Abstract
The economic important medicinal plant, Panax ginseng, encompasses a diverse array of pharmacologically beneficial ginsenosides governed by complex metabolic pathways. The cultivation of P. ginseng over an extended period of time poses many challenges to fulfill market requirements. The utilization of in vitro tissue culture presents a viable alternative approach for the generation of P. ginseng root biomass and metabolites. Harnessing the root inducing plasmid (Ri plasmid) of Agrobacterium rhizogenes in the transformation process to create hairy roots in P. ginseng could be a useful metabolic engineering technique. In this investigation, the transformation efficiency and biomass production of five distinct ginseng genotypes were evaluated. Of them, the ‘Yunpoong’ cultivar, and a local landrace ‘Ganghwa’ had the highest transformation efficiencies of 66.11% and 65.00%, respectively. The biomass production of transgenic hairy roots was 1.5–2.1 times faster than that of non-transgenic adventitious roots without hormone supplementation. Various ginsenosides such as Rg1, Rf, Rh1, Rb1, Rb2, Rd, F2, and Rg3, were found to be similar or greater in the hairy roots when compared to the concentrations observed in adventitious roots. Furthermore, the ginsenoside contents of cultivated roots are similar to those cultured in a bioreactor. The findings provide fundamental insights into the metabolic engineering of ginseng, facilitating the in vitro production of ginsenosides.
Key message
In vitro root culture is an ideal method for the large-scale production of biomass for the slowly growing ginseng plant. We report the improved transgenic hairy root culture system for production of biomass and ginsenosides via Ri plasmid transformation using five different ginseng genotypes. This transformation system provides a basic tool for the future metabolic engineering of ginseng.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panax ginseng, a medicinal plant with high economic value, has been used as a traditional medicine in Oriental nations for thousands of years. Its effectiveness is mostly attributed to the presence of ginsenoside, a highly prized saponin compound with notable pharmacological properties. To date, more than 150 distinct ginsenosides have been separated and identified from different species of the Panax genus (Christensen 2008). Among 17 Panax species, P. ginseng is well study for its wide diversity of ginsenoside compounds and pharmacological effects (Choi 2008; Kim et al. 2016). However, the production and supply of wild P. ginseng remains limited because of difficulty in reproducing their growing conditions, overharvesting, and habitat destruction. Wild ginseng requires 4–6 years to mature in forests with 75–80% canopy cover or shade (Han et al. 2017; Pritts 2010). In perennial the cultivated ginseng also faces many threats by biotic and abiotic stresses eventually leads to yield loss (Yu et al. 2003; Yu and Ohh 1994). It is challenging to meet the growing market demand for ginseng due to its specific growing conditions, lengthy growth cycle, and the need for comprehensive disease and pest management.
In recent years, the demand for herbal medicines has been steadily growing across the world as a direct result of the renewed interest in natural products (Chen et al. 2016; Hishe et al. 2016; Verma and Singh 2008). Thus, various in vitro tissue culture strategies have been developed to improve the biomass production and extraction of secondary metabolites in plants (Murthy et al. 2014). Various approaches have been undertaken to enhance the production of ginseng biomass and improve ginsenosides production in vitro including callus, suspensions and adventitious root cultures (Ali et al. 2006; Furuya et al. 1984; Langhansova et al. 2005; Nguyen and Paek 2010; Woo et al. 2004; Yu et al. 2002). In vitro culture systems have also been developed for biomass production, including the use of bioreactors, liquid shaking cultures, and temporary immersion cultures are being used (Baque et al. 2012; Paek et al. 2005; Vaněk et al. 2005). However, the practicality of these methods are limited in ginsenoside production because of the slow growth rate of ginseng tissues, and the need for supplementation with expensive plant growth hormones.
Genetic engineering allows the manipulation of genes in plant species. Insertion of transgenes into a host genome can result in altered genotype and/or phenotype traits (Council 1987). Conventionally, genetic engineering of ginseng involves six major steps: (1) standardization of plant regeneration protocols, (2) identification of desired gene to incorporate the target characteristics, (3) transformation of the desired gene into plants using Agrobacterium or other efficient method, (4) regeneration of putative transformed plants in selection media, (5) confirmation of transgene integration in the ginseng genome, and (6) characterization of transgenic plant phenotypes for the new trait.
Agrobacterium rhizogenes is a soil bacterium classified as gram-negative, which is responsible for the initiation of hairy roots. These hairy roots are formed as a consequence of the expression of recombinant proteins derived from the Ri T-DNA (Ghiasi et al. 2012; Ono and Tian 2011). A. rhizogenes-mediated hairy root induction has many advantages, including faster growth in hormone-free media, strong root branching, genetic and biosynthetic stability, plagiotropic growth and a biosynthetic capacity comparable to native plant roots (Pratap Chandran and Potty 2011; Tao and Li 2006). Transformed root cultures have attracted considerable attention because of their genetic and biochemical stability, rapid growth rate, and their ability to synthesize secondary products at levels similar to wild-type roots (Giri and Narasu 2000).
