Abstract
The kinetics of oxidation of riboflavin (RFH) by sodium metaperiodate (IO4 −) in aqueous acidic medium have been studied. The reaction showed first-order dependence on both reactants and inverse dependence on [H+] over the pH range 1.4–2.6. The deprotonated form of riboflavin was found to be more reactive than its conjugate acid, (RFH2 +). The polymerization of acrylonitrile provided evidence for an inner-sphere mechanism involving iodine(VI) free radicals. The main oxidation products were identified by TLC and mass spectra as lumichrome and 1-acetylglycerol. The effect of iron(II) on the rate of oxidation was studied over the range (0.96–6.0) × 10−6 mol dm−3, and the rate was found to decrease with [Fe2+] over the range studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Periodate oxidations play an important role in biological determinations [1, 2]. They have been used in the spectrophotometric determination of glucose and fructose in invert sugar syrups [1]. Sodium metaperiodate [IO4 −] has been used for the oxidation of many inorganic [3–6] and organic substrates [7, 8] through two-electron transfer, [iodine(VII) → iodine(V)]. Symons reported that the oxidation of iron(II) by periodate proceeds via a series of one-electron transfer steps, involving iodine(VI) free radicals [9]. The polymerization of added acrylonitrile was taken as evidence for the formation of free radicals, resulting from one-electron transfer oxidation. Oxidations of inorganic and organic substrates by periodate are reported to proceed through an inner-sphere mechanism [3–5]. In all cases, IO4 − was reduced to IO3 − [4–6, 10]. The kinetics of oxidation of l-arginine by periodate have been studied spectrophotometrically in alkaline medium at λ = 280 nm [11]. The reaction was first order with respect to [substrate], [alkali], and [periodate]. The oxidation product of the reaction was found to be the α-keto acid. The anionic form of arginine (Arg−) was considered to be the reactive species. The kinetics and mechanism of oxidation of [CoII(HDTA)]4− (HDTA) = hexamethylenediaminetetraacetic acid by periodate have been studied spectrophotometrically in aqueous acidic medium [12] under pseudo-first-order conditions by taking a large excess of [IO4 −] at pH 4.0 and temperature = 30 °C. The electron transfer reaction between [CoII(HDTA)]4− and [IO4 −] obeys an inner-sphere reaction pathway, in which a long-lived intermediate complex is converted into the corresponding [CoIII(HDTA)]3− complex as final reaction product. Osmium(VIII)-catalyzed oxidation of dl-methionine by sodium periodate (Per) was studied spectrophotometrically at λ = 280 nm in aqueous alkaline medium at 30 °C [13]. A microamount of osmium(VIII) was sufficient to catalyze the reaction, which was first order in both [periodate] and [osmium(VIII)]. The reaction rate decreased with increasing [Met] and was independent of ionic strength and [OH−]. Methionine sulfone was found to be the main oxidation product. The kinetics and mechanism of oxidation of norfloxacin (NF) by diperiodato-argentate(III) (DPA) in alkaline medium were studied spectrophotometrically [14]. The reaction exhibits 1:1 DPA– NF stoichiometry, being first order in DPA, fractional order in both NF and alkali, and negative fractional order with respect to periodate. The main reaction products were hydroxylated NF and Ag(I). A mechanism involving free radicals was proposed. The kinetics of oxidation of chloramphenicol(CHP) by diperiodato-cuprate(III) (DPC) in aqueous alkaline medium were studied spectrophotometrically [15]. This reaction exhibits 1:2 stoichiometry (CHP–DPC) in alkaline medium. The reaction was first order in both DPC and CHP and showed fractional order dependence on alkali concentration. Increasing periodate concentration decreased the reaction rate.
Riboflavin is a water-soluble vitamin involved in a range of biologically important redox reactions. In the present work, we report on a study of the kinetics and mechanism of oxidation of riboflavin by sodium metaperiodate in aqueous acid medium.
Experimental
Materials and methods
All chemicals used in this study were of reagent grade (Analar, BDH, Fluka). Buffer solutions were prepared using Na2HPO4 (0.1 mol dm−3) and citric acid (0.2 mol dm−3). NaCl solution was used to adjust the ionic strength. Doubly distilled water was used in all kinetic runs and preparations. A stock solution of sodium metaperiodate (NaIO4, Aldrich) was prepared by accurate weighing and wrapped with aluminum foil to avoid photochemical decomposition [9]. Riboflavin (Sigma) was used without further purification. A stock solution of riboflavin was prepared by dissolving the required weight in doubly distilled water, and NaOH (0.1 mol dm−3) was added dropwise to form a clear yellow to orange solution, which was kept in the dark to avoid its photolysis. Riboflavin is light sensitive and unstable in alkaline solutions, but neutral and acidic solutions are stable in the dark (3 % decomposition per month at 27 °C, pH 6.0).
