Abstract
The reaction of Fe(acac)3 and 2-(2′-hydroxyphenyl)oxazoline (Hphox) as a bidentate O,N donor ligand afforded a six-coordinated iron(III) complex [Fe(phox)2(acac)] with distorted octahedral configuration. The complex was isolated as an air-stable crystalline solid and characterized by elemental analysis, FTIR, solution electrical conductivity, and by single-crystal X-ray structure analysis. The structure, electronic properties, and vibrational normal modes of the complex were investigated by DFT. The use of this complex as a catalyst for the oxidation of sulfides to their corresponding sulfoxides using urea hydrogen peroxide as the primary oxidant was investigated. The catalyst shows very efficient reactivity, giving high yields and selectivities at room temperature under air.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective oxidation of sulfides to sulfoxides is an essential step in many biological and industrial processes. The resulting sulfoxides are essential intermediates for a number of applications. A number of methods have been developed for the conversion of sulfides into sulfoxides [1–4]. Iron compounds are not only low cost and environmentally friendly, but also used by nature in a variety of metalloenzymes and especially in oxidation reactions. Thus, in the last two decades, considerable effort has been invested in the search for synthetic iron catalysts with various ligands to promote oxidation reactions [5–8].
Compounds containing an oxazoline ring have become one of the most successful, versatile, and commonly used classes of ligands for catalysis of various reactions, due to their accessibility from readily available amino alcohols in short and high yielding syntheses, as well as their modular nature and applicability in a wide range of metal-catalyzed transformations [9–11]. Many complexes of such ligands show excellent catalytic activity, and there have been many recent reports on their applications in homogeneous and heterogeneous catalysis [12–14].
Density functional theory (DFT) has been widely used in the prediction of structure, electronic properties, and spectroscopic properties of metal complexes [15–17]. Electron transfer process in iron complexes with cyanide, pyrazine, 4,4′-bipyridine, and azide ligands has been studied by the B3LYP hybrid density functional method [15]. Jaworska et al. [16] have investigated the electronic structures and UV–Vis spectra of iron complexes with EDTA and NO ligands using the B3LYP functional. Conradie et al. [17] have employed DFT calculations using different functionals in order to study the properties of isomers of tris(beta-diketonato)3 iron complexes, and their results showed that the B3LYP functional provides the best description of both the spin state and geometry of such complexes.
Continuing our studies on the oxidation of sulfides using five-coordinated iron(III) complexes with ligands based on oxazoline, oxazine, and thiazoline rings [18–20], herein we describe the synthesis of a new six-coordinated Fe(III) complex [Fe(phox)2(acac)] (Hphox = 2-(2′-hydroxyphenyl)oxazoline and acac = acetylacetonate). The complex has been characterized by X-ray crystallography as well as DFT calculations and examined as a catalyst in the oxidation of sulfides in the presence of urea hydrogen peroxide (UHP) as an oxidant under air at room temperature (Scheme 1).
Experimental section
General procedures
Chemicals and solvents were purchased from Fluka and Merck. 2-(2′-Hydroxyphenyl)oxazoline (Hphox) was synthesized according to a published procedure [21].
Elemental analyses (C, H, N) were obtained with a Carlo ERBA Model EA 1108 analyzer. FTIR spectra were obtained with a Unicam Matson 1000 FTIR spectrophotometer using KBr disks at room temperature. Molar conductances were determined in methanol (ca. 10−3 M) at room temperature using a Toa CM 405 conductivity meter. The products of oxidation reactions were determined and analyzed with an HP Agilent 6890 gas chromatograph equipped with a HP-5 capillary column and flame ionization detector.
Synthesis of the complex
To a solution of Fe(acac)3 (0.353 g, 1.00 mmol) in ethanol (15 mL) was added a solution of 2-(2′-hydroxyphenyl) oxazoline and (0.326 g, 2.00 mmol) of the ethanol (10 mL). After stirring at room temperature for 4 h, the solution was filtered and evaporated under reduced pressure to give a precipitate. Recrystallization from acetonitrile yielded the complex as dark brown crystals. Yield: 0.361 g, 75 %. Anal. Calcd for C23H23FeN2O6: C, 57.6; H, 4.8; N, 5.8. Found: C, 57.5; H, 4.8; N, 5.9 %. Selected IR frequency (KBr disk, cm−1): 1611 (νC=N); ΛM (Ω−1 cm2 mol−1): 11.5.