This work presents a highly effective technique for promoting hairy root formation in multiple cultivars of ginseng. Additionally, we investigated the effectiveness of mass production of biomass in a hormone-free medium and the profiling of ginsenosides using the hairy root culture system.
Materials and methods
Plant materials and tissue culture conditions
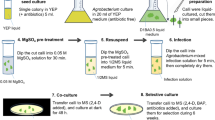
The calluses of five P. ginseng accessions, including one cultivar (‘Gumpoong’), three collections from local landraces (‘Yunpoong’, ‘Ganghwa’, and ‘Jakyung’), and one wild collection (‘Kangwon’), were cultured and generated in a basic Murashige and Skoog medium (MS). The medium was supplemented with 1.0 mg/L of 2,4-D (2,4-dichlorophenoxyacetic acid, Duchefa), 30 g/L of sucrose (Duchefa), and 7 g/L of plant agar (Duchefa). The cultures were maintained at a temperature of 23 ± 1 °C in the absence of light. The pH of the medium was adjusted to 5.8 before the addition of agar, followed by sterilization in an autoclave at a temperature of 121 °C for a duration of 15 min. Following a period of 30 days of culturing, calluses were utilized in a transformation experiment.
Agrobacteriumstrains and binary vector
A. rhizogenes strain R1000 was kindly provided by Prof. Sang Un Park, Department of Crop Science, Chungnam National University, Deajeon, South Korea. The binary vector pBI121 (Jefferson 1987), containing a CaMV 35 S promoter-GUS gene fusion, and the NPT II gene as a selectable marker, was transferred into A. rhizogenes R1000 by electroporation. Transformed A. rhizogenes were cultured in LB medium containing 50 mg/L kanamycin at 28 °C with shaking (220 rpm) to the mid-log phase (OD A600 = 0.5). Bacterial cells were collected by centrifugation at 3000×g for 10 min and resuspended in half-strength MS (½ MS) liquid medium containing 30 g/L sucrose for inoculation.
Plant transformation
Subcultured calluses were excised by scalpel and directly used for co-cultivation with A. rhizogenes. Excised explants were dipped into the liquid inoculation medium containing the A. rhizogenes for 10 min, then dried on sterilized filter paper, and incubated in the dark at 23 ± 1 °C on solid ½ MS medium. After 2 days of co-cultivation, explants were washed several times with sterilized water to remove A. rhizogenes on their surfaces and dried on sterilized filter paper. Co-cultivated explants were transferred to hormone-free solid ½ MS medium containing 250 mg/L cefotaxime to eliminate residual bacteria and incubated in the dark at 23 ± 1 °C for 1 week. Explants were then transferred to hormone-free solid ½ MS medium containing 30 g/L sucrose, 250 mg/L cefotaxime, and 50 mg/L kanamycin to select transgenic hairy roots. Putative transgenic hairy roots were observed emerging from explant wound sites after 4 weeks of incubation. Subsequently, the induced putative transgenic hairy roots were isolated and transferred to 30 mL of hormone-free Schenk and Hildebrandt (SH) liquid medium containing 30 g/L sucrose, 250 mg/L cefotaxime, and 50 mg/L kanamycin, in a 100-mL flask on a rotary shaker (100 rpm) at 23 ± 1 °C in the dark. Subculture was done every 30 days. After three rounds of subculture, five individual hairy roots from each cultivar were selected and grown separately. After 30 days, the number of roots increased, and half of them were used for subculture, while the other half was utilized for DNA and RNA isolation. Subsequently, cDNA synthesis was performed to identify the presence of the rol and gus genes. Only the hairy roots containing foreign genes were retained for further subculture.
Histochemical GUS assay
The transgenic hairy roots which is induced by Ri plasmid transformation (hairy roots) and the adventitious roots which is induced in vitro without transformation (adventitious roots) were examined in staining solution (100 mM sodium phosphate, 1 mg/mL X-gluc, 0.1% Triton X-100, 10 mM EDTA, 2 mM potassium ferricyanide, and 2 mM potassium ferrocyanide) for histochemical GUS activity. Hairy roots were soaked in staining solution and incubated overnight at 37 °C. Stained roots were rinsed extensively in 70% ethanol to remove residual phenolic compounds, and observed using a microscope equipped with a camera.
Isolation of DNA and RNA, and cDNA synthesis
Total genomic DNA and RNA of hairy and adventitious roots were extracted using the DNeasy and RNeasy Plant Mini Kits (Qiagen, Hilden, Germany), respectively, according to the manufacturer’s protocol. cDNAs were synthesized from 5 µg of total RNA using the SMARTer cDNA Synthesis Kit (Clontech Laboratories, Inc.), in accordance with the guidelines provided by the manufacturer.