Measurement of rate constant
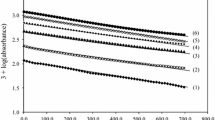
As reported, riboflavin (RFH) shows absorption maxima at 225, 275, 370, and 450 nm at pH 7 [16]. The UV–visible absorption spectra of the oxidation products of (RFH) by IO4 − were followed spectrophotometrically for a definite period of time using a Shimadzu 1700 p.c. spectrophotometer. Separate solutions of riboflavin and periodate in the required buffers were allowed to equilibrate separately at the required temperatures in a water bath for ca. 20 min, then thoroughly mixed and quickly transferred to an absorption cell. The rate of reaction was followed by measuring the absorption of the oxidation product (lumichrome) versus time at λ max = 380–385 where the absorption was maximal at the pH’s used (Fig. 1 ). In our pH range (pH 1.40–2.60), riboflavin shows an absorption maximum at λ max = 445 nm, whereas the band at 385 nm belongs to lumichrome as shown in Fig. 1. On oxidation, the peak at 445 nm decreased, while the peak at 385 nm increased with time. The presence of one isosbestic point at 420 nm indicates the presence of two absorbing species at equilibrium. Pseudo-first-order conditions were maintained in all kinetic runs using a large (tenfold) excess of [IO4 −] over riboflavin. The pH of the reaction mixture was measured using a 3505 Jenway pH meter.
Absorption spectra of the oxidation products at different times. Curves (1–11) were recorded at 0.5, 1.5, 3.0, 4.0, 6.0, 7.0, 8.0, 10.0, 15.0, 20.0, and 25.0 min. from the time of initiation of reaction. pH 2.20, I = 0.10 mol dm−3, T = 30 °C, [IO4 −] = 1.30 × 10−4 mol dm−3, and [RFH] = 1.30 × 10−5 mol dm−3
Stoichiometry
A known excess of riboflavin (at least threefold) was added to IO4
− in the required buffer. The concentration of the unreacted riboflavin was measured after 24 h from the onset of the reaction by dividing the absorbance at 445 nm by its molar absorptivity at the employed pH. This experiment showed that one mole of periodate was consumed per mole of riboflavin, consistent with; Scheme 1.
Product analysis
The products of the oxidative degradation of riboflavin in aqueous acidic solution were studied by a combination of UV–vis spectrophotoscopy, TLC, and mass spectra. TLC of the oxidation product was carried out on 250-μm silica gel G plates using the solvent system: (a) 1-butanol-acetic acid–water (40:10:50 v/v) [17] organic phase and (b) chloroform–methanol (9:2 v/v) [18]. The degradation products were significantly influenced by pH. Ahmed et al. [19] reported that lumichrome was the only major riboflavin degradation product in neutral to acidic pHs. When the pH of the solution was between 7 and 9, lumiflavin was also one of the major degradation products. Formylmethyl-flavin (FMF) and carboxymethyl-flavin (CMF) were reported as the other minor products [19].
Results and discussion
Oxidation of riboflavin by periodate was studied over the pH range (1.40–2.60), (0.1–0.5) mol dm−3 ionic strength and temperature range 20–40 °C for a range of periodate and riboflavin concentrations. The reaction rate was measured at the onset of the slow reaction at fixed [IO4 −], ionic strength, pH, and temperature. Plots of ln (A ∞–A t) versus time where A ∞ and A t are the absorbance of the oxidation products at infinity and at time t, respectively, were linear up to ≥90 % of the reaction. Values of the pseudo-first-order constant, k obs, were calculated from the slopes of the first-order plots. The stability of riboflavin toward the buffer was checked by measuring the absorbance at 445 nm for different periods of time. The constancy of the absorbance values indicates that there was no reaction between riboflavin and the reaction medium.
The effect of riboflavin concentration on the reaction rate was studied by varying the concentration over the (0.80–5.00) × 10−5 mol dm−3 range while keeping other parameters at constant values. The constancy of k obs at different riboflavin concentrations over the range studied indicates that the reaction is first order with respect to riboflavin concentration (Table 1). A plot of log (rate) versus log [riboflavin] was linear with slope = 1.0 ± 0.1. The effect of temperature on the reaction rate was studied over the range 20–40 °C while keeping other parameters constant. The kinetic data (Table 1) indicate that the reaction rate increased with temperature over the range studied. At constant [H+] and ionic strength, 1/k obs varies linearly with 1/[IO4 −] at different temperatures (Fig. 2) over the 20–40 °C range, with correlation coefficients r 20 = 0.9988, r 25 = 0.9996, r 30 = 0.9998, r 35 = 0.9999, and r 40 = 0.9998, where the subscripts are the temperatures in °C. The rate of reaction is thus represented as,
where [IO4 −]T and [RHF]T represent the total periodate and riboflavin concentrations. Then,
or
values of a and b were calculated at different temperatures from the slopes and intercepts of the plots, respectively (Table 2). Thermodynamic activation parameters including the enthalpy ∆H ‡ and entropy ∆S ‡ associated with the factor a were calculated using a least-squares fit to the transition state theory equation as 12.56 kJ mol−1 and −177.17 J K−1 mol−1, respectively. The electron transfer step is endothermic, as indicated by the positive value of ∆H ‡. The parameter ∆H ‡ is a composite value including the enthalpy of formation associated with the precursor intermediate, [RFH − (IVII)], and the enthalpy of activation of the intramolecular electron transfer step. The composite negative value of ∆S ‡ may be attributed largely to the substantial mutual ordering of the solvated water molecules of the equilibrium reactions and intramolecular electron transfer step [20].
