Abstract
Around 1.7 million people die annually due to enteric infections, which are mainly caused by ETEC (Enterotoxigenic E. coli), V. cholera, V. parahaemolyticus, and Salmonella. There are currently licensed vaccines against cholera and salmonella, whose distribution is chain-dependent making difficult to efficiently distribute them in poor countries. In this context plants are attractive hosts for the synthesis and delivery of subunit vaccines that could be produced at very low costs and widely distributed ensuring vaccination coverage. Subunit vaccines often demand the use of adjuvants to reach proper immunogenicity. Several bacterial toxins have been used as mucosal and systemic adjuvants and a potential molecule for this purpose is the PirA-like toxin (ToxA) from V. parahaemolyticus, which was deemed highly immunogenic in some species. In this study a protein named ToxAentero was expressed in tobacco plants to initiate the development of accessible vaccines against enteric diseases. ToxAentero is based on ToxA, as adjuvant carrier, and epitopes from ETEC, V. cholerae, V. parahaemolyticus, and S. typhimurium. The production yields reached up to 5.46 µg g− 1 fresh leaf tissue. The plant-made ToxAentero was found immunogenic in mice immunized by oral or subcutaneous routes in terms of the induction of IgG (sera) and IgA (feces) humoral responses against most of the target epitopes from the enteric pathogens. This study opens the path for the development of a promising oral plant-based multiepitopic vaccine candidate in the fight against enteric diseases.
Key message
A multiepitopic protein targeting several enteric pathogens, was expressed in plants and characterized in mice; revealing that it is immunogenic and thus is proposed as an attractive vaccine candidate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diarrhea is still a major sanitation disease problem associated to around 1.7 million of deceases each year. There are several pathogens causing diarrhea; however, ETEC and Vibrio cholerae are the commonest bacteria-causing diarrhea. These bacteria are responsible for around 500,000 deaths of children annually (WHO 2017). Other important enteric pathogens are: Salmonella that causes 1.4 million infections each year in the United States (The Center for Disease Control and Prevention, 2020) and Vibrio parahaemolyticus that is associated with 30% of the food-related poisonings in Japan (Broberg et al. 2011). Both pathogens persist as major public health threats in developed and low-income countries.
There are two vaccines used to fight against cholera: (1) Shanchol® killed bivalent (O1 & O139) whole cell oral cholera vaccine and (2) Dukoral® composed of inactivated V. cholerae serotype O1 and recombinant CTB (Bi et al. 2017). Currently, an ETEC vaccine is not available in the market, nonetheless advanced clinical trials have been performed. Meanwhile, Dukoral® has been used against ETEC with a 75% protection rate. There are two vaccines licensed for use against Salmonella. These are the live attenuated vaccines: Ty21a and Vi capsular polysaccharide (Vi CPS), which only protect about 50% immunized people; having poor immunogenicity in young children (Benoun et al. 2018). However, both vaccines target S. typhi infection and no vaccines are available against S. paratyphi, S. typhimurium, and S. enteritidis (MacLennan et al. 2014). Similarly, vaccines to prevent V. parahaemolyticus infection have not been licensed and the field is limited to some candidates evaluated at the preclinical level (Li et al. 2014). Overall, these vaccines require cold chain for storage and distribution; limiting vaccine coverage in low- and middle-income countries (Ashok et al. 2017).
In this arena, plants are well-proven hosts for the production and oral delivery immunogens; several plant-made proteins have been assessed as vaccine candidates against enteric and respiratory diseases (Azegami et al. 2020). The use of plant-made immunologically active antigens offers several advantages that include low production costs, cold chain-free formulations, easy scalability, no replication of human pathogens, and proper synthesis of complex heterologous proteins (Rosales-Mendoza et al. 2016; Criscuolo et al. 2019; Kurup and Thomas 2020). Today; molecular biology, genetic engineering, and epitope mapping allow for the effective design and recombinant expression of multiepitopic recombinant antigens capable of triggering immunity against several pathogens using a single antigen (Ruan et al. 2015).
Vaccine subunits usually require immunostimulant elements to potentiate the immune response, especially in oral immunization schemes (Chauhan et al. 2017). In this regard, several bacterial toxins have been used as mucosal adjuvants; including the B subunits of the cholera toxin (CTB) or the heat labile enterotoxin (LTB) (Kang et al. 2004; Adkins et al. 2012; Al-Barwani et al. 2014). Both subunits increase secretory antigen-specific IgA responses and cellular immunity (Kang et al. 2004). Recently another subunit toxin, the PirA-like toxin (ToxA) from Vibrio parahaemolyticus; has been reported to elicit immunostimulatory and protective effects in shrimp and fish against bacterial challenges (Campa-Córdoba et al. 2017; Reyes-Becerril et al. 2016; 2017). In fish (Lutjanus peru and Sparus aurata), an intraperitoneal injection of ToxA induced mucosal and systemic humoral responses (IgM) with the expression of proinflammatory cytokines (IL-1β, TNF-α) (Reyes-Becerril et al. 2016, 2017). ToxA is safe upon oral or intraperitoneal administration in shrimp and fish, respectively. Interestingly, ToxA has almost an identical conformational structure to the Cry toxin of Bacillus thuriengiensis (Lee et al. 2015), which is recognized as a potent systemic and mucosal adjuvant assessed in several vaccine candidate formulations (Moreno-Fierros et al. 2003, 2015; Rubio-Infante and Moreno-Fierros 2016). In line with these findings, Monreal-Escalante et al. (2019) demonstrated that a plant-made ToxA is immunogenic in orally immunized mice through the induction of systemic and mucosal antibody responses. The immunogenic properties of ToxA and its functional production in plant cells for oral delivery have opened an attractive path for several applications in human health. Therefore, the objective of this study was developing a plant-based multiepitopic protein (ToxAentero) based on antigenic determinants from enteric pathogens and evaluate its immunogenicity in mouse. ToxAentero comprised PirA-like toxin as adjuvant/carrier and epitopes from ETEC, V. cholerae, V. parahaemolyticus, and S. typhimurium. The expression levels of the recombinant ToxAentero produced in tobacco plants were determined and its immunogenicity assessed in terms of the induction of humoral responses in mice.
