable for X-ray diffraction analysis were obtained after several days. Yield: 68 % (based on Co). IR (KBr, cm−1): 545.27(w), 624.34(w), 694.73(w), 754.51(m), 992.20(w), 1095.37(s), 1158.04(m), 1226.51(m), 1305.57(w), 1346.55(w), 1368.73(w), 1406.33(s), 1451.65(m), 1519.63(m), 1608.82(s), 3055.17(w), 3318.41(s). Anal. Calcd for Co(C19H15N5O)2·(ClO4)2·H2O: H, 3.5 %; C, 48.8 %; N, 15.0 %. Found: H, 3.5 %; C, 48.9 %; N, 15.1 %.
Preparation of complex 2
Light brown block-shaped crystals of [Ni(PPQ)2](ClO4)2·2H2O, complex 2, suitable for X-ray diffraction were obtained in a similar way to that described for complex 1, except that Co(ClO4)2·6H2O was used instead of Ni(ClO4)2·6H2O. Yield: 52 % (based on Ni). IR (KBr, cm−1): 504.29(w), 542.86(w), 624.34(m), 692.32(w), 753.07(m), 795.49(w), 992.68(m), 1016.78(m), 1091.11(s), 1108.87(s), 1156.63(m), 1228.43(m), 1343.18(w), 1365.84(w), 1389.94w), 1406.82(m), 1445.87(m), 1482.51(w), 1510.47(w), 1608.34(s), 1666.20(m), 3399.41(s). Anal. Calcd for Ni(C19H15N5O)2·(ClO4)2·2H2O: H, 3.6 %; C, 47.9 %; N, 14.7 %. Found: H, 3.6 %; C, 47.9 %; N, 14.8 %.
Preparation of complex 3
Deep brown block-shaped crystals of [Cu(PPQ)2](ClO4)2·CH3OH·H2O, complex 3, suitable for X-ray diffraction were obtained in a similar manner to that described for complex 1, except that Co(ClO4)2·6H2O was used instead of Cu(ClO4)2·6H2O. Yield: 46 % (based on Cu). IR (KBr, cm−1): 498.99(w), 541.42(w), 622.41(s), 692.80(m), 754.03(s), 923.25(w), 996.10(m), 1097.30(s), 1160.94(s), 1227.47(s), 1290.14(w), 1341.73(w), 1400.07(m), 1446.83(m), 1509.51(m), 1609.31(s), 1647.39(m), 2009.94(w), 3067.71(w), 3365.66(s), 3493.90(m). Anal. Calcd for Cu(C19H15N5O)2·(ClO4)2·(CH4O)·H2O: H, 3.7 %; C, 48.2 %; N, 14.4 %. Found: H, 3.8 %; C, 48.3 %; N, 14.3 %.
Preparation of complex 4
Light brown block-shaped crystals of [Zn(PPQ)2](ClO4)2·CH3OH·3CH3CN·2H2O, complex 4, suitable for X-ray diffraction were obtained in a similar way to that described for complex 1, except that Co(ClO4)2·6H2O was used instead of Zn(ClO4)2·6H2O, together with a solvent mixture of MeOH/MeCN(1:1). Yield: 43 % (based on Zn). IR (KBr, cm−1): 545.27(w), 624.34(m), 696.66(w), 754.99(m), 847.56(w), 891.92(w), 927.11(w), 987.38(w), 1090.55(s), 1156.12(s), 1227.95(s), 1304.13(w), 1346.07(w), 1406.33(m), 1449.24(m), 1516.74(w), 1612.68(s), 3058.55(w), 3345.41(s). Anal. Calcd for Zn(C19H15N5O)2·(ClO4)2·(CH4O)·3(C2H3N)·2H2O: H, 4.3 %; C, 48.5 %; N, 16.3 %. Found: H, 4.3 %; C, 48.5 %; N, 16.4 %.