General procedure for sulfide oxidation
The following standard procedure was used for sulfide oxidation experiments. To a solution of sulfide (0.2 mmol), chlorobenzene (0.2 mmol) as internal standard and [Fe(phox)2(acac)] (0.01 mmol) in a 1:1 mixture of CH3OH/CH2Cl2 (1 mL), was added UHP (0.4 mmol). The mixture was stirred at room temperature, and the reaction progress was monitored by GC. Products were identified by comparison with authentic samples.
X-ray crystallography
The crystal evaluation and data collection were performed on the Bruker SAINT Software package using a narrow-frame algorithm. A red block-like crystal of C23H23FeN2O6, approximate dimensions 0.090 mm × 0.170 mm × 0.700 mm, was used for the X-ray crystallographic analysis. The frames were integrated with the Bruker SAINT software package using a narrow-frame algorithm. The integration of the data using a monoclinic unit cell yielded a total of 23118 reflections to a maximum θ angle of 25.25° (0.83 Å resolution), of which 3941 were independent (average redundancy 5.866, completeness = 99.5 %, R int = 9.15 %) and 2560 (64.96 %) were >2σ(F 2). The final cell constants of a = 11.809(2) Å, b = 14.117(3) Å, c = 13.887(3) Å, β = 108.789(3)°, and volume = 2191.7(7) Å3 are based upon the refinement of the XYZ-centroids of 2674 reflections above 20 σ(I) with 4.647° < 2θ < 43.13°. Data were corrected for absorption effects using the multi-scan method (SADABS). The ratio of minimum to maximum apparent transmission was 0.851. The calculated minimum and maximum transmission coefficients (based on crystal size) were 0.6290 and 0.9370. The final anisotropic full-matrix least-squares refinement on F 2 with 291 variables converged at R 1 = 4.55 %, for the observed data and wR 2 = 12.05 % for all data. The goodness-of-fit was 1.007. The largest peak in the final difference electron density synthesis was 0.503 e−/Å3, and the largest hole was −0.437 e−/Å3 with an RMS deviation of 0.066 e−/Å3. On the basis of the final model, the calculated density was 1.452 g/cm3 and F(000), 996 e−.
Computational methods
The structure of the complex was geometry-optimized using the B3LYP hybrid density functional and 6−31+G* basis set. A frequency calculation was performed on the optimized structure in order to confirm that it was a local minimum on the potential energy surface. All calculations were performed using the Gaussian03 program [22].
Results and discussion
Complex characterization
The complex was obtained by the reaction of two equivalents of 2-(2′-hydroxyphenyl) oxazoline with one equivalent of Fe(acac)3 (Scheme 2). The fast color change from cherry red to a dark brown solution during the synthesis indicated coordination of ligand.
The new iron(III) complex is dark brown, stable in air and light and soluble in methanol, ethanol, acetonitrile, methylene chloride, DMF, and DMSO. The elemental analyses and spectroscopic data for the complex agree very well with its formulation as [Fe(phox)2(acac)].
In order to study the binding mode of the oxazoline ligand, the IR spectrum of free Hphox was compared with the spectrum of the complex. The shift of the band assigned to C=N from 1624 (free ligand) to 1611 cm−1 (complex) indicates coordination of the azomethine nitrogen to iron [23, 24].
The molar conductivity of the complex in methanol was 11.5 Ω−1 cm2 mol−1, indicating a non-electrolytic nature [25].
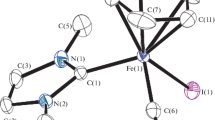
The structure of the complex has been determined by X-ray crystallography, and an ORTEP view of the complex with atom numbering scheme is shown in Fig. 1. A suitable single crystal of the complex was obtained by slow evaporation from MeCN solution. A summary of the X-ray structure refinement is shown in Table 1, and a crystal packing diagram of the complex is shown in Fig. 2.