Validation of transformants
Polymerase chain reaction (PCR) was used to confirm the presence of transgenes and introgressed rol genes. The oligonucleotide sequences used to amplify fragments of the GUS (GenBank accession number AF485783.1) and rol (GenBank accession number X03433.1) genes are provided in Table 1. PCR was conducted in a 25-µL reaction mixture containing 20 ng of DNA template, 5 pmol of each primer, 1.25 mM deoxynucleotide triphosphate (dNTP), 1.25 units of Taq DNA polymerase (Inclone, Korea), and 2.5 µL of 10× reaction buffer. PCR cycling parameters were 94 °C (5 min); 35 cycles of 94 °C (30 s), 54–58 °C (30 s); 72 °C (30–60 s), with a final extension of 72 °C (7 min). PCR products were visualized on agarose gels (1.0%) containing a safe gel stain.
Gene expression analysis by RT-PCR
Expression of transgenes was analyzed by reverse transcriptase PCR (RT-PCR). Synthesized cDNA was tenfold diluted and used as a template for PCR analysis. PCR conditions and primers for GUS, rol and Actin7 (GenBank accession number DC03005B05) genes were the same as those described for transformation validation, and in Table 1.
Production of liquid culture and bioreactor for biomass production
Transgenic hairy roots, selected in hormone-free SH liquid medium containing 30 g/L sucrose, 250 mg/L cefotaxime, and 50 mg/L kanamycin, were transferred to hormone-free SH liquid medium containing 50 g/L sucrose without cefotaxime and kanamycin for amplification. Subsequently, these hairy roots were used to compare growth rates. One gram (fresh weight) of hairy roots were inoculated into 30 mL of SH liquid medium containing 50 g/L sucrose with or without 3 mg/L IBA in a 100-mL flask with shaking (100 rpm) at 23 ± 1 °C in the dark. Data was collected after 30 days of culture. Biomass production of transgenic hairy roots was performed in air-lift balloon-type bioreactors. To begin with, 12 g hairy roots (fresh weight) were inoculated into a 3-L air-lift balloon-type bioreactor containing 1 L of hormone-free SH liquid medium supplemented with 5% sucrose. During cultivation, the airflow rate was adjusted at 0.1 vvm (100 mL/min). The bioreactor was maintained in the dark at 23 ± 1 °C. Hairy root biomass was harvested after 30 days of culture.
Sample preparation and quantification of ginsenosides
Pulverized, freeze-dried adventitious and hairy roots (25 mg each) were extracted by sonication with 1 mL of 70% methanol for 180 min at room temperature. The crude extract was centrifuged at 13,000 rpm for 5 min, then the supernatant was diluted with water (1:3) and filtered using a 0.2-µm RC-membrane filter (Minisart RC15, Sartorius Stedim Biotech, Gottingen, Germany). Reference standards for the ginsenosides Rf, Rb1, Rb2, Ro, Rh1, Rg2, Rg3, and F2 were purchased from Chengdu Biopurify Phytochemicals Co., Ltd. (Chengdu, China), and Rb3, Rc, Rd, and Re from Chromadex (Irvine, CA, USA). Ginsenoside standard Rg1 was kindly provided by The Korean Food and Drug Administration agency (KFDA). Stock solutions of the 13 ginsenoside reference standards were prepared in methanol and working solutions were prepared to a series of known concentrations. Solutions were filtered through a 0.2-µm RC-membrane filter before quantitative analysis. All solutions were stored at 4 °C until required for analysis.
Ginsenoside quantification using LC–MS
Ginsenoside content was quantified using an Agilent 6460 Triple Quadrupole liquid chromatography–mass spectrometry (LC–MS) system coupled with an Agilent 1290 Infinity II LC system (Agilent Technologies, Palo Alto, CA, USA). Chromatographic separation was obtained using an ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm; Waters Co., Milford, MA, USA) operated at 40 °C. The mobile phase, which comprised water (A) and acetonitrile (B), both of which were added with 0.1% formic acid, was delivered at a flow rate of 0.3 mL/min with the following gradient program: 0–9 min, 20% (B); 9–14 min, 20–30% (B); 14–17 min, 30% (B); 17–21 min, 30–32% (B); 21–26 min, 32–42.5% (B); 26–29 min, 42.5–90%; 29–31 min, 90% (B); 31–32 min, 90–20%; 32–35 min, 20% (B). The sample injection volume was set to 2.0 µL. The triple quadrupole MS system was equipped with an electrospray ionization (ESI) interface and operated in the negative ion mode. Parameters for MS were set as follows: capillary voltage 3.5 kV, Vcharge 2.0 kV, drying and sheath gas temperature 320 °C, drying gas flow 10 L/min, sheath gas flow 11 L/min, and nebulizer pressure 50 psi.
Statistical analysis
All statistical analysis was conducted using R (version 4.2.0). Data were analyzed by one-way analysis of variance (ANOVA), and significant differences between values were calculated using Tukey’s multiple comparison test. P-values ≤ 0.05 were considered statistically significant.