Preparation of complex 5
Cd(NO3)2·2H2O (18.1 mg, 0.05 mmol) and PPQ (32.9 mg, 0.1 mmol) were added to acetonitrile (10 mL), and the mixture was stirred for 20 min at room temperature. The suspension was then filtered, and the bright yellow filtrate was left undisturbed in air. Light yellow block-shaped single crystals of [Cd(PPQ)2·NO3](NO3)·CH3CN, complex 5, suitable for X-ray diffraction analysis were obtained after several days. Yield: 48 % (based on Cd). IR(KBr, cm−1): 488.87(w), 525.03(w), 545.76(w), 617.59(w), 636.88(w), 696.66(m), 762.71(s), 791.64(m), 848.53(w), 914.58(w), 935.31(w), 980.63(m), 1028.84(m), 1103.08(m), 1151.30(s), 1209.15(m), 1236.63(m), 1293.04(s), 1322.57(s), 1373.55(s), 1400.07(s), 1454.55(s), 1567.84(w), 1505.17(m), 1612.20(s), 1646.43(s), 2251.97(w), 3034.92(m), 3069.64(m), 3216.68(s), 3341.07(s). Anal. Calcd for Cd(C19H15N5O)2·(NO3)2·(C2H3 N): H, 3.6 %; C, 51.3 %; N, 19.5 %. Found: H, 3.5 %; C, 51.3 %; N, 19.4 %.
X-ray crystallography
Single-crystal X-ray diffraction measurements of all the complexes were obtained on a Bruker Apex II-CCD diffractometer at the ambient temperature (complexes 2, 3 and 5) or the atmosphere of liquid nitrogen (complexes 1 and 4) with graphite-monochromated Mo-Ka radiation (λ = 0.71073 Å). Absorption corrections were applied using the multiscan program of SADABS. All structures were solved by direct methods and refined anisotropically through full matrix least-squares methods on F 2 with the SHELXL program. Hydrogen atoms were located geometrically, whereas those of solvent molecules were found in Fourier difference maps, and all of the hydrogen atoms were refined in riding mode. In complex 1, four oxygens of ClO4 were distributed over two positions, i.e., O11(O11A), O12 (O12A), O13 (O13A) and O14 (O14A), and they were refined with site occupation factors (SOF) of 50 %. In addition, there is also disorder of perchlorate across an inversion center for Cl2 and Cl3, with SOF 50 %, respectively. In complex 3, the four oxygen atoms attached on Cl(3) are disordered and distributed into two parts (SOF 0.50 for each one) around a symmetric center. Final crystallographic parameters for complexes 1– 5 are listed in Table 1. Selected bond lengths and angles are listed in Table 2.
Results and discussion
Synthesis and general characterization
We have been systematically investigating the reactions of PPQ ligands with different transition metal salts for the synthesis of mononuclear and multinuclear compounds. Reactions of the metal perchlorides with PPQ in 1:2 molar ratio in MeOH/MeCN gave [Co(PPQ)2](ClO4)2·H2O (1), [Ni(PPQ)2](ClO4)2·2H2O (2), [Cu(PPQ)2](ClO4)2·CH3OH·H2O (3), [Zn(PPQ)2](ClO4)2·CH3OH·3CH3CN·2H2O (4) and [Cd(PPQ)2·(NO3)](NO3)·CH3CN (5), respectively. Complexes 1– 5 are soluble in common solvents such as methanol, ethanol and acetonitrile.
Crystal structure of complex 1
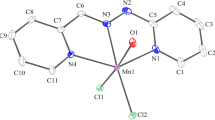
Crystals of complex 1 were grown from methanol solution. Crystallographic refinement data at 173 K suggested that 1 crystallized in the triclinic space group P ī (Table 1). The asymmetric unit is comprised of two PPQ ligands, one Co(II) center, two disordered perchlorate anions, a water molecule and a methanol molecule (Fig. 1a). The Schiff base provides O1, N1 and N2 for cobalt(II) coordination and therefore acts as a tridentate ligand, with the Co–O bond distances 2.098(6), 2.103(5) Å and Co–N bond distances in the range of 2.057(7)–2.128(7) Å and the cis-L–M–L bond angles around the metal center in the ranges of 75.4(3)°–108.8(3)°, thus leading to the formation of a distorted octahedral mononuclear cobalt complex. Configuration analyses were carried out with SHAPE [29], confirming the polyhedral shapes (Table 3 and Table S1). The crystal packing structure revealed a two-dimensional supramolecular framework. The crystal lattice is stabilized by π–π stacking interactions between the phenyl rings of HL, with a centroid–centroid distance of 3.4789 (7) Å (Fig. 2 left).