The complex crystallizes in the monoclinic crystal system and space group P 1 21/n 1 and has a tetragonally compressed octahedral FeN2O4 chromophore. The iron atom is ligated by four donor atoms [N(1), N(2), O(2) and O(4)] from two chelating phox ligands plus two oxygen atoms [O(5) and O(6)] of one acac ligand. The complex is mononuclear, consisting of discrete monomeric units of [Fe(phox)2(acac)], in which the acetate ligand is in an all-equatorial position, with bond distances of 2.014(3) and 2.030(3) Å for Fe1–O5 and Fe1–O6, respectively, while the two donor atoms of each oxazoline ligand occupy one equatorial and one axial position. The rather short Fe1–O2 and Fe1–O4 distances of 1.934(2) and 1.951(2) Å compared to the equatorial coordination distances are indications of tetragonal compression [26].
DFT results
The geometry-optimized structure of the complex obtained from DFT calculations has a distorted octahedral configuration, in accordance with the X-ray crystallographic analysis. Table 2 presents some selected calculated and experimental bond lengths and bond angles for the complex. The calculated bond lengths are almost all slightly shorter than the corresponding experimental values. The error is in the 2–7 % range for Fe–O(N) bond lengths and <1 % for other bond lengths. Both shorter and longer calculated bond lengths, compared to experimental values, have been reported previously [27–29]. The differences have been attributed to environmental effects as well as the choice of DFT method [27–29].
The calculated natural bond orbital (NBO) atomic charge on Fe1 is +0.717, while those on O1, O2, O3, O4, O5, O6, N1, and N2 are −0.529, −0.592, −0.529, −0.592, −0.573, −0.573, −0.443, and −0.443, respectively. The charge on the iron atom is thus significantly lower than the formal charge of +3 indicating charge transfer from the ligands to the metal center. Figure 3 shows the HOMO−1 (−5.06 eV), HOMO (−5.59 eV), LUMO (−1.38 eV), and LUMO+1 (−1.28 eV) of the complex. It is clear that while the HOMO-1 and HOMO orbitals show smaller contributions on Fe, the LUMO and LUMO+1 orbitals show greater contributions on and around the Fe atom. The calculated HOMO–LUMO energy gap is 4.21 eV, indicating good stability and chemical hardness of the complex.
A frequency calculation on the complex at the B3LYP/6-31G* level of theory confirmed that the optimized structure is a minimum on the potential energy surface. Some of the experimental and corresponding calculated vibrational frequencies along with the probable assignments are reported in Table 3. It should be added that the calculated vibrational frequencies have been scaled by a factor of 0.9613 in order to correct for theoretical errors such as electron correlation and basis set deficiencies [30]. Good agreement between experimental and calculated vibrational frequencies is observed. According to the computational results, vibrations in the region of 3093–3054 cm−1 are related to the aromatic C–H stretching of benzene groups in agreement with the results reported in the literature [31]. Vibrations in the region of 3035–2936 cm−1 are related to the C–H stretching modes of the oxazoline and acac rings. The calculated C=N stretching frequency of the complex is 1610 cm−1, in very good agreement with the experimental value of 1611 cm−1. The aromatic C–H in-plane and out-of-plane bending modes of benzene and its derivatives have been reported in the region of 1300–1000 and 1000–675 cm−1, respectively [32]. Our calculated frequencies for the aromatic C–H in-plane bending and C–H out-of-plane bending modes are 1144 and 745 cm−1, respectively, in good agreement with the reported values.
Catalytic reactivity
In order to evaluate the catalytic activity of this complex for the oxidation of sulfides, the reaction conditions were optimized with respect to the oxidation of methylphenyl sulfide (MPS) with respect to solvent, quantity of catalyst, and the amount of UHP.
