Abstract
Two new cobalt–radical complexes, namely [Co(IM-5-Br-2Py)2(N3)2]·MeOH (1) and [Co(NIT-5-Br-2Py)2(N3)2] (2) (IM-5-Br-2Py = 2-(5-bromo-2-pyridyl)-4,4,5,5-tetramethyl-limidazoline-1-oxyl, NIT-5-Br-2Py = 2-(5-bromo-2-pyridyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) have been synthesized and characterized by magnetic and single-crystal X-ray diffraction studies. X-ray diffraction analysis indicates that both complexes possess mononuclear structures, in which two radical ligands coordinate to the Co(II) atom in chelating fashion and two azides act as terminal ligands. The magnetic properties for complexes 1 and 2 have been investigated in the temperature range 2–300 K. DC magnetic measurements show that the Co(II) atom of complex 1 interacts ferromagnetically with the directly bonding imino nitroxide, while the Co(II) atom strongly antiferromagnetically couples with the directly coordinated N–O group in complex 2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coordination complexes of paramagnetic metal atoms with nitroxide radical ligands have recently attracted considerable interest for the purpose of exploiting new molecular-based magnetic materials [1–4]. By using this approach, various highly interesting species such as molecular-based ferromagnets [5–7], single-chain magnets (SCMs) [8–13], single-molecule magnets (SMMs) [14–19] and molecule spin transition species [20–23] have been achieved so far. However, most of the work performed in this area has involved in copper(II), nickel(II), manganese(II) or lanthanide compounds, and the reported cobalt(II)–radical compounds are currently very scarce [24–26]. It is well known that the high-spin cobalt(II) atom may exhibit large magnetic anisotropy due to strong spin–orbital coupling. On the other hand, the direct binding of the nitroxide radicals with transition metal atoms can yield strong magnetic exchange coupling. Thus, the combination of the cobalt(II) atom and the nitroxide radical into a molecular entity will be an appealing way to obtain interesting magnetic materials. As part of our continuing interest in molecular-based magnetic materials by means of nitroxide radicals, here, we have chosen cobalt(II) atom and imino and nitronyl nitroxide radicals to construct new metal–radical complexes. In this paper, we report the crystal structures and magnetic properties of two cobalt(II)–radical complexes [Co(IM-5-Br-2Py)2(N3)2]·MeOH (1) and [Co(NIT-5-Br-2Py)2(N3)2] (2) (IM-5-Br-2Py = 2-(5-bromo-2-pyridyl)-4,4,5,5-tetramethyl-limidazoline-1-oxyl, NIT-5-Br-2Py = 2-(5-bromo-2-pyridyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide).
Experimental section
Materials and general methods
All reagents and chemicals were purchased from commercial sources and used without further purification. The imino radical ligand IM-5-Br-2Py and radical ligand NIT-5-Br-2Py were prepared by the literature methods [27, 28]. Elemental analysis for carbon, hydrogen and nitrogen were carried out on a PerkinElmer elemental analyzer model 240. The infrared spectra were recorded from KBr pellets in the range 4000–400 cm−1 on a Bruker Tensor 27 IR spectrometer. Variable-temperature magnetic susceptibilities were measured on a VSM SQUID magnetometer. Diamagnetic corrections were made using Pascal’s constants for all the constituent atoms.
Synthesis of [Co(IM-5-Br-2Py)2(N3)2]·MeOH (1)
NaN3 (0.065 g, 1.0 mmol) was added to a mixture of Co(ClO4)2·6H2O (0.1829 g, 0.5 mmol) and IM-5-Br-2Py(0.3 g, 1.0 mmol) in methanol (25 mL) at room temperature. The resulting solution was stirred for 10 min to obtain a dark blue solution, filtered and the filtrate was evaporated at room temperature for 3 days to give deep-blue crystals suitable for X-ray analysis. Yield 0.18 g (48 %). Anal. calc. for C25 H34Br2CoN12O3 (%): C 39.02; H 4.45; N 21.84. Found: C 38.44, H 4.07, N 21.11. IR (KBr, cm−1): 3414 (m), 2985 (m), 2030 (s), 1445 (s), 1363 (s), 1255 (s), 1161 (s), 1024 (s), 659 (m).