Crystal structures of complexes 2 and 3
Single-crystal X-ray diffraction analysis reveals that complex 3 belongs to the monoclinic system in C 2/c space group. The asymmetric unit of 3 consists of a Cu(II) center, two PPQ ligands, two disordered perchlorate anions and two free methanol molecules. The Cu(II) is coordinated to the N2 imino nitrogen, the N1 pyridine nitrogen and one atom O1 from an anionic PPQ ligand, while the second anionic PPQ ligand provides N6, N7 and N10 for coordination (Fig. 1c). The Cu–O bond distance is 2.292(3), and Cu–N bonds range from 1.974(4) to 2.269(4) Å. The bond angles around Cu are in the range of 72.12(13)°–107.76(15)°, giving a distorted octahedral mononuclear geometry. In the crystal structure, π–π stacking with a centroid distance of 3.8481(13) Å, which is larger than the stacking interaction of 1, leads to a one-dimensional framework (Figure S1b). The asymmetric unit of complex 2 shows a similar coordination environment to 3, such that the Ni(II) center is coordinated by two PPQ ligands in different modes; one ligand provides NNO and the other NNN coordination to the center metal atom (Fig. 1b). Similar to 3, the complex 2 exhibits a π–π packing framework with a centroid–centroid distance of 3.6905(8) Å, giving rise to a more close packing in this construction (Figure S1a).
Crystal structure of complex 4
Crystals of complex 4 were grown from methanol/acetonitrile mixed solution. Crystallographic refinement data at 120 K revealed that 4 crystallized in the triclinic space group P ī (Table 1).The asymmetric unit comprises of two PPQ ligands, a Zn(II) center, two perchlorate anions, two methanol molecules, three acetonitrile molecules and a water molecule (Fig. 1d). The Schiff base provides O1, N1 and N2 donors for zinc(II) coordination, thereby behaving as a tridentate ligand with the Zn–O bond distances of 2.142(3), 2.145(3) Å and Zn–N bond distances in the range of 2.081(4)–2.139(3) Å. The cis-bond angles around the zinc are in the ranges of 73.75(12)°–109.51(14)°, giving distorted octahedral coordination (Table 3). The crystal packing structure shows a two-dimensional supramolecular framework, stabilized by π–π stacking interactions between the phenyl rings of HL with a centroid–centroid distance of 3.4385 (4) Å (Figure S1c).
Crystal structure of complex 5
Complex 5 crystallizes in the monoclinic system with space group P 2(1)/c. As illustrated in Fig. 1e, each Cd(II) center is coordinated by two PPQ ligands plus one nitrate ion, forming an octa-coordinate mode CdN4O4. The asymmetric unit contains a Cd(II) center, two PPQ ligands, an acetonitrile molecule, a coordinate nitrate ligand and a free nitrate anion. Both PPQ ligands utilize the NNO coordination mode, while the bidentate nitrate provides two oxygen atoms to the metal center. The Cd–O bond distances in the range of 2.433(2)–2.5779(18) Å together with Cd–N of 2.371(2)–2.438(2) Å provide a distorted triangular dodecahedral geometry. The packing diagram shows three layers of molecules, which are independently arranged in the unit cell. Molecules in each layer are connected through the nitrate anion by intermolecular hydrogen bonding. In each unit, there are hydrogen bonds with D549A distances ranging from 3.511(4)–3.590(4) Å, which support the stabilization of the crystal packing (Fig. 2 right).
In previous work by Pelizzi, the PPQ ligand was found to act as a tridentate donor through either NNN or NNO donor sets when coordinated to Mn or Cu chlorides. This resulted in a five-coordinate environment provided by two chloride ligands plus the tridentate PPQ ligand. The bonding geometry in the resulting coordination polyhedra was distorted trigonal bipyramidal [30]. In our present study of PPQ, perchlorate or nitrate salts of transition metals were used instead of chlorides. It is interesting to note that the change of anions lead to different coordination behavior. In addition, the ligand configuration in these complexes is also dependent upon the type of metal center (Scheme 2). In the complexes of Co, Cu, Ni and Zn, the metal atom is six-coordinated by two PPQ ligands, resulting in a distorted octahedral coordination polyhedron. However, the Cd complex is eight-coordinated by a nitrate and two PPQ ligands, forming a distorted triangular dodecahedral configuration. We suggest that the different anions give rise to the different coordinate modes.