The effect of pH on the reaction rate was studied by varying the pH between (1.40 − 2.60) at constant temperature and ionic strength. The values of k obs (Table 3) show that the reaction rate increased with pH over the range studied, suggesting involvement of the deprotonated form of riboflavin in the rate determining step. Plots of 1/k obs versus 1/[IO4 −] at different pH values were linear with definite intercept, correlation coefficients 0.9991, 0.9998, 0.9998, and 0.9998, at pHs 1.40, 1.70, 2.2, and 2.6, respectively (Fig. 3).
The effect of ionic strength was studied by varying the ionic strength values using standard NaCl solution over the (0.10–0.50) mol dm−3 range while maintaining other parameters constant. Values of k obs obtained at different ionic strengths (Table 4) indicate that the reaction rate was independent of this variable, supporting the conclusion that the reaction occurs between charged and uncharged species.
When acrylonitrile was added to the reaction mixture in a separate experiment, polymerization was observed after 1 day, indicating the presence of free radicals (IVI), consistent with the oxidation taking place through a one-electron transfer pathway. When acrylonitrile was added to riboflavin in the required buffer but in the absence of periodate, no polymerization was observed after the same period; hence, the polymerization is attributed only to the presence of iodine(VI) free radicals.
As reported previously [16], riboflavin exists in cationic form (RFH2 +) at low pH (<4.0), neutral form (RHF) at intermediate pH, and anionic form (RF−) at high pH (>9.7). In our pH range (1.40–2.6), the most probable reactive form of riboflavin is therefore (RHF2 +). From the reported equilibrium constants of aqueous periodate solution, it is assumed that over the pH range used, the periodate species likely to be present are IO4 − and H4IO4 −. Kustin and Lieberman [21] reported that at pH 4.2 and I = 0.1 mol dm−3, 99.8 % of periodate is present as H4IO6 − plus IO4 − and the remaining 0.2 % as H5IO6 −.
The mechanistic pathway for the oxidation of riboflavin, (RFH2 +) by periodate over the pH range studied, (1.40–2.60) may therefore be represented as follows (IVII represents [IO4 −] and H4IO6 −).
The main oxidation products of riboflavin were identified as lumichrome and 1-acetylglycerol, CH3–CO–(CHOH)3H by TLC and mass spectra.
From the above mechanism,
If [RFH]T represents all the different forms of riboflavin, then
Substitution into Eq. (8) then gives
and
Since the deprotonated form of riboflavin, (RFH) is considered to be more reactive than its conjugate acid in this system, Eq. (10) reduces to Eq. (11)
and
At constant [H+], Eq. (12) is consistent with Eq. (2), where
and
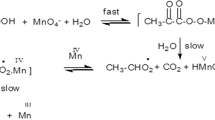
A plot of 1/a versus [H+] was linear with correlation coefficient 0.9998, slope = 0.302 and intercept = 0.0389. K 1 was calculated by dividing the intercept by the slope as 0.128 mol−1 dm3 s. A plot of b/a versus [H+] was also linear with correlation coefficient 0.9963, slope = 167.92 and intercept = 6.37. The value of k 2 was calculated from the reciprocal of the intercept as 0.156 mol−1 dm3 s−1, while K 3 was calculated by dividing the intercept of the plot of b/a versus [H+] by the intercept of the plot of 1/a versus [H+] as 163.8 mol dm−3. Substituting for the values of K 1, k 2, and K 3, the value of K 2 was calculated from the slope of the plot of b/a versus [H+] as 549.1 mol dm−3. Indeed an inner-sphere mechanism seems to be the preferred, if not the only pathway, in all periodate oxidations. Failure of periodate to oxidize [Fe(Phen)3]2+ to [Fe(Phen)3]3+ is in keeping with the requirement for an inner-sphere mechanism [22].