Results
Genotype effect of A. rhizogenes-mediated transformation in P. ginseng
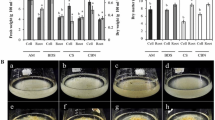
The genetic transformation of plants can be affected by plant genotype, type of explant, physical and chemical factors, and the strain of A. rhizogenes used (Kumar et al. 1991; Sharafi et al. 2014). We found that calluses were suitable explants for A. rhizogenes-mediated transformation of P. ginseng and had a transformation efficiency of 16.33–66.11% among five ginseng genotypes. The cultivar ‘Yunpoong’, and the local landrace ‘Ganghwa’ showed greater transformation efficiency than the other three genotypes (Fig. 1A, C). The number of hairy roots differed between samples, ranging from 1.71 to 3.36 roots per explant while ‘Yunpoong’ and ‘Ganghwa’ explants showed significantly higher rooting capacity than others (Fig. 1B, C).
Efficiency of Agrobacterium rhizogenes-mediated transformation of five Panax ginseng accessions. A Hairy root induction rate; B number of roots per explant; C transformation-related induction of hairy roots using calluses of five P. ginseng accessions on solid selection media. GU, P. ginseng cv. ‘Gumpoong’; YP, P. ginseng cv. ‘Yunpoong’; GH, P. ginseng local landrace ‘Ganghwa’; KW, P. ginseng wild collection ‘Kangwon; JK, P. ginseng local landrace ‘Jakyung’. The different lowercase letters (a, b, c) indicate significant differences between values based on Tukey’s multiple comparison test (P-values ≤ 0.05)
Molecular analysis of transgenic hairy roots
Based on above results, we selected two putative transgenic hairy root accessions, ‘Yunpoong’ (T-YP) and ‘Ganghwa’ (T-GH), for molecular verification and further analysis. The rol genes of the Ri-plasmid are responsible for inducing hairy roots (Gorpenchenko et al. 2006). The genomic DNA PCR for rolB and rolC genes confirmed genetic transformation in the DNA of selected hairy root lines suggesting the stable incorporation of pRi T-DNA fragments of A. rhizogenes (Fig. 2A). At the transcription level, our results confirmed that rolB and rolC genes were actively expressed in the transgenic roots (Fig. 2B), confirming the role of rol genes in hairy root induction. Besides, the GUS gene incorporation and expression were identified in hairy roots of T-YP however in T-GH GUS gene presence neither expression was not found (Fig. 2A, B). The observed results were correlated with the GUS activity observed in the transgenic hairy roots of T-YP, but not in T-GH (Fig. 3B). The absence of the GUS gene in the hairy roots of T-GH might attributed to the loss or elimination of transgenes into ‘Ganghwa’ callus.
Analysis of transgenic hairy ginseng root. A Genotyping PCR analysis for the presence of rolB, rolC, and GUS genes in transgenic hairy roots. B RT-PCR analysis for rolB, rolC, GUS and Actin7 transcript accumulation in the transgenic hairy roots. T-YP, transgenic hairy roots of ‘Yunpoong’; T-GH, transgenic hairy roots of ‘Ganghwa’; A-YP, adventitious roots of ‘Yunpoong’; A-GH, adventitious roots of ‘Ganghwa’. M, 100-bp DNA ladder
GUS histochemical analysis of transgenic hairy ginseng root, transformed using Agrobacterium rhizogenes R1000 (pBI121). A Transient GUS spots on callus (bar = 2 mm); B–D transgenic hairy roots after 2 months on selection medium (bar = 2 mm); E lateral root hair of a hairy root (bar = 0.2 mm). T-YP, transgenic hairy roots of ‘Yunpoong’; T-GH, transgenic hairy roots of ‘Ganghwa’
Hairy root growth in shaking liquid culture
Hairy roots were cultured in a medium with and without 3 mg/L indole-3-butyric acid (IBA). At first, the growth rate of transgenic hairy roots (hairy roots) was significantly faster than the non-transgenic adventitious roots. The growth rate of hairy roots remained the same regardless of the presence or absence of IBA in the medium, but the adventitious roots did not grow without IBA (Fig. 4A, B; Fig. S1). This result indicates that the growth rate of hairy roots is not influenced by auxin (IBA), and they could also grow in an auxin-free medium. Among the hairy roots, T-GH grew significantly faster than T-YP with or without supplementation IBA (Fig. 4A, B; Fig. S1).