Thermal stability studies
The thermal stabilities of complexes 1–3 were investigated by thermogravimetric analysis (TGA) from room temperature up to 600 °C (Fig. 3). In all cases, the TGA curves indicate two steps; the first is attributed to the release of guest solvents (MeOH, H2O), while the second, at higher temperatures, is due to elimination of the PPQ ligands. Thus, for complex 1 the first step is observed in the temperature range 30–130 °C, while for complex 2 it occurs from 30 to 105 °C. In the case of complex 3, the weight loss is in accordance with the loss of MeOH solvent from 30 to 90 °C. The overall solvent losses for 1, 2 and 3 observed values are in agreement with the calculated values (1.9, 3.7 and 5.1 %, respectively). The second mass loss occurs very sharply which is assigned to elimination of the PPQ ligands for all the compounds.
Magnetic susceptibility studies
Variable-temperature magnetic susceptibility studies were performed on complexes 1–3 between 300 and 5 K, and a plot of χ m T versus T is shown in Fig. 4. At room temperature, octahedral Co(II) complexes typically maintain a large contribution due to the 4 T g ground term and exhibit µ eff values in the range 4.8–5.6 B.M [31, 32]. The magnetic moment of complex 1 is ca. 2.693 cm mol−1 K at 300 K, which is much higher than the expected value of 1.875 cm mol−1 K for a spin-only Co(II) ion with S = 3/2 and g = 2.0, owing to the important orbital contribution to the susceptibility of the Co(II) ion in a high-spin octahedral configuration [33, 34]. The χ m T value gradually decreases with cooling, reaching a minimum value of 1.81 cm mol−1 K at 5 K. The χ m value was obtained by best fitting to the Curie–Weiss law above 50 K with C m = 2.90 cm mol−1 K and θ = −11.21 K. The value of C is very close to the expected value of 2.8–3.4 cm mol−1 K for a mononuclear Co(II) ion in octahedral ligand field [35]. For complex 2, the χ m T product at 300 K (ca. 1.13 cm mol−1 K) is consistent with Ni(II) ions (1.0 cm3 mol−1 K for g = 2.0), and this value remains almost constant over all the measurement temperature ranges. The decrease in χ m T at low temperatures could be due to the zero field splitting. For complex 3, the χ m T value at room temperature is 0.50 cm mol−1 K for the Cu(II) center with S = 1/2, which is slightly larger than the uncoupled, spin-only value of 0.375 cm mol−1 K. On lowering the temperature, the χ m T value decreases slowly, reaching 0.485 cm mol−1 K at 2 K.
Fluorescence properties
Complexes of d10 metal centers with organic ligands are promising candidates for potential luminescence materials. Accordingly, the emission spectra of complexes 4 and 5 as well as those of the free ligand PPQ were studied in the solid state at room temperature to explore their potential applications as luminescent crystalline materials (see Fig. 5). All these microcrystalline samples have similar excitation maxima at ca. 350 nm. Intense fluorescence emissions were observed at 466 nm for free PPQ and at 557 nm for 4 and 583 nm for 5, as shown in Fig. 5. Compared to the emission of free PPQ, complexes 4 and 5 showed obvious redshifts of 91 and 117 nm, respectively. The strong fluorescence emission of free PPQ can be ascribed to π → π* and/or n → π* transitions. For complexes 4 and 5, the fluorescence may be attributed to intraligand transitions. We conclude that the overall architectures of complexes 4 and 5 increase to the rigidity of the ligand’s aromatic backbone and therefore maximize the intramolecular interactions among the organic moieties for energy transfer, effectively reducing the intraligand HOMO–LUMO energy gap [36].
Conclusion
In summary, five quinazoline-containing carbohydrozone complexes of five different metals have been synthesized and characterized by X-ray single-crystal diffraction. The ligand exhibits two different coordination modes, namely NNO and mixed NNO/NNN. Variable-temperature magnetic susceptibility measurements on complexes 1, 2 and 3 indicated paramagnetic nature with S = 3/2, 1 and ½, respectively. Complexes 4 and 5 showed strong ligand-based fluorescence with obvious redshifts compared to the free ligand.
Supplementary material
CCDC 1063922, 1063923, 1063924, 1063925 and 1063926 contain the supplementary crystallographic data for complexes 1–5. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 3360- 33; e-mail: deposit@ccdc.cam.ac.uk).