Materials and methods
Multiepitopic ToxAentero gene and molecular cloning
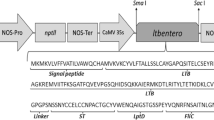
The multiepitopic gene was in silico designed based on a scrutiny of previously reported antigens and epitopes. The resulting gene coding for the target multiepitopic comprises epitopes located at ST (epitope sequence: SNSSNYCCELCCNPACTGCYV) from ETEC, CT (epitope sequence: VEVPGSQHIDSQKCT) from V. cholerae, LptD (epitope sequence: WENQAIGSTGSSPEY) from V. parahaemolyticus, and FliC (epitope sequence: VQNRFNSAITNLGNT) from S. typhimurium (Jacob et al. 1983, 1985; Newton et al. 1989; Bergman et al. 2005; Kremer et al. 2011; Rosales-Mendoza et al. 2011; Zha et al. 2016) (Table 1). The coding sequence of PirA-like toxin (ToxA) was used as immunogenic carrier; followed by a proline-containing linker (GPGP) to favor appropriate folding and displaying of the target epitopes. Finally, the coding sequence of the signal peptide from the Glycine max vegetative storage protein was incorporated at the N-terminus of the chimeric protein, whereas the sequence coding the SEKDEL endoplasmic reticulum retention signal was included at the C-terminus. The gene was flanked by the Sacl and SmaI restriction sites to allow direct cloning into the pBI121 binary vector, driven by the CaMV 35 S promoter (Fig. 1). The ToxAentero coding gene (639 bp) was codon-optimized according to the expression host and synthetized by GenScript® (Piscataway, NJ.). Subcloning was carried out by conventional molecular cloning techniques to obtain the plasmid called pBI121ToxAentero (Sambrook and Russell 2006).
a Description of the expression vector used to produce the target antigen in tobacco plants. The ToxAentero gene was inserted into the pBI121 vector in which the expression is driven by the constitutive 35SCaMV promoter. The vector possesses nptII as selectable marker for kanamycin resistance. The multiepitopic gene ToxAentero contains the signal peptide from the Glycine max vegetative storage protein, the full-length sequence of the V. parahaemolyticus PirA-like toxin(ToxA), and a four-amino acid linker; followed by the target epitopes from LT/CT, ST, LptD, and FliC. The SEKDEL endoplasmic reticulum retention signal was linked at the C-terminus. b Detection of the ToxAentero gene in transformed plants. PCR analysis was performed for total DNA samples from candidate lines (P1-P11), WT (C-) plant and a purified pBI121-ToxAentero vector (C+) using specific primers targeting the 35 S promoter and the NOS terminator to detect the transgene, which results in a 769 bp amplicon
(a)
Agrobacterium-mediated tobacco transformation
The recombinant plasmid obtained from E. coli Top 10 cultures was identified by ClaI restriction analysis and subsequently mobilized into Agrobacterium tumefaciens (GV3101 strain) by electroporation. A positive A. tumefaciens clone was propagated and used for the tobacco transformation procedure.
Transgenic tobacco lines (Nicotiana tabacum cv. Petite Havana SR1) were transformed according to the method of Horsch et al. (1985). Briefly, tobacco leaf sections from in vitro-generated plants were infected with an overnight-grown culture of recombinant Agrobacterium (OD600nm = 0.5) and co-cultured onto RMOP medium MS medium containing 3% sucrose, 1 mg/L benzyladenine, 0.1 mg/L naphthaleneacetic acid, 1 mg/L thiamine, and solidified with 3% agar; pH 5.8) at 25 °C for 48 h. Thereafter, selection and shoot development were accomplished by a selective medium (RMOP medium plus 250 mg/L cefotaxime and 100 mg/L kanamycin). Shoots were rooted in MS medium and regenerated plants were moved to soil and grown under a 16 h photoperiod, light intensity of 100 µmol m− 2 s− 1, and 30% of relative humidity).
ToxAentero transgene detection
Total DNA was isolated from the candidate lines or wild-type (WT) plants by the method of Dellaporta et al. (1983). Detection of the transgene was performed by PCR analysis using the following primers: forward 5’CGCACAATCCCACTATCCTTCGC 3’, targeting the 35 S promoter, and reverse 5’AGGGTTTCGCTCATGTGTTGAGC 3’; targeting the terminator NOS. Twenty five µL PCR reactions were set with 100 ng of test DNA, 1× PCR buffer, 1.5 mM magnesium chloride, 2.5 U Taq DNA polymerase (Vivantis), 1 mM dNTPs, and 1 µM of each sense and antisense primers designed to yield 769 bp amplicon. Temperature cycling conditions were: 94 °C for 5 min (initial denaturation), 35 cycles comprising 30 s at 94 °C (denaturation), 60 s at 56 °C (annealing), and 60 s at 72 °C (elongation); and a final extension at 72 °C for 5 min. Thermal cycling was performed in a MultiGene™ Mini Personal Thermal Cycler (Labnet). The presence of ToxAentero amplicons was assessed by electrophoresis using 1% agarose gels.