Growth rate of transgenic hairy and adventitious roots in liquid medium. A Fresh weight of hairy and adventitious roots in medium with or without 3 mg/L IBA. B Morphology of adventitious and transgenic hairy roots in SH liquid medium supplemented with or without 3 mg/L indole-3-butyric acid (I3 and I0, respectively). C Production of T-GH hairy roots in a bioreactor in hormone free SH medium supplemented with 50 mg/L sucrose. T-YP, hairy roots of ‘Yunpoong’; T-GH, hairy roots of ‘Ganghwa’; A-YP, adventitious roots of ‘Yunpoong’; A-GH, adventitious roots of ‘Ganghwa’. The different lowercase letters (a, b, c) indicate significant differences between values based on Tukey’s multiple comparison test (P-values ≤ 0.05)
Ginsenoside profiles in hairy roots and adventitious roots of two ginseng accessions
By using LC–ESI–MS/MS in multiple reaction monitoring (MRM) acquisition mode, we analyzed the contents of ginsenosides in two transgenic hairy root lines (T-GH and T-YP), and their wild type adventitious roots (A-GH and A-YP). We chose major ginsenosides such as Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rh1, Rg1, Rg2, Rg3, Ro, and F2, for the analysis. The relative abundance of each ginsenoside was similar in both A-GH, A-YP and T-GH, T-YP (Fig. S2). T-GH, hairy roots were predominantly enriched with clinically important ginsenosides Rg1, Rh1, and Rd while A-GH was rich in ginsenosides Re, Rg2, Rc, Rb2, Rb3, and Ro. The ginsenosides Rb1, F2, and Rg3 were found in similar levels in A-GH and T-GH (Fig. 5). However, T-YP hairy roots had significantly lower concentrations of six major ginsenosides such as Rb1, Re, Rf, Rg1, Rg2, and Ro than A-YP similarly, the other seven ginsenosides contents were not significantly different as well (Fig. 5). The total ginsenoside contents of A-GH and A-YP were significantly higher than those of T-GH and T-YP (Fig. 5). However, among the transgenic hairy roots T-GH had a significantly higher concentration than T-YP and a tad lesser concentration to A-GH (Fig. 5). Thus, based on the above results we suggest T-GH was superior in total ginsenoside and individual ginsenosides content than T-YP.
Quantitative analysis of 13 major ginsenosides in adventitious and transgenic hairy roots. DW, dry weight; T-YP, transgenic hairy roots of ‘Yunpoong’; T-GH, transgenic hairy roots of ‘Ganghwa’; GH, adventitious roots of ‘Ganghwa’; YP, adventitious roots of ‘Yunpoong’. The different lowercase letters (a, b, c) indicate significant differences between values based on Tukey’s multiple comparison test (P-values ≤ 0.05)
Mass production of hairy roots in bioreactors
We established a bioreactor system to mass produce T-GH hairy roots by using an air-lift bioreactor. From an initial inoculum of 12 g fresh weight of hairy roots, 141.33 g was harvested after 30 days of growth in the bioreactor. Accordingly, the biomass was increased by 11.75 times in fresh weight, and by 11.77 times in dry weight. We noticed the phenotype of transgenic hairy roots cultivated in a bioreactor and the liquid culture system were uniform and identical (Fig. 4C). This result showed that transgenic hairy roots might be a novel hormone-free material for the biomass and ginsenoside production in ginseng.
Discussion
In vitro production of plant secondary metabolites
In vitro tissue culture-based production of secondary metabolites from plants is a promising alternative method because of its many advantages, including low cost, safety to human health, and simple procedure for large-scale industrial application. Many economically important secondary metabolites are used in pharmaceutics, natural medicine, as flavoring and coloring agents (Hussain et al. 2012). Efforts were focused on improving plant cell culture techniques or conditions to commercially exploit these compounds (DiCosmo and Misawa 1995) and the use of transgenics has revolutionized the plant tissue culture techniques in secondary metabolite production (Hussain et al. 2012). In this study, we developed a new transgenic hairy root system and evaluated the efficiency in different ginseng cultivars to improve the yield characteristics and potential cultivar for ginseng tissue culture method. We further demonstrate the mass production of transgenic hairy roots using a bioreactor system with hormone free media.
Ginseng genotypes for hairy root transformation
Genetic transformation for the production of novel metabolites in vitro is widely practiced. A. rhizogenes (popularly known as ‘natural genetic engineer’) mediated transformation technique has been considered advantageous due to its simple, rapid and relatively short time to identify the transformants (Liu et al. 2016). Many plant tissue explants have been used for Agrobacterium-mediated transformation, including hypocotyl, leaves, cotyledons (Aarrouf et al. 2012; Bae et al. 2012), adventitious roots (Han and Choi 2009), and calluses (Gorpenchenko et al. 2006; Ismael and Antar 2014). Among all, callus tissue has been the preferred material for plant transformation, because of its higher rate of infection by Agrobacterium and ease of selection and regeneration procedure (Al Abdallat et al. 2011; Ismael and Antar 2014; Komari 1989). Thus, we used callus tissue from five P. ginseng accessions as an explant for transformation and compared the transformation efficiency between them to select the best materials for future studies and biomass production. We obtained higher transformation efficiency for three out of five accessions (Fig. 1A), and the hairy root induction rate of 66.11% by A. rhizogenes strain R1000 (Fig. 1A) in ‘Yunpoong’ cultivar followed by 65.00% for ‘Ganghwa’ local landrace cultivar. ‘Ganghwa’ accession has produced higher number of roots per explant followed by Yunpoong (Fig. 1B) suggesting that these two accessions were best suitable for A. rhizogenes mediated hairy root induction in ginseng. Transformed calluses did not only show increased induction of hairy root growth, but also somatic embryogenesis, and shoot organogenesis as well (Fig. 1C). These findings were consistent with a previous report that identified the role of rol genes from Agrobacterium (Gorpenchenko et al. 2006). Based on above transformation event the accessions Ganghwa (T-GH) and Yunpoong (T-YP) were used for further studies.