References
Tsuchida E, Oyaizu K (2003) Coord Chem Rev 237:213
Drozdzak R, Allaert B, Ledoux N, Dragutan I, Dragutan V, Verpoort F (2005) Coord Chem Rev 249:3055
Gupta KC, Sutar AK (2008) Coord Chem Rev 252:1420
Woodruff DN, Winpenny REP, Layfield RA (2013) Chem Rev 113(7):5110
Wu D-Y, Sato O, Einaga Y, Duan C-Y (2009) Angew Chem Int Ed 48:1475
Zheng Z-P, Wei Q, Yin W-X, Wan L-T, Huang X, Yu Y, Cai Y-P (2015) RSC Adv 5:27682
Vaughn AE, Bassil DB, Barnes CL, Tucker SA, Duval PB (2006) J Am Chem Soc 128(33):10656
Frederick FC, Coleman WM, Taylor LT (1983) Inorg Chem 22(5):792
Sarkar B, Konar S, Gómez-García CJ, Ghosh A (2008) Inorg Chem 47(24):11611
Rajput A, Mukherjee R (2013) Coord Chem Rev 257:350
Sacconi L, Bertini I, Morassi R (1967) Inorg Chem 6(8):1548
Lions F, Martin KV (1957) J Am Chem Soc 79(11):2733
Kong W-L, Chai Z-Y, Wang Z-X (2014) Dalton Trans 43:14470
Lumsden SEA, Durgaprasad G, Thomas Muthiah KA, Rose MJ (2014) Dalton Trans 43:10725
Fernández JJ, Fernández A, Vázquez-García D, López-Torres M, Suárez A, Gómez-Blanco N, Vila JM (2007) Eur J Inorg Chem 34:5408
Abdel-Aziz AA, Salem ANM, Sayed MA, Aboaly MM (2012) J Mol Struct 1010:130
Narang KK, Lal RA (1976) Indian J Chem 14A:442
Narang KK, Yadav US (1981) Indian J Chem 20A:404
Aminabhavi TM, Biradar NS, Roddabasanagoudar VL, Rudzinski WE, Hoffman DE (1986) Inorg Chim Acta 121:L45
Hunoor RS, Patil BR, Badiger DS, Vadavi RS, Gudasi KB, Chandrashekhar VM, Muchchandi IS (2011) Appl Organometal Chem 25:476
Hunoor RS, Patil BR, Badiger DS, Vadavi RS, Gudasi KB, Dandawate PR, Ghaisas MM, Padhye SB, Nethaji M (2010) Eur J Med Chem 45:2277
Gudasi KB, Vadavi RS, Shenoy RV, Patil MS, Patil SA, Nethaji M (2005) Trans Met Chem 30:661
Gudasi KB, Vadavi RS, Shenoy RV, Patil SA, Nethaji M (2006) Tran Met Chem 31:374
Badiger DS, Hunoor RS, Patil BR, Vadavi RS, Mangannavar CV, Muchchandi IS, Gudasi KB (2012) J Mol Struct 1019:159
Mangia A, Nardelli M, Pelizzi C, Pelizzi G (1974) Acta Cryst B30:17
Nardelli M, Pelizzi C, Pelizzi G (1977) Trans Met Chem 2:174
Mangia A, Nardelli M, Pelizzi G (1974) Acta Cryst B30:487
Gudasi KB, Vadavi RS, Shenoy RV, Patil MS, Patil SA (2005) Trans Met Chem 30:661
Llunell M, Casanova D, Cirera J, Alemany P, Alvarez S (2010) Shape program, version 2. Universitat de Barcelona, Barcelona
Pelizzi C, Pelizzi G (1974) Acta Cryst B30:2421
Figgis BN, Nyholm RS (1958) J Chem Soc 4190
Yamada S (1966) Coord Chem Rev 1:415
Zeng MH, Zhou YL, Wu MC, Sun HL, Du M (2010) Inorg Chem 49:6436
Zhang ZC, Zheng LM (2008) Inorg Chem Comm 11:1243
Sengupta O, Mukherjee PS (2010) Inorg Chem 49:8583
Zheng SL, Yang JH, Yu XL, Chen XM, Wong WT (2004) Inorg Chem 43:830
Acknowledgments
We thank the financial support by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). This experiment work is financially funded by NSFC program (21371010 and 21471023) and sponsored by Jiangsu Provincial QingLan Project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, F., Huang, W., Huang, X. et al. Transition metal complexes of a dihydroquinazoline-containing ligand: synthesis, crystal structures and physical properties. Transition Met Chem 40, 681–689 (2015). https://doi.org/10.1007/s11243-015-9962-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9962-x