Synthesis of [Co(NIT-5-Br-2Py)2(N3)2] (2)
An identical procedure as that for 1 was followed to prepare 2, except that IM-5-Br-2Py was replaced by NIT-5-Br-2Py. Yield 0.23 g (60 %). Anal. calc. for C24H30Br2CoN12O4 (%): C 37.46; H 3.93; N 21.84; Found: C 37.04; H 3.87; N 21.19. IR (KBr, cm−1): 3117 (m), 2985 (m), 2040 (s),1574 (s),1555 (s), 1445 (s), 1349 (s), 1132 (s), 1028 (s), 845 (s).
X-ray crystallography
X-ray single-crystal diffractions of complexes 1 and 2 were performed on a Rigaku Saturn CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 113 K. The structures were solved by direct methods using the program SHELXS-97 and refined using full matrix least squares methods on F 2 with the use of the SHELXL-97 program package [29]. Anisotropic thermal parameters were assigned to all non-hydrogen atoms. The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter. Detailed data collection and refinement of the compounds 1 and 2 are summarized in Table 1. Selected bond lengths and angles are listed in Tables 2 and 3, respectively. Crystallographic data for the structural analyses have been deposited with the Cambridge Crystallographic Data Centre, CCDC Nos. 1059321for 1, and 1059322 for 2. The copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB21EZ, UK (Fax: t44 1223336 033; E-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
Results and discussion
Structural description of [Co(IM-5-Br-2Py)2(N3)2]·MeOH(1)
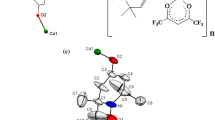
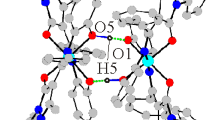
Complex 1 crystallizes in the monoclinic space group P21/c. An ORTEP drawing of 1 is shown in Fig. 1. The asymmetric unit of compound 1 contains one Co(IMNIT-5-Br-2Py)2(N3)2 moiety and one methanol solvent molecule. The coordination geometry of the cobalt(II) atom is distorted octahedral with the six coordination sites being occupied by four nitrogen atoms [N(2), N(3), N(4), N(6)] of the two bidentate imino nitroxide radicals and two nitrogen atoms [N(7), N(10)] of two azide groups. Two radical ligands act as bidentate ligands in a chelating manner toward cobalt(II) through the nitrogen atoms of the pyridine ring and imino nitrogen atoms. The Co–N(pyridyl) bond distances are 2.150(5) and 2.130(6) Å for Co(1)–N(3) and Co(1)–N(6), respectively, which are slightly shorter than the Co–N(imino) bond distances [2.158(6) and 2.147(6) Å for Co(1)–N(2) and Co(1)–N(4), respectively]. The dihedral angles between the N–O–C–N moieties and pyridyl rings for the two im-2-py ligands are 2.733(5)° and 1.967(5)°, respectively. Two azide groups coordinate to metal atom as terminal ligands in cis-positions. The Co(1)–N(7) and Co(1)–N(10) bond lengths are 2.078(7) and 2.092(6) Å, respectively. The azide ligands are quasi-linear [177.4(8)° and 179.4(8)°for N(7)–N(8)–N(9) and N(10)–N(11)–N(12), respectively]. The packing arrangement of complex 1 is shown in Fig. S1. The shortest intermolecular contact between nitroxide groups of O(2)···O(2) is 4.857(9) Å, implying the complex units are well separated.