Screening of transgenic hairy root lines
Selection of transformed cells and tissues is a key step in the transformation process which is facilitated by antibiotic or herbicide resistance genes present in the fusion vectors. The use of reporter genes is an alternative selection technique including the use of gus reporter genes. In this study, we used GUS reporter gene, and the NPT II (Kanamycin resistance) gene as a selectable marker. We found that all representative transformed lines showed kanamycin resistance, and hairy roots were able to grow well in medium supplemented with 50 mg/L kanamycin. Additionally, the results of genotyping-PCR and RT-PCR clearly showed the presence and the expression of rol genes in all the transformed roots (Fig. 2A, B). However, the GUS gene was present only in the hairy roots of T-YP and it was missing or absent in T-GH. In the hairy roots of T-GH, the loss or elimination of GUS transgene might have occurred during transformation selection or during subculture. Similar phenomenon was also reported in other transgenic experiments involving tobacco, Nicotiana, and soybean species (Hodal et al. 1992; Risseeuw et al. 1997). We assayed the GUS expression in the selected transformants. As evident T-GH transformants doesn’t show any GUS activity, whereas T-YP had shown GUS spots in the transformed callus, the hairy roots as well (Fig. 3A-E). Besides, variation in gene expression in the transformant is a general phenomenon, the elimination of transgene might have occurred by intrachromosomal recombination or genetic instability resulting from tissue culture practices (Romano et al. 2005).
Mass production of transgenic hairy roots and ginsenosides profiling
In vitro production of plant biomass usually requires the application of plant hormones; however, this increases the production cost. In this study, we found both the hairy root lines grew fast in hormone-free medium, with growth rates 1.5–2.1 times higher than adventitious roots (Fig. 4, Fig. S1). A previous study reported differences in ginsenoside contents between P. ginseng cultivars (Lee et al. 2017) whereas we observed the ginsenoside profiles of hairy and adventitious roots, and cultivated roots, were the same (Fig. S2). Additionally, we found that the local landrace ‘Ganghwa’ contained significantly higher levels of ginsenosides than the ‘Yunpoong’ cultivar in adventitious roots as well as in transgenic hairy roots (Fig. 5). Thus we suggest that the local landrace ‘Ganghwa’ a superior line compared to ‘Yunpoong’. Surprisingly, the transgenic hairy roots of ‘Ganghwa’ and ‘Yunpoong’ had a lower total ginsenoside content than their adventitious roots (Fig. 5). Yet, levels of some ginsenosides were higher (Rg1, Rh1, and Rd) or similar (Rf, Rb1, F2, and Rg3) in ‘Ganghwa’ hairy roots (Fig. 5). The bioreactor system has been successfully applied for the large-scale production of many horticultural and medicinal plants (Paek et al. 2005). The use of bioreactor technology is a key step towards the commercial production of bioactive molecules in plant and animal cells (Baque et al. 2012; Warnock and Al-Rubeai 2006). In this study, we used a bioreactor system for the mass production of hairy roots, and over a period of 1 month, we obtained high amounts and quality of hairy roots in a hormone-free medium (Fig. 4C). The fresh biomass of T-GH hairy roots were increased to 11.7 fold (141.33 ± 5.20 g fresh weight, 11.98 g dry weight) in 1 month from an initial inoculum of 12 g. It should be noted that the transgenic hairy roots were grown on a hormone-free media compared to the adventitious roots. Several studies have proven the fact that plant hormones such as MeJA, SA, and IBA or the combination of these hormones would improve ginsenoside production in adventitious roots (Kim et al. 2007; Kim et al. 2015). Besides, during rapid cell growth ginsenosides accumulation tends to reach only half production and the final half occurs during later slow growing phase in the bioreactor (Kim et al. 2015). This could be a reason why the transgenic hairy roots accumulated half the ginsenosides content than their adventitious roots. Recently, our group obtained the whole genome sequence for ginseng and annotated 59,352 functional genes (Kim et al. 2017). We identified several genes related to metabolic pathways. Based on the hairy root transformation system, metabolic engineering can be used to unveil unknown metabolic pathway genes and can also be used for in vitro mass production of biomass and secondary metabolites.
Conclusions
P. ginseng transformation is a demanding task and highly dependent on genotypes. We screened five different cultivars for the better transformation efficiency and found ‘Yunpoong’ and local landrace ‘Ganghwa’ having high A. rhizogenes-mediated transformation efficiency and hairy root induction. Transgenic hairy roots grew faster than adventitious roots without hormone application and will become an ideal material for the mass production of biomass and ginsenosides. There was a significant difference in ginsenoside contents between adventitious roots and transgenic hairy roots of these two accessions. Yet the transgenic hairy roots of ‘Ganghwa’ accession contains similar ginsenoside profiles of few important ginsenosides such as Rg1, Rb1, Rd, F2 and Rg3 with adventitious and cultivated roots. Our study provides a basic transformation tool and has showed the suitable cultivars for the mass production of ginseng hairy roots and shed light on the metabolic engineering of ginseng and future studies in ginseng.