Detection of plant-expressed ToxAentero
In order to detect and quantify the recombinant ToxAentero in tobacco plants, a hyperimmune serum was generated in a mouse using pure recombinant ToxA; following a previously reported protocol (Ríos-Huerta et al. 2017). Recombinant ToxA was obtained as Monreal-Escalante et al. (2019) previously reported. Briefly, The E. coli BL21 strain carrying the pET-28a_ToxA expression vector, was used to produce recombinant ToxA. The E. coli BL21 strain was grown in Luria-Bertani (LB) medium supplemented with 50 mg/L ampicillin, at 37 °C, until it reached an OD600nm = 0.5. Expression was induced with 1 mM IPTG for 24 h at 37 °C. Cells were subsequently lysed by sonication at a 30% amplitude for eight 32 s-periods using an GEX130PB ULTRASONIC Processor. After clarification, the supernatant was collected and used for purification of the recombinant ToxA protein by Immobilized-Metal Affinity Chromatography (IMAC), using a BioRad NGC Quest equipment.
Animal handling was in accordance with the Guide for Care and Use of Laboratory Animals of the National Institute of Health (USA) and experiments accepted by the Committee on Research Ethics of the Faculty of Chemistry/University of San Luis Potosi (Permit Number: CEID-2013-004). The recombinant protein was detected in the transformed plants by Dot and Western blot analyses. Leaf tissues from transgenic or WT plants (100 mg) were pulverized in 300 µL of cold protein extraction buffer (750 mM Tris-HCl, pH = 8.0, 15% sucrose, and 1 mM PMSF. For Dot blot analysis extracted proteins were placed onto a BioTrace PVDF membrane (Pall Corporation, NY) and pure recombinant ToxA was used as positive control. After blocking (incubation in a fat-free dry milk solution, 5%, at 25 °C for 5 h), the membrane was incubated at 4 °C for 12 h with the anti-ToxA serum (1:500). For Western blot analysis, protein samples were mixed with 1× reducing buffer (50 mM Tris-HCl, pH 6.8, 100 mM DTT, 2% SDS, 0.1% bromophenol blue, and 10% glycerol). The samples were boiled at 95 °C for 5 min and subsequently subjected to denaturing SDS-PAGE (4–12% acrylamide gels). The gel was transferred onto a BioTrace PVDF membrane (Pall Corporation, NY) using a TV100-EBK Electroblotter (AlphaMetrix Biotech, GER) in a methanol-based transfer buffer at 150 V for 1 h. After blocking (incubation in 5% fat-free dry milk at 25 °C for 5 h), the membrane was incubated overnight at 4 °C with the anti-ToxA serum (1:500). Pure recombinant ToxA was used as positive control. Immunoreactivity in both analyses was revealed by labeling with a horseradish peroxidase-conjugated goat anti-mouse antibody (1:2000; Sigma, St. Louis MO, USA) upon incubation for 2 h at room temperature; with a subsequent incubation with the SuperSignal West Dura solution according to the instructions from the supplier (Thermo Scientific, Waltham MA, USA).
ELISA was conducted to quantify ToxAentero levels in plant leaf tissues. Fifty milligrams of leaf tissue from transgenic or wild-type plants were pulverized with 500 µL of cold protein extraction buffer. Extracts were clarified by centrifugation (16,000×g at 4 °C for 15 min) and the supernatants diluted (1:16) in phosphate buffer saline (PBS) to be adsorbed on ELISA plates (4 °C, overnight incubation). Three washes with PBS + 0.1% Tween (PBST) were conducted after completing each step. The plates were blocked at room temperature for 2 h with a PBS solution containing fat-free dry milk (5%). Thereafter, anti-ToxA serum (diluted 1:500) was added and plates incubated at 4 °C overnight. Secondary labeling was performed with a goat anti-mouse horseradish peroxidase-conjugated antibody diluted 1:2000 (Sigma, St. Louis, MO; incubation at 25 °C for 2 h). The final step comprised a 30 min-incubation with an ABTS solution (0.3 mg/mL 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Sigma, St. Louis, MO) and 0.1 mM H2O2). Optical density (OD) readings were obtained at 405 nm in a Multiskan® FC microplate reader (Thermo Scientific, Waltham, MA, USA). Pure recombinant ToxA was used as standard to obtain a calibration curve to determine ToxAentero levels in tobacco leaf tissues (expressed as µg per gram of fresh leaf tissue, µg g− 1 FW). The background OD values from the negative control (extract from WT plant) was subtracted from those of the test extracts. Data derived from ELISA studies were analyzed by one-way ANOVA using SPSS v.19.0 software (SPSS, Richmond, VA, USA).