Kinetics in the presence of iron(II)
The oxidation with periodate can be catalyzed by copper(II) and iron(II) [23]. At constant [RFH], [IO4 −], pH, ionic strength and temperature, the effect of iron(II) on the reaction rate was studied over the (0.96–6.0) × 10−6 mol dm−3 range. The obtained values of k obs (Table 5; Fig. 4) indicate that the reaction rate was inhibited by iron(II). This is attributed to the formation of an iron(III)-periodate complex which is less reactive than free periodate. The dependence of k obs on iron(II) concentration is in agreement with Eq. (13);
A plot of 1/k obs versus [Fe2+] was linear with a definite intercept (Fig. 4). Values of k 3 and k 4 were calculated from the intercept and the slope of the plot as 1.61 s and 2.21 mol−1 dm3 s, respectively. The reaction mechanism in the presence of Fe2+ may be represented by the following reaction scheme:
where IVII and IVI refer to periodate [IO4 −] and the free radical [IVI], respectively.
From the above mechanism,
If [RFH]T represents all the different forms of riboflavin present, then
and
At constant periodate, Eq. (14) is consistent with Eq. (13), such that k 3 = 1/k 5[IVII] and k 4 = K 4/k 5[IVII]. The value of K 4 was calculated by dividing the slope by the intercept of (Fig. 4), as 1.37 mol−1 dm3. The reported ability of periodate to coordinate copper(III) and nickel(IV) [24] may confirm the role by which Fe2+ inhibits the reaction rate via the formation of an (Fe3+ − IVII) species.
Conclusion
The kinetics of oxidative degradation of riboflavin by periodate have been studied under various conditions. The reaction rate showed first-order dependence on both reactants and increased with pH over the (1.4–2.9) range. In an acid medium, riboflavin exists in its protonated form, RFH2 +. However, the deprotonated form of riboflavin, RFH was regarded as most reactive species over the pH range studied. Periodate oxidation may occur through one- or two-electron transfer pathways, but the detection of free radicals is consistent with a one-electron transfer reaction. The main oxidation products were lumichrome and 1-acetylglycerol. An inner-sphere mechanism in which the deprotonated form of riboflavin bridges to periodate in a step preceding the rate determining steps was suggested. The reaction rate was inhibited by added Fe2+, probably due to the formation of a Fe3+ periodate complex which is less reactive than free periodate.
References
Josian M, Tolot C, Crisiti DB, Elias ZA, Santos ML (2005) Anal Chim Acta 531:279
Slyke DDV, Hiller A, Macfadyen DA, Hastings AB, Kemperer W (1994) J Biol Chem 133:287
Sulfab Y (1976) J Inorg Nucl Chem 38:2271
Sulfab Y, Abu-Shadi AI (1977) Inorg Chim Acta 21:115
Kasim AY, Sulfab Y (1977) Inorg Chem Acta 24:247
El-Eziri FR, Sulfab Y (1977) Inorg Chem Acta 25:15
Buist GJ (1972) In: Boneford CH, Tripper CFH (eds) Comprehensive chemical kinetics, vol 6. Elsevier, Amsterdam, p 435
Indelli A, Ferranti F, Secco F (1966) J Phys Chem 70:631
Symons MCR (1955) J Chem Soc. doi:10.1039/jr9550002794
Hussein MA, Sulfab Y (1981) Transition Met Chem 7:181
Garapati S, Parvataneni V (2010) RJPBCS 1:977
Abdel-Khalek AA, Khalil MM, Khalid IS (1993) Transition Met Chem 18:153
Rao BD, Sridevi M, Vani P (2013) Indian J Appl Res 3:585
Padavathil HT, Mavalangi S, Nandibewoor ST (2013) AIJRSTEM 3:63
Rajeshwari VH, Kirthi SB, Sharanappa TN, Shivamurti AC (2010) Z Phys Chem 225:79
Drossler P, Holzer W, Penzkofer A, Hagamann P (2003) Chem Phys 286:409
Treadwell GE, Cairns WL, Metzler DE (1968) J Chromatogr 35:376
Schunan JN, Schollnhammer G, Hemmerich P (1975) Eur J Biochem 57:35
Ahmed I, Fasihullah Q, Noor A, Ansari IA, Ali QNN (2004) Int J Farm 280:199
Weaver MJ, Yee EL (1980) Inorg Chem 19:1936
Kustin K, Lieberman EC (1964) J Phys Chem 68:3869
Kasim AY, Sulfab Y (1997) Inorg Chem Acta 22:169
Bridgart GI, Fuller MW, Wilson IR (1973) J Chem Soc Dalton Trans 1274.doi:10.1039/DT9730001274
Hadinecm I, Jenšovský L, Linek A, Syneček V (1960) Naturwiss Enschaften 47:377
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Hady, A.E.M., Taha, A.M. Kinetics and mechanism of oxidation of riboflavin by periodate in aqueous acidic medium: evidence for the inhibiting effect of iron(II). Transition Met Chem 40, 379–385 (2015). https://doi.org/10.1007/s11243-015-9927-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9927-0