Data availability
The authors confirm that data supporting the findings of this study are available within the article or its supplementary material.
References
Aarrouf J, Castro-Quezada P, Mallard S, Caromel B, Lizzi Y, Lefebvre V (2012) Agrobacterium rhizogenes-dependent production of transformed roots from foliar explants of pepper (Capsicum annuum): a new and efficient tool for functional analysis of genes. Plant Cell Rep 31(2):391–401
Al Abdallat A, Sawwan J, Al Zoubi B (2011) Agrobacterium tumefaciens-mediated transformation of callus cells of Crataegusaronia. Plant Cell Tissue Organ Cult (PCTOC) 104(1):31–39
Ali MB, Yu KW, Hahn EJ, Paek KY (2006) Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep 25(6):613–620
Bae H, Kim YB, Park NI, Kim HH, Kim YS, Lee MY, Park SU (2012) Agrobacterium rhizogenes-mediated genetic transformation of radish (Raphanus sativus L. cv. Valentine) for accumulation of anthocyanin. Plant Omics 5(4):381
Baque MA, Moh SH, Lee EJ, Zhong JJ, Paek KY (2012) Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol Adv 30(6):1255–1267
Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A (2016) Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med 11(1):37
Choi KT (2008) Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol Sin 29(9):1109
Christensen LP (2008) Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55:1–99
NR Council (1987) Agricultural biotechnology: strategies for national competitiveness. Natl Acad Press, Washington, DC
DiCosmo F, Misawa M (1995) Plant cell and tissue culture: alternatives for metabolite production. Biotechnol Adv 13(3):425–453
Furuya T, Yoshikawa T, Orihara Y, Oda H (1984) Studies of the culture conditions for Panax ginseng cells in jar fermentors. J Nat Prod 47(1):70–75
Ghiasi SM, Salmanian AH, Sharafi A, Kazemi R, Jafari M, Chinikar S, Zakeri S (2012) Molecular farming, an effective system for the production of immunogenic Crimean-Congo hemorrhagic fever virus glycoprotein. Prog Biol Sci 2(1):12–29
Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18(1):1–22
Gorpenchenko T, Kiselev K, Bulgakov V, Tchernoded G, Bragina E, Khodakovskaya M, Koren O, Batygina T, Zhuravlev YN (2006) The Agrobacterium rhizogenes rolc-gene-induced somatic embryogenesis and shoot organogenesis in Panax ginseng transformed calluses. Planta 223(3):457–467
Han JY, Choi YE (2009) Rapid induction of Agrobacterium tumefaciens-mediated transgenic roots directly from adventitious roots in Panax ginseng. Plant Cell Tissue Organ Cult (PCTOC) 96(2):143
Han H, Jang KM, Chung JS (2017) Selecting suitable sites for mountain ginseng (Panax ginseng) cultivation by using geographically weighted logistic regression. J Mt Sci 14(3):492–500
Hishe M, Asfaw Z, Giday M (2016) Review on value chain analysis of medicinal plants and the associated challenges. J Med Plants Stud 4(3):45–55
Hodal L, Bochardt A, Nielsen JE, Mattsson O, Okkels FT (1992) Detection, expression and specific elimination of endogenous β-glucuronidase activity in transgenic and non-transgenic plants. Plant Sci 87(1):115–122
Hussain MS, Fareed S, Saba Ansari M, Rahman A, Ahmad IZ, Saeed M (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4(1):10
Ismael KA, Antar EBN (2014) Establishment of high-efficiency Agrobacterium-mediated transformation conditions of soybean callus. Indian J Biotechnol 13:459–463
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5(4):387–405
Kim YS, Yeung EC, Hahn EJ, Paek KY (2007) Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng C.A. Meyer. Biotechnol Lett 29:1789–1792. https://doi.org/10.1007/s10529-007-9442-2
Kim YJ, Zhang D, Yang DC (2015) Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv 33:717–735
Kim YJ, Silva J, Zhang D, Shi J, Joo SC, Jang MG, Kwon WS, Yang DC (2016) Development of interspecies hybrids to increase ginseng biomass and ginsenoside yield. Plant Cell Rep 35(4):779–790
Kim NH, Jayakodi M, Lee SC, Choi BS, Jang WJ, Lee J, Kim HH, Waminal NE, Lakshmanan M, Nguyen VB, Lee YS, Park HS, Koo HJ, Park JY, Perumal S, Joh HJ, Lee H, Kim JK, Kim IS, Kim KH, Koduru L, Kang KB, Sung SH, Yu Y, Park DS, Choi D, Seo E, Kim S, Kim YC, Hyun DY, Park YI, Kim C, Lee TH, Kim HU, Soh MS, Lee Y, In JG, Kim HS, Kim YM, Yang DC, Wing RA, Lee DY, Paterson A, Yang TJ (2017) Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol J. https://doi.org/10.1111/pbi.12926