Immunogenicity assay
The immunogenic potential the plant-made ToxAentero was assessed in 12-week old female BALB/c mice as described by Rosales-Mendoza et al. (2008). Test mice groups (n = 4) were orally or subcutaneously immunized with the plant-made antigen from the transgenic line L9 (ToxAentero group) or the wild-type line (WT group) as negative control. For subcutaneous immunization, the test doses consisted of 10 mg of fresh tobacco leaves (containing ≈ 55 ng of ToxAentero) milled in 300 µL of PBS and clarified by centrifugation. Oral immunization consisted of 50 mg of the tobacco tissues (containing ≈ 275 ng of ToxAentero) pulverized in 300 µL of PBS and intragastrically administered. The immunization regime scheme consisted of four weekly immunizations (on days 1, 8, 15, and 22). Sera and feces samples were collected weekly for antibody content analysis.
The humoral response against ToxAentero was measured in serum and feces by ELISA as previously described by Rosales-Mendoza et al. (2009). Plates were coated overnight at 4 °C with pure recombinant ToxA or each peptide (LTB, ST, LptD, or FliC) using 1 µg per well and later blocked with 5% milk PBS-based solution at 25 °C for 2 h. Plates were incubated overnight at 4 °C with sera samples (1:20 to 1:160 dilutions). For feces analysis, samples were mixed in PBST supplemented with 1 mM PMSF and 5% non-fat milk, clarified by centrifugation at 16,000×g and 4 °C for 15 min, and placed in the wells (1:2 to 1:16 dilutions). Secondary labeling was performed with goat horseradish peroxidase-conjugated anti-mouse antibodies against IgG for sera or IgA for feces (1:2000 dilution, Sigma, St. Louis MO, USA) at 25 °C for 1 h. Immunodetection was revealed by adding an ABTS substrate (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic) (ABTS, Sigma, St. Louis MO, USA) plus 1 mM H2O2) for 60 min. OD values were measured at 405 nm in a microplate reader. Antibody titers were defined as the reciprocal of the highest sample dilution leading to a mean OD value above the corresponding value from the WT group plus 2 × SD. Data generated were analyzed by a one-way ANOVA using SPSS v.19.0 software (SPSS, Richmond, VA, USA).
Results
Design and construction of the pBI121 ToxAentero vector
In a previous work, epitopes reported with high immunoprotective efficacy against the target enteric pathogens were identified (Trujillo et al. 2020). Among them, the LT and ST antigens from ETEC, FliC from S. typhimurium, and LptD from V. parahaemolyticus have been reported as effective immunoprotective antigens. Epitopes of these antigens were selected and used to in silico assemble the multiepitopic gene named ToxAentero, which was obtained by synthetic approaches and successfully cloned into the pBI121 vector (Fig. 1a).
Presence of ToxAentero transgene in transformed tobacco lines
Several putative transgenic lines were obtained from the kanamycin-resistant recovered shoots after performing the standard protocol for tobacco transformation. Transgenic plants were regenerated after two months following co-cultivation; obtaining eleven independent lines (L1-L11) selected from individual explants. DNA samples from transformed lines were analyzed by PCR for the presence of the ToxAentero transgene (769 bp amplicon) showing a positive amplification in nine of the eleven lines, whereas the wild-type control plant sample showed no amplification (Fig. 1b).
Tobacco plants express and accumulate the ToxAentero recombinant protein
To confirm the presence and integrity of the recombinant protein, anti-ToxA Dot and Western blot analyses were performed; revealing the presence of a ≈ 28 kDa- immunoreactive protein in six tobacco lines. No signal was observed for the protein sample of the wild type control plant, which validates the specificity of the assay (Fig. 2). The accumulation levels of the ToxAentero recombinant protein in the obtained tobacco lines were determined by ELISA using anti-ToxA serum for labeling and a standard curve made with pure recombinant E. coli-made ToxA. The results revealed that the ToxAentero yields in tobacco lines ranged from 0.8 to 5.46 µg g− 1 fresh leaves weight; with lines 9 and 6 as the highest producers (Fig. 3).
Detection of the plant-made ToxAentero protein. a Dot Blot analysis for protein extracts from transgenic positive lines or the WT line as negative control and pure recombinant ToxA as positive control. b Western blot analysis for protein extracts from transgenic positive lines or the WT line as negative control and pure recombinant ToxA as positive control (13 kDa). A mouse anti-ToxA serum was used in both analyses for labeling
Expression levels of the ToxAentero protein in tobacco transformed lines determined by ELISA. The quantification was performed in protein extracts from transformed plants or the WT plant as negative control. Labeling was performed with a mouse anti-ToxA serum. A calibration curve was made with pure ToxA was used to determine the recombinant protein yields. The asterisk denotes significant differences versus the WT (P < 0.05)
Plant-made ToxAentero induces humoral responses in mice
Immunogenicity of the plant-made ToxAentero protein was assessed in terms of its capacity to induce humoral responses in BALB/c mice, which were either subcutaneously (s.c.) or orally (p.o.) immunized with the plant-made ToxAentero produced by the transgenic tobacco line 9. Significant anti-LT IgG levels (mean titer = 160) were observed in sera from both subcutaneously (s.c.) or orally (p.o.) immunized mice (Fig. 4a). Interestingly, the response was similar in magnitude regardless of the immunization route. The measurement of anti-LT IgA showed higher levels in feces from mice orally-immunized (mean titer = 8) compared to s.c.-immunized mice (mean titer = 4) (Fig. 5a). Concerning anti-ST antibodies, the p.o-.immunized mice showed higher IgG (mean titer = 160) (Fig. 4b) and IgA (mean titer = 16) levels compared to the IgG (mean titer = 80) and IgA (mean titer = 2) levels from s.c.-immunized mice (Fig. 5b). In addition, similar seric anti-LptD IgG responses were induced (mean titer = 160) in mice s.c.- or p.o.-immunized with the plant-made ToxAentero (Fig. 4c), whereas anti-LptD IgA levels (mean titer = 2) in feces from s.c.-immunized mice were slightly lower than those for the p.o.-immunized group (mean titer = 4, Fig. 5c). Moreover, significant anti-FliC IgG systemic responses were detected at similar levels in both s.c.- or p.o.-immunized mice (mean titer = 40) (Fig. 4d). Measurements of intestinal IgA responses against FliC, measured in feces, revealed a positive response in both s.c.- or p.o.-immunized mice (mean titers of 2 and 4, respectively; Fig. 5d).