Structural description of [Co(NIT-5-Br-2Py)2(N3)2] (2)
Complex 2 crystallizes in the triclinic space group Pī. An ORTEP drawing of 2 is shown in Fig. 2. The asymmetric unit of complex 2 is composed of two crystallographically independent Co0.5(NIT-5-Br-2Py)(N3) moieties. The cobalt atom lies on an inversion center and adopts a distorted octahedral coordination. The coordination sphere of the cobalt(II) atom is formed by two nitrogen atoms from two pyridyl rings, two oxygen atoms from two N–O groups and two nitrogen atoms of two azide ligands. The two azido anions acting as terminal ligands are ligated to a cobalt(II) atom at axial positions in the trans-form. The Co–O(nitroxide) distances are 2.115(4) and 2.107(4) Å for the two crystallographically independent moieties, respectively. The Co–N(pyridine) bond lengths are 2.144(5) and 2.127(5) Å for Co(1)–N(1) and Co(2)–N(7), respectively, which are longer than the axial Co–N(azide) bond distances [2.090(5) and 2.101(5) Å], suggesting the coordination geometry is a compressed distorted octahedron. The coordinated N–O bond lengths of the nitronyl nitroxide radicals are 1.300(6) and 1.318(6) Å, indicating the existence of the nitronyl nitroxide radical. The dihedral angles defined by the pyridine ring and the O–N–C–N–O plane are 20.298(2)° for Co(1) and 18.568(3)° for Co(2). The packing arrangement of complex 2 is shown in Fig. S2. The shortest intermolecular contact between nitroxide groups of O(2)···O(4) is 4.184(6) Å, implying the complex units are well separated.
Magnetic properties
The temperature-dependent magnetic susceptibilities of the two complexes were measured in the range of 2–300 K in an external magnetic field of 1 kOe, and the plots of χ M T versus T of the two compounds 1 and 2 are provided in Fig. 3, respectively. For compound 1, the χ M T values gradually increased as the temperature was lowered from 300 K, reaching a maximum value of 3.83 cm3 K mol−1 at 64 K, and then decreased sharply to 1.19 cm3 K mol−1 at 2.0 K. The χ M T value (3.66 cm3 K mol−1) at 300 K is larger than the expected value (2.62 cm3 K mol−1 with g = 2) for one uncoupled system for one cobalt(II) atom (S = 3/2) and two radicals (S = 1/2), which is due to the orbital contribution of the cobalt(II) atom. The gradual increase in the χ M T value in the high-temperature region suggests the occurrence of an intramolecular ferromagnetic interaction between the cobalt(II) atom and the coordinated radical. The χ M T value decreases rapidly in the low-temperature region, which can be attributed to the spin–orbit coupling of the cobalt(II) atom and/or the intermolecular antiferromagnetic interactions in the complex. It would be difficult to incorporate the spin–orbit coupling and magnetic interactions into a model to fit the magnetic data over the whole temperature range. However, the higher temperature magnetic data may be approximately interpreted by the isotropic spin Hamiltonian. For the present magnetic unit, the spin Hamiltonian can be expressed as \( \hat H = - 2J\sum {({{\hat S}_{\text{Co}}}} {\hat S_R} + {\hat S_{\text{Co}}}{\hat S_R}) \), where J represents the magnetic interaction between cobalt(II) atom and the coordinated radical ligand. The expression of the magnetic susceptibility is given below [30]:
with x = J/kT, g 1 = (3g Co + 2g R )/5, g 2 = (11g Co + 4g R )/15, g 3 = (5g Co − 2g R )/3 and g 4 = g Co.
The best fit for magnetic data (70–300 K) gave g Co = 2.37, g rad = 2.00, J = 6.46 cm−1, R = 1.20 × 10−3 (the residual value R is defined as ∑[(χ M)obs − (χ M)calc]2/∑[(χ M)obs]2). The fitting results indicate that the magnetic coupling between the cobalt(II) atom and the coordinated imino nitroxide radical is weakly ferromagnetic. Furthermore, the field dependence of magnetization has been determined at 2 K in the 0–70 kOe field range (Fig. 4). Upon increasing in the applied field, M increases up to 3.30 Nβ at 70 kOe, which does not reach the saturation, indicating the presence of magnetic anisotropy in the system [31, 32].