Initially, oxidation of MPS was examined without catalyst and with either Fe(acac)3 or [Fe(phox)2(acac)]. As reported in Table 4, the maximum activity was observed with [Fe(phox)2(acac)], whereas oxidation of MPS without catalyst gave almost no conversion. The conversion of MPS increased with increasing amount of catalyst from 0 to 0.01 mmol. When the amount of catalyst was further increased to 0.015 mmol, the selectivity for sulfoxide product was reduced from 96 to 89 %. Next, dichloromethane, chloroform, acetonitrile, acetone, methanol, and 1:1 mixtures of CH2Cl2/CH3OH and CH2Cl2/CH3CN were investigated as solvents. Among these choices, the 1:1 mixture of CH3OH/CH2Cl2 was found to be the best for this protocol (Table 4). The amount of UHP also had a significant effect on both the conversion and methylphenyl sulfoxide selectivity (Table 4, entries 12–16). When the amount of UHP was increased from 0.1 to 0.4 mmol, the conversion of MPS increased drastically from 22 to 73 %. With a further increase in UHP to 0.5 mmol, the selectivity for methylphenyl sulfoxide decreased from 96 to 89 %, while the conversion of MPS increased from 73 to 80 %. In other words, the selectivity for sulfoxide would appear to be better for reactions with 2 equivalents of the oxidant compared to higher amounts.
In order to compare the present catalytic system with recently reported protocols, we compared the results obtained for MPS oxidations in the presence of other five-coordinated iron(III) complexes. As shown in Table 5, [Fe(phox)2(acac)] gave lower oxidation yield but higher selectivity than the other complexes. Although the mechanism of these oxidation reactions is not yet known, it is probably related to the availability of a site for coordination of the substrate or oxo-intermediate in five-coordinated iron(III) complexes. Hence, the six-coordinated iron(III) complex [Fe(phox)2(acac)] must gain a free site before the reaction can proceed.
The present complex [Fe(phox)2(acac)] proved to be capable of catalyzing the oxidation of a range of structurally diverse sulfides (Table 6). Arylalkyl (Table 6, entries 1,2), arylbenzyl (Table 6, entry 3), dibenzyl (Table 6, entry 4), diaryl (Table 6, entry 5) and dialkyl (Table 6, entries 6–8) sulfides underwent clean and selective oxidation to the corresponding sulfoxide under air, with impressive selectivities. Very good conversions of substrates were obtained for all cases. Aromatic sulfides were found to undergo oxidation more easily than aliphatic substrates. In the case of dialkyl sulfides (Table 6, entries 6–8), no over-oxidation to the sulfone was observed.
In general, the reactivity of the catalytic system completely ceased after 15 min. Upon addition of UHP to a solution of the iron complex for the oxidation of methylphenyl sulfide, the absorption band at 230 nm assigned to LMCT [phenolate (p π ) → iron(III) (d σ*)] [33, 34] of [Fe(phox)2(acac)] disappeared gradually, and the solution turned from brown to pale yellow. The marked decrease in the absorption intensity at 230 nm after 15 min (Fig. 4) indicates the destruction of the catalyst, resulting in loss of reactivity. In the presence of greater amounts of the oxidant the decomposition of the catalyst increased, for example when a large excess of UHP (1 mmol) was added to the solution, the absorption band disappeared immediately and the brown solution became almost colorless.
Conclusions
The iron(III) complex [Fe(phox)2(acac)] has been synthesized and characterized by physicochemical methods and by X-ray crystallography. The complex proved to be an effective catalyst for the oxidation of sulfides to their corresponding sulfoxides. However, the lack of a free site may explain the lower oxidation yields of the present complex compared to others reported previously. Nevertheless, easy preparation, mild reaction conditions, high yields of the products, short reaction times, and high selectivity make this catalytic system of potential interest for oxidation reactions.