Systemic humoral response elicited in mice upon subcutaneously (s.c.) or oral (p.o.) administration of plant-made ToxAentero. a anti-LT/CT, b anti-ST, c anti-LptD, and d anti-FliC IgG titers were measured by ELISA. Mice were subjected to a scheme comprising four weekly doses. Samples (sera) were taken one week after each immunization. The asterisk denotes significant differences between s.c. and p.o. routes (P < 0.05)
Mucosal humoral response induced in mice upon subcutaneous (s.c.) or oral (p.o.) administration of the plant-made ToxAentero. a anti-LT/CT, b anti-ST, c anti-LptD, and d anti-FliC IgA titers were measured by ELISA. Mice were subjected to a scheme comprising four weekly doses. Samples (feces) were taken one week after each immunization. The asterisk denotes significant differences between s.c and p.o. routes (P < 0.05)
Discussion
In the present study a multiepitopic protein containing the PirA-like toxin (ToxA) as innovative adjuvant/carrier was designed and expressed in plant cells. This plant-based multiepitopic protein was designed based on antigenic determinants from the enteric pathogens ETEC, V. cholerae, V. parahaemolyticus, and S. typhimurium (Jacob et al. 1983, 1985; Newton et al. 1989; Bergman et al. 2005; Kremer et al. 2011; Rosales-Mendoza et al. 2011; Zha et al. 2016). The selected epitopes with known protective capacity were linked to the ToxA sequence to obtain a chimera called ToxAentero.
Transgenic tobacco lines carrying the ToxAentero gene were confirmed by PCR. Dot and Western blot analyses showed the presence of ToxAentero and validated its integrity according to the observed molecular weight (28 kDa). The ToxAentero expression levels determined by ELISA ranged from 0.8 to 5.46 µg g− 1 FW, which were similar to those reported for other multiepitopic proteins expressed in plants (Soria-Guerra et al. 2007; Rubio-Infante et al. 2015; Nieto-Gómez et al. 2019; Trujillo et al. 2020). However, the differential protein accumulation among modified lines might be associated to the transgene random insertion site into the plant tobacco genome (Kim et al. 2007). Importantly; no phenotypic alterations were observed in the modified tobacco lines, which is a key observation given the fact that other plant-expressed bacterial toxin subunits exerted toxicity in some plant species. For instance, Rawat et al. (2011) demonstrated that the expression of the Cry1Ac endotoxin has detrimental effects on both the in vitro and in vivo growth and development of cotton and tobacco transgenic plants. All the lines that showed appreciable levels of expression were found to be phenotypically abnormal.
Since alkaloids with potential toxic effects are present in tobacco plants, one important aspect to evaluate in preclinical settings is the potential toxicity of the plant-based immunization scheme. The lack of obvious toxic effects in mice subjected to such schemes supports the use of tobacco for a preliminary assessment of plant-based vaccines (Rosales-Mendoza et al. 2009; Ríos-Huerta et al. 2017; Monreal-Escalante et al. 2019). A common path in the molecular farming field consists in proving the concept in tobacco and subsequently producing the target antigen in hosts that are appropriate for immunization such as edible crops (carrot, corn, and lettuce) (Appaiahgari et al. 2017; Rosales-Mendoza et al. 2017; Daniell et al. 2019; Arevalo-Villalobos et al. 2020).
The immunogenicity assessment performed in mice revealed that the plant-made ToxAentero is immunogenic using low doses (≈ 55 and ≈ 275 ng for s.c. and p.o. immunization, respectively). Significant specific IgG production in sera against the four epitopes (CT/LT, ST, LptD, and FliC) was observed in subcutaneously and orally immunized mice. A similar IgA response in feces was detected against the four epitopes (CT/LT, ST, LptD, and FliC) in mice immunized by both routes. The mucosal immunogenic activity of the plant-made ToxAentero in terms of IgA induction was similar to that of a chimeric protein based on LTB as carrier (LTBentero; Trujillo et al. 2020). The immunogenic properties of ToxAentero are considered highly attractive due to its capacity to induce high levels of systemic IgG antibodies against CT/LT, ST, and LptD (Mean titer = 160) and significant IgG anti-FliC (Mean titer = 40). In contrast, the LTBentero candidate induced lower IgG responses, particularly anti-LptD IgG (Mean titer = 20), and failed to induce anti-FliC IgG responses. This might due to the particular folding of the two proteins that led to a differential display and processing of the target epitopes (Nyambi et al. 2000). Another hypothesis is that ToxA possesses higher adjuvanticity for the induction of humoral responses than LTB. Further studies are need to assess these hypotheses. Previous studies have demonstrated the high immunogenicity of recombinant ToxA and its protective potential against a V. parahaemolyticus challenge in fish (Pacific red snapper (Lutjanus peru), gilt head bream (Sparus aurata), and shrimp (Reyes-Becerril et al. 2016, 2017; Campa-Córdoba et al. 2017). Moreover, Monreal-Escalante et al. (2019) found strong IgG and IgA antibody induction against ToxA when mice were orally immunized with tobacco plant-based ToxA. Interestingly, this study found that the IgG levels in orally immunized mice were higher than those in s.c. immunized mice. This finding is particularly relevant to propose the oral route of administration to induce immune responses and protection against enteric pathogen infections. Mechanistic studies are envisioned to decipher the potential of ToxA as mucosal adjuvant/carrier in which comparisons against LTB should be considered as it is a well know potential mucosal adjuvant (da Hora et al. 2011; De Haan et al. 1998; Nashar et al. 2001).