Komari T (1989) Transformation of callus cultures of nine plant species mediated by Agrobacterium. Plant Sci 60(2):223–229
Kumar V, Jones B, Davey M (1991) Transformation by Agrobacterium rhizogenes and regeneration of transgenic shoots of the wild soybean Glycine argyrea. Plant Cell Rep 10(3):135–138
Langhansova L, Marsik P, Vanek T (2005) Production of saponins from Panax ginseng suspension and adventitious root cultures. Biol Plant 49(3):463–465
Lee YS, Park HS, Lee DK, Jayakodi M, Kim NH, Koo HJ, Lee SC, Kim YJ, Kwon SW, Yang TJ (2017) Integrated transcriptomic and metabolomic analysis of five Panax ginseng cultivars reveals the dynamics of ginsenoside biosynthesis. Front Plant Sci 8:1048
Liu S, Su L, Liu S, Zeng X, Zheng D, Hong L, Li L (2016) Agrobacterium rhizogenes-mediated transformation of Arachis hypogaea: an efficient tool for functional study of genes. Biotechnol Biotechnol Equip 30:869–878
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult (PCTOC) 118(1):1–16
Nguyen TT, Paek KY (2010) Cell suspension culture Panax ginseng CA Meyer: role of plant growth regulators and medium composition on biomass and ginsenoside production. VNU J Sci Nat Sci Technol 26:191–196
Ono NN, Tian L (2011) The multiplicity of hairy root cultures: prolific possibilities. Plant Sci 180(3):439–446
Paek K, Chakrabarty D, Hahn E (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Berlin
Pratap Chandran R, Potty V (2011) Initiation of hairy roots from Canavalia sp. using Agrobacterium rhizogenes 15834 for the co-cultivation of Arbuscular mycorrhizal fungi, Glomus Microcarpum. J Agric Technol 7(2):235–245
Pritts KD (2010) Ginseng: how to find, grow, and use America’s forest gold, 2nd edn. Stackpole Books, Mechanicsburg
Risseeuw E, Dijk ME, Hooykaas PJ (1997) Gene targeting and instability of Agrobacterium T-DNA loci in the plant genome. Plant J 11(4):717–728
Romano E, Soares A, Proite K, Neiva S, Grossi M, Faria JC, Rech EL, Aragão FJ (2005) Transgene elimination in genetically modified dry bean and soybean lines. Genet Mol Res 4(2):177–184
Sharafi A, Sohi HH, Azadi P, Sharafi AA (2014) Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants 20(2):257–262
Tao J, Li L (2006) Genetic transformation of Torenia fournieri L. mediated by Agrobacterium rhizogenes. S Afr J Bot 72(2):211–216
Vaněk T, Langhansová L, MaršÍk P (2005) Cultivation of root cultures of Panaxginseng in different bioreactors and in temporary immersion—comparison of growth and saponin production. Liq Culture Syst In Vitro Plant Propag. https://doi.org/10.1007/1-4020-3200-5_40
Verma S, Singh S (2008) Current and future status of herbal medicines. Vet World 1(11):347–350
Warnock JN, Al-Rubeai M (2006) Bioreactor systems for the production of biopharmaceuticals from animal cells. Biotechnol Appl Biochem 45(1):1–12
Woo SS, Song JS, Lee JY, In DS, Chung HJ, Liu JR, Choi DW (2004) Selection of high ginsenoside producing ginseng hairy root lines using targeted metabolic analysis. Phytochem 65(20):2751–2761
Yu Y, Ohh S (1994) Problems and present status of research on ginseng diseases in Korea. Proc Int Ginseng Conf Vancouver, Canada, 120–130
Yu KW, Gao W, Hahn EJ, Paek KY (2002) Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng CA Meyer. Biochem Eng J 11(2):211–215
Yu KW, Hahn EJ, Paek KY (2003) Ginsenoside production by hairy root cultures of Panax ginseng C.A. Meyer in bioreactors. Acta Hortic 597:237–243
Funding
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development, Rural Development Administration (Grant number PJ01311901), Republic of Korea.
Author information
Authors and Affiliations
Contributions
VBN, MJK, and TJY conceived and designed the experiments. VBN, MJK, VNLG, YSL, BR, EJP, and TKP prepared the samples and performed the experiments. VBN, MJK, KBK, HSP, and BR analyzed the data. VBN, MJ, PM and TJY wrote and revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Yi Li.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Binh, N., Kim, M.J., Giang, V.N.L. et al. Improved biomass and metabolite production in hairy root culture in various genotypes of Panax ginseng through genetic transformation. Plant Cell Tiss Organ Cult 156, 43 (2024). https://doi.org/10.1007/s11240-023-02644-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02644-x