Supplementary data
The CIF file of crystal structure complex [Fe(phox)2(acac)] has been deposited with the CCDC, No.1030742. This data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk
References
Amini M, Haghdoost MM, Bagherzadeh M (2014) Coord Chem Rev 268:83–100
Amini M, Haghdoost MM, Bagherzadeh M (2013) Coord Chem Rev 257:1093–1121
Jeyakumar K, Chakravarthy RD, Chand DK (2009) Catal Commun 10:1948–1951
Muthupandi P, Alamsetti SK, Sekar G (2009) Chem Commun 2009:3288–3290
Comba P, Lee Y-M, Nam W, Waleska A (2014) Chem Commun 50:412–414
Hong D, Mandal S, Yamada Y, Lee Y-M, Nam W, Llobet A, Fukuzumi S (2013) Inorg Chem 52:9522–9531
Bayat A, Shakourian-Fard M, Ehyaei N, Hashemi MM (2014) RSC Adv 4:44274–44281
Bagherzadeh M, Amini M (2009) Inorg Chem Commun 12:21–25
Hussain SMS, Ibrahim MB, Fazal A, Suleiman R, Fettouhi M, Ali BE (2014) Polyhedron 70:39–46
Amini M, Bagherzadeh M, Moradi-Shoeili Z, Boghaei DM, Ellern A, Woo LK (2013) J Coord Chem 66:464–472
Chen M-T, Chang P-J, Huang C-A, Peng K-F, Chen C-T (2009) Dalton Trans 2009:9068–9074
Bagherzadeh M, Amini M, Ellern A, Woo LK (2012) Inorg Chim Acta 383:46–51
Amini M, Bagherzadeh M, Atabaki B, Derakhshandeh PG, Ellern A, Woo LK (2014) J Coord Chem 67:1429–1436
Hoogenraad M, Ramkisoensing K, Driessen WL, Kooijman H, Spek AL, Bouwman E, Haasnoot JG, Reedijk J (2001) Inorg Chim Acta 320:117–126
Ene CD, Lungu A, Mihailciuc C, Hillebrand M, Ruiz-Perez C, Andruh M (2012) Polyhedron 31:539–547
Jaworska M, Stopa G, Stasicka Z (2010) Nitric Oxide 23:227–233
Conradie MM, van Rooyen PH, Conradie J (2013) J Mol Struct 1053:134–140
Amini M, Arab A, Derakhshandeh PG, Bagherzadeh M, Ellern A, Woo LK (2014) Spectrochim Acta A Mol Biomol Spectrosc 133:432–438
Amini M, Haghdoost MM, Bagherzadeh M, Ellern A, Woo LK (2013) Polyhedron 61:94–98
Amini M, Bigdeli M, Delsouz-Hafshejani S, Ellern A, Woo LK (2014) Z Anorg Allg Chem 640:385–389
Hoveyda HR, Kamnaname V, Rettig SJ, Orvig C (1992) Inorg Chem 31:5408–5416
Frisch MJ (2003) Gaussian 03 Revision B 03. Gaussian Inc, Pittsburgh, PA
Maurya MR, Dhaka S, Avelilla F (2014) Polyhedron 67:145–159
Crichton RR (2008) Biological inorganic chemistry, an introduction, vol 1. Elsevier, Amsterdam, The Netherlands
Bhattacharjee CR, Goswami P, Pramanik HAR, Paul PC, Mondal P (2011) Spectrochim Acta A Mol Biomol Spectrosc 78:1408–1415
Koner S, Iijima S, Mizutani F, Harata K, Watanabeb M, Nagasawa A, Sato M (1999) Polyhedron 18:2201–2204
Cappelli C, Duce C, Formica M, Fusi V, Ghezzi L, Giorgi L, Micheloni M, Paoli P, Rossi P, Rosaria Tine M (2014) Inorg Chim Acta 417:230–238
Qu Y (2012) Spectrochim Acta A Mol Biomol Spectrosc 94:205–209
Sayin K, Karakas D (2014) J Mol Struct 1076:244–250
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc., Pittsburgh, PA
Rastogi VK, Palafox MA, Tanwar RP, Mittal L (2002) Spectrochim Acta A Mol Biomol Spectrosc 58:1987–2004
Bellamy LJ (1975) The infrared spectra of complex molecules, 3rd edn. Wiley, New York
Shongwe MS, Al-Rashdi BA, Adams H, Morris MJ, Mikuriya M, Hearne GR (2007) Inorg Chem 46:9558–9568
Davis MI, Orville AM, Neese F, Zaleski JM, Lipscomb JD, Solomon EIJ (2002) J Am Chem Soc 124:602–614
Acknowledgments
Authors thank the Research Council of the University of Maragheh (M.A), Semnan University (A. A), and the National Science Foundation (L.K.W) for funding of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amini, M., Khaksar, M., Arab, A. et al. Catalytic behavior of an iron(III) complex containing an N,O-type bidentate oxazoline ligand for selective oxidation of sulfides. Transition Met Chem 41, 97–105 (2016). https://doi.org/10.1007/s11243-015-0001-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-0001-8