Several studies have shown that epitope-based vaccines exhibit substantial advantages over conventional vaccines. However, epitope vaccines are associated with a poor immunogenicity, which can be overcome by conjugating the selected epitopes with built-in adjuvants (e.g., some carrier proteins or new biomaterials). When designing epitope-based vaccines, the following types of built-in adjuvants are typically used: (1) pattern recognition receptor ligands (i.e., toll-like receptors); (2) virus-like particles; (3) bacterial toxin proteins; and (4) novel delivery systems (e.g., self-assembled peptide nanoparticles, lipid core peptides, and polymeric or inorganic nanoparticles). (Lei et al. 2019). Although some adjuvants are currently used in vaccines licensed for human use, they are usually used as mixtures with antigens. In contrast, the use of built-in adjuvants make the vaccine formulation a simpler process and can significantly improve immunogenicity, thus offers a substantial potential to aid in the induction of a potent antigen-specific immune response (Chen et al. 2017; Rueda et al. 2017). New and efficient adjuvants for mucosal immunization are required; consequently, bacterial toxins have been explored as a potential adjuvants (i.e. LTB fron enterotoxigenic E. coli, CTB from V. cholerae, and Cry1Ac from B. thuringiensis; Guerrero Manriquez and Tuero 2021). The findings of this study provide evidence on the potential of ToxA as a new mucosal adjuvant and open the path for more research. It should be considered that the design of multieptopic vaccines implies some immunological challenges; for instance (i) epitope selection, which requires a deep understanding of the pathogen’s antigenic diversity, its immune evasion mechanisms, and the validation of conserved epitopes capable to elicit strong immune responses across multiple strains or variants (Lundegaard et al. 2011). (i) addressing proper immunogenicity since subunit vaccines often result in poor immunogens (Flower 2003). (iii) epitope interference, meaning that epitopes might compete for immune recognition or negatively impact their immunogenicity (Ndifon et al. 2009). (iv) immune dominance, meaning that some epitopes could dominate the immune response induced while for others a poor adaptive immune response is induced (Kedl et al. 2003). It must be pointed out that in this study significant levels of IgG and IgA against all the target epitopes were successfully induced. Future efforts will be focused on assessing other immunological parameters, such as immunoprotective effects of the vaccine candidate upon a challenge with the target enteric pathogens.
In conclusion, plant cells were transformed and able to synthesize significant levels of the functional multiepitopic protein ToxAentero, which triggered strong systemic (IgG) and mucosal (IgA) antibody responses against antigenic determinants from E. coli ETEC, V. cholerae, V. parahaemolyticus, and S. typhimurium, which are relevant enteric pathogens. Furthermore, PirA-like toxin from V. parahaemolyticus stands as a potential adjuvant for vaccines administered by either oral or parenteral routes.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adkins I, Holubova J, Kosova M, Sadilkova L (2012) Bacteria and their toxins tamed for immunotherapy. Curr Pharm Biotechnol 13(8):1446–1473. https://doi.org/10.2174/138920112800784835
Al-Barwani F, Donaldson B, Pelham SJ, Young SL, Ward VK (2014) Antigen delivery by virus-like particles for immunotherapeutic vaccination. Ther Deliv 5(11):1223–1240. https://doi.org/10.4155/tde.14.74
Appaiahgari MB, Kiran U, Ali A, Vrati S, Abdin MZ (2017) Plant-based edible vaccines: issues and advantages. Plant Biotechnology: principles and applications. Springer, Singapore, pp 329–366. https://doi.org/10.1007/978-981-10-2961-5_13
Arevalo-Villalobos JI, Govea-Alonso DO, Rosales-Mendoza S (2020) Using carrot cells as biofactories and oral delivery vehicles of LTB-Syn: a low-cost vaccine candidate against synucleinopathies. J Biotechnol 309:75–80. https://doi.org/10.1016/j.jbiotec.2019.12.007
Ashok A, Brison M, LeTallec Y (2017) Improving cold chain systems: Challenges and solutions. Vaccine 35(17):2217–2223. https://doi.org/10.1016/j.vaccine.2016.08.045
Azegami T, Yuki Y, Kiyono H (2020) Plant-based mucosal vaccine delivery systems. In Mucosal Vaccines (pp. 357–370). Academic Press. https://doi.org/10.1016/B978-0-12-811924-2.00020-1
Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT (2005) CD4+-T-Cell responses generated during murine Salmonella enteric serovar typhimurium infection are Directed towards multiple epitopes within the Natural Antigen FliC. INFECT IMMUN 73:11: 72267235. https://doi.org/10.1128/IAI.73.11.7226-7235