For compound 2, the χ M T value at room temperature is 2.34 cm3 K mol−1, which is slightly lower than the spin-only value of 2.62 cm3 K mol−1 for an uncoupled system for one cobalt(II) atom (S = 3/2) and two radicals (S = 1/2). Upon cooling, the χ M T value decreases more and more rapidly to reach 0.64 cm3 K mol−1 at 2.0 K. These indicate the presence of strong antiferromagnetic interactions in 2. Using the same magnetic model as in complex 1, the least squares analysis of magnetic data (60–300 K) led to g Co = 2.39, g rad = 2.00, J = −64.20 cm−1, R = 3.68 × 10−3. This shows that the cobalt(II)–nitronyl nitroxide interaction is a strong antiferromagnetic interaction. The field-dependent magnetization had been determined at 2 K in the 0–70 kOe field range (Fig. 4), upon increasing in the applied field, M increases up to 1.22 Nβ at 70 kOe for complex 2, suggesting the presence of a strong antiferromagnetic interaction in the complex.
As seen, the cobalt(II) atom ferromagnetically interacts with the imino nitroxide, whereas the antiferromagnetic interaction is observed between the cobalt(II) and nitronyl nitroxide. Because the imino and nitronyl nitroxides have similar related electronic structures, the reversal of the sign of the interaction should originate from the different coordination geometries around the cobalt(II) atoms. For the present two compounds, the magnetic orbitals of both imino and nitronyl nitroxide radicals are π* orbitals. The cobalt(II) atom has two magnetic orbitals (\( {d_{{x^2} - {y^2}}} \) and \( {d_{z^2}} \)) with σ symmetry and one magnetic orbital (either of d xy , d xz , d yz ) with π symmetry. Thus, the imino radical ligand and metal magnetic orbitals are orthogonal, which results in ferromagnetic coupling as observed in complex 1 [33, 34]. The observed weak ferromagnetic coupling can be attributed to the overlap of the π* orbital and another magnetic orbital of cobalt(II) (either of d xy , d xz , d yz ) leading to antiferromagnetic coupling. This antiferromagnetic contribution will lead to the reduction in the ferromagnetic interaction. For complex 2, two nitronyl nitroxide radicals are coordinated to the cobalt(II) atoms in equatorial positions. The Co–O–N angle is about 117°, which favors the overlap of the radical magnetic orbital (π*) and the cobalt(II) magnetic orbitals (\( {d_{{x^2} - {y^2}}} \) and \( {d_{z^2}} \)), resulting in strong antiferromagnetic interaction [35].
Conclusion
Two rare cobalt(II)-nitroxide complexes have been obtained by using imino and nitronyl nitroxide radicals. The radical ligands are bonded to the metal ion in a chelating fashion. Magnetic studies show that the cobalt(II) interacts ferromagnetically with the imino nitroxide. In contrast, antiferromagnetic coupling is observed between the cobalt(II) and nitronyl nitroxide. This can be ascribed to the different structural features for two complexes.