Benoun JM, Peres NG, Wang N, Pham OH, Rudisill VL, Fogassy ZN, Whitney PG, Fernandez-Ruiz D, Gebhardt T, Pham Q, Puddington L, Bedoui S, Strugnell RA, McSorley SJ (2018) Optimal protection against Salmonella infection requires noncirculating memory. Proc Natl Acad Sci 2018:08339. https://doi.org/10.1073/pnas.1808339115
Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, Lessler J (2017) Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 17(10):1080–1088. https://doi.org/10.1016/S1473-3099(17)30359-6
Broberg CA, Calder TJ, Orth K (2011) Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect 13(12–13):992–1001. https://doi.org/10.1016/j.micinf.2011.06.013
Campa-Córdova AI, León-Gallo AF, Romero-Maldonado A, Ibarra-Serrano AC, Rosales-Mendoza S, Hirono I, Angulo C (2017) Recombinant PirA-like toxin protects shrimp against challenge with Vibrio parahaemolyticus, the aetiological agent of acute hepatopancreatic necrosis disease. J Fish Dis 40(11):1725–1729. https://doi.org/10.1111/jfd.12625
Centers for Disease Control and Prevention (2020) Salmonella. http://www.cdc.gov/salmonella/. Accessed 10 May 2020
Chauhan N, Tiwari S, Iype T, Jain U (2017) An overview of adjuvants utilized in prophylactic vaccine formulation as immunomodulators. Expert Rev Vaccines 16(5):491–502. https://doi.org/10.1080/14760584.2017.1306440
Chen Q, Li W, Wang P, Shao H, Ding Y, Wang W, Cen D, Cai Y, Xue X, Zhang L (2017) Induction of humoral and cellular immune responses in mice by multiepitope vaccines composing of both T and B lymphocyte epitopes of MAGE-A3 which are recombined into HBcAg. Protein & Peptide Letters 24(10):947–954. https://doi.org/10.2174/0929866524666170621094921
Criscuolo E, Caputo V, Diotti RA, Sautto GA, Kirchenbaum GA, Clementi N (2019) Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. Journal of immunology research, 2019. https://doi.org/10.1155/2019/8303648
da Hora VP, Conceição FR, Dellagostin OA, Doolana DL (2011) Review: Non-toxic derivatives of LT as potent adjuvants. Vaccine 29 (2011) 1538–1544. https://doi.org/10.1016/j.vaccine.2010.11.091
Daniell H, Rai V, Xiao Y (2019) Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. Plant Biotechnol J 17(7):1357–1368. https://doi.org/10.1111/pbi.13060
De Haan L, Verweij WR, Feil IK, Holtrop M, Hol WG, Agsteribbe E (1998) Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit. Immunology 94(3):424–430. https://doi.org/10.1046/j.1365-2567.1998.00535.x
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Flower DR (2003) Towards in silico prediction of immunogenic epitopes. Trends Immunol 24(12):667–674. https://doi.org/10.1016/j.it.2003.10.006
Guerrero Manriquez GG, Tuero I (2021) Adjuvants: friends in vaccine formulations against infectious diseases. Hum vaccines immunotherapeutics 17(10):3539–3550. https://doi.org/10.1080/21645515.2021.1934354
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231. https://doi.org/10.1126/science.227.4691.1229
Jacob CO, Sela M, Arnon R (1983) Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proceedings of the National Academy of Sciences, 80(24), 7611–7615. https://doi.org/10.1073/pnas.80.24.7611
Jacob CO, Leitner M, Zamir A, Salomon D, Arnon R (1985) Priming immunization against cholera toxin and E. coli heat-labile toxin by a cholera toxin short peptide‐beta‐galactosidase hybrid synthesized in E. coli. The EMBO journal, 4(12), 3339–3343. https://doi.org/10.1002/j.1460-2075.1985.tb04086.x
Kang TJ, Han SC, Jang MO, Kang KH, Jang YS, Yang MS (2004) Enhanced expression of B-subunit of Escherichia coli heat-labile enterotoxin in tobacco by optimization of coding sequence. Appl Biochem Biotechnol 117:175–187. https://doi.org/10.1385/ABAB:117:3:175
Kim SI, Veena JH, Gelvin SB (2007) Genome-wide analysis of AgrobacteriumT-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 51:779–791. https://doi.org/10.1111/j.1365-313X.2007.03183.x
Kedl RM, Kappler JW, Marrack P (2003) Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol 15(1):120–127. https://doi.org/10.1016/S0952-7915(02)00009-2
Kremer CJ, O’Meara KM, Layton SL, Hargis BM, Cole K (2011) Evaluation of recombinant Salmonella expressing the flagellar protein fliC for persistence and enhanced antibody response in commercial turkeys. Poult Sci 90:752–758. https://doi.org/10.3382/ps.2010-01076
Kurup VM, Thomas J (2020) Edible vaccines: Promises and challenges. Molecular Biotechnology, 1–12. https://doi.org/10.1007/s12033-019-00222-1
Lee C, Chen T, Yang Y, Ko T, Huang Y, Huang J, Huang M, Lin L, Chen C, Lin S, Lightner DV, Wang H, Wang A, Wang H, Horb L, Lo C (2015) The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. PNAS 112:10798–10803. https://doi.org/10.1073/pnas.1503129112