References
Caneschi A, Gatteschi D, Sessoli R, Rey P (1989) Acc Chem Rev 22:392
Iwamura H, Inoue HK, Hayamizu T (1996) Pure Appl Chem 68:243
Ovcharenko VI, Sagdeev RZ (1999) Russ Chem Rev 68:345
Benelli C, Gatteschi D (2002) Chem Rev 102:2369
Caneschi A, Gatteschi D, Renard JP, Rey P, Sessoli R (1989) Inorg Chem 28:1976
Caneschi A, Gatteschi D, Laugier J, Rey P (1987) J Am Chem Soc 109:2191
Ovcharenko V, Fursova E, Romanenko G, Ikorskii V (2004) Inorg Chem 43:3332
Caneschi A, Gatteschi D, Lalioti N, Sangregorio C, Sessoli R, Venturi G, Vindigni A, Rettori A, Pini MG, Novak MA (2001) Angew Chem Int Ed 40:1760
Ishii N, Okamura Y, Chiba S, Nogami T, Ishida T (2008) J Am Chem Soc 130:24
Bernot K, Bogani L, Caneschi A, Gatteschi D, Sessoli R (2006) J Am Chem Soc 128:7947
Maria GFV, Cassaro RAA, Akpinar H, Schlueter JA, Lahti PM, Novak MA (2014) Chem Eur J 20:5460
Han T, Shi W, Niu Z, Na B, Cheng P (2013) Chem Eur J 19:994
Ishii N, Ishida T, Nogami T (2006) Inorg Chem 45:3837
Kanegawa S, Karasawa S, Maeyama M, Nakano M, Koga N (2008) J Am Chem Soc 130:3079
Gass IA, Tewary S, Nafady A, Chilton NF, Gartshore CJ, Asadi M, Lupton DW, Moubaraki B, Bond AM, Boas JF, Guo SX, Rajaraman G, Murray KS (2013) Inorg Chem 52:7557
Bernot K, Pointillart F, Rosa P, Etienne M, Sessoli R, Gatteschi D (2010) Chem Commun 46:6458
Wang XL, Li LC, Liao DZ (2010) Inorg Chem 49:4735
Coronado E, Giménez-Saiz C, Recuenco A, Tarazón A, Romero FM, Camón A, Luis F (2011) Inorg Chem 50:7370
Poneti G, Bernot K, Bogani L, Caneschi A, Sessoli R, Wernsdorferc W, Gatteschia D (2007) Chem Commun 18:1807
Lanfranc de Panthou F, Luneau D, Musin R, Öhrström L, Grand A, Turek P, Rey P (1996) Inorg Chem 35:3484
Lanfranc de Panthou F, Belorizky E, Calemzuk R, Luneau D, Marcenat C, Ressouche E, Turek P, Rey P (1995) J Am Chem Soc 117:11247
Fokin S, Ovcharenko VI, Romanenko G, Ikorskii V (2004) Inorg Chem 43:969
Maryunina K, Fokin S, Ovcharenko VI, Romanenko G, Ikorskii V (2005) Polyhedron 24:2094
Yamamoto Y, Suzuki T, Kaizaki S (2001) J Chem Soc Dalton Trans 10:1566
Yamamoto Y, Suzuki T, Kaizaki S (2001) J Chem Soc Dalton Trans 19:2943
Wang LY, Zhao B, Zhang CX, Liao DZ, Jiang ZH, Yan SP (2003) Inorg Chem 42:5804
Tretyakov EV, Eltsov IV, Fokin SV, Shvedenkov YG, Romanenko GV, Ovcharenko VI (2003) Polyhedron 22:2499
Rajadurai C, Ostrovsky S, Falk K, Enkelmann V, Haase W, Baumgarten M (2004) Inorg Chim Acta 357:581
Sheldrick GM (2007) Acta Cryst A 64:112
Xu YH, Qu XN, Song HB, Jiang ZH, Li LC, Liao DZ (2007) Polyhedron 26:741
Murrie M, Teat SJ, Stoeckli-Evans H, Güdel HU (2003) Angew Chem Int Ed 42:4653
Hong CS, Koo J, Son SK, Lee YS, Kim YS, Do Y (2001) Chem Eur J 7:4243
Luneau D, Rey P, Laugier J, Belorizky E, Cognefn A (1992) Inorg Chem 31:3578
Luneau D, Rey P, Laugier J, Fries P, Caneschi A, Gatteschi D, Sessoli R (1991) J Am Chem Soc 113:1245
Cogne A, Laugier J, Luneau D, Rey P (2000) Inorg Chem 39:5510
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21471083) and MOE Innovation Team (IRT13022) of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Zhang, J. & Li, L. Synthesis, crystal structure and magnetism of two cobalt(II) complexes with imino and nitronyl nitroxides. Transition Met Chem 40, 631–636 (2015). https://doi.org/10.1007/s11243-015-9956-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9956-8