Li C, Ye Z, Wen L, Chen R, Tian L, Zhao F, Pan J (2014) Identification of a novel vaccine candidate by immunogenic screening of Vibrio parahaemolyticus outer membrane proteins. Vaccine 32(46):6115–6121. https://doi.org/10.1073/pnas.1503129112
Lei Y, Zhao F, Shao J, Li Y, Li S, Chang H, Zhang Y (2019) Application of built-in adjuvants for epitope-based vaccines. PeerJ 6:e6185. https://doi.org/10.7717/peerj.6185
Lundegaard C, Lund O, Nielsen M (2011) Prediction of epitopes using neural network based methods. J Immunol Methods 374(1–2):26–34. https://doi.org/10.1016/j.jim.2010.10.011
MacLennan CA, Martin LB, Micoli F (2014) Vaccines against invasive Salmonella disease: current status and future directions. Hum vaccines immunotherapeutics 10(6):1478–1493. https://doi.org/10.4161/hv.29054
Monreal-Escalante E, Rosales-Mendoza S, Govea-Alonso DO, Campa-Córdova AI, Angulo C (2019) Genetically-engineered plants yield an orally immunogenic PirA-like toxin from Vibrio parahaemolyticus. Int J Biol Macromol 137:126–131. https://doi.org/10.1016/j.ijbiomac.2019.06.159
Moreno-Fierros L, Ruiz-Medina E, Esquivel R, López-Revilla R, Piña-Cruz S (2003) Intranasal Cry1Ac Protoxin is an effective mucosal and systemic carrier and adjuvant of Streptococcus pneumoniae polysaccharides. Scand J Immunol 57(1):45–55. https://doi.org/10.1046/j.1365-3083.2003.01190.x
Moreno-Fierros L, Verdín-Terán S, García-Hernández A (2015) Intraperitoneal immunization with Cry1Ac protoxin from Bacillus thuringiensis provokes upregulation of Fc-Gamma-II/and Fc-gamma-III receptors associated with IgG in the intestinal epithelium of mice. Scand J Immunol 82:35–47. https://doi.org/10.1111/sji.12305
Nashar TO, Betteridge ZE, Mitchell RN (2001) Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4(+) T cells. Int Immunol 13(4):541–551. https://doi.org/10.1093/intimm/13.4.541
Ndifon W, Wingreen NS, Levin SA (2009) Differential neutralization efficiency of hemagglutinin epitopes, antibody interference, and the design of influenza vaccines. Proceedings of the National Academy of Sciences, 106(21), 8701–8706. https://doi.org/10.1073/pnas.0903427106
Newton SM, Jacob CO, Stocker BA (1989) Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 244(4900):70–72. https://doi.org/10.1126/science.246818
Nieto-Gómez R, Angulo C, Monreal-Escalante E, Govea-Alonso DO, De Groot AS, Rosales-Mendoza S (2019) Design of a multiepitopic Zaire ebolavirus protein and its expression in plant cells. J Biotechnol 295:41–48. https://doi.org/10.1016/j.jbiotec.2019.02.003
Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nádas A, Zolla-Pazner S (2000) Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol 74:7096–7107. https://doi.org/10.1128/JVI.74.15.7096-7107.2000
Reyes-Becerril M, Maldonado-García M, Guluarte C, León-Gallo AF, Rosales-Mendoza S, Ascencio F, Hirono I, Angulo C (2016) Evaluation of ToxA and Vibrio parahaemolyticus lysate on humoral immune response and immune-related genes in Pacific red snapper. Fish Shellfish Immunol 56:310–321. https://doi.org/10.1016/j.fsi.2016.07.014
Reyes-Becerril M, Guluarte C, Ceballos-Francisco D, Angulo C, Esteban MA (2017) Enhancing gilthead seabream immune status and protection against bacterial challenge by means of antigens derived from Vibrio parahaemolyticus. Fish Shellfish Immunol 60:205–218. https://doi.org/10.1016/j.fsi.2016.07.014
Ríos-Huerta R, Monreal-Escalante E, Govea-Alonso DO, Angulo C, Rosales-Mendoza S (2017) Expression of an immunogenic LTB-based chimeric protein targeting Zaire ebolavirus epitopes from GP1 in plant cells. Plant Cell Rep 36(2):355–365. https://doi.org/10.1007/s00299-016-2088-6
Rosales-Mendoza S, Soria-Guerra RE, López-Revilla R, Moreno-Fierros L, Alpuche-Solís ÁG (2008) Ingestion of transgenic carrots expressing the Escherichia coli heat-labile enterotoxin B subunit protects mice against cholera toxin challenge. Plant Cell Rep 27:79–84. https://doi.org/10.1007/s00299-007-0439-z
Rosales-Mendoza S, Alpuche-Solís AG, Soria-Guerra RE, Moreno- Fierros L, Martínez-González L, Herrera-Díaz A, Korban SS (2009) Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J 57:45–54. https://doi.org/10.1111/j.1365-313X.2008.03666.x
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Govea-Alonso DO, Herrera-Díaz A, Korban SS, Alpuche-Solís AG (2011) Immunogenicity of nuclear-encoded LTB:ST fusion protein from Escherichia coli expressed in tobacco plants. Plant Cell Rep 30:1145–1152. https://doi.org/10.1007/s00299-011-1023-0
Rosales-Mendoza S, Angulo C, Meza B (2016) Food-Grade Organisms as Vaccine Biofactories and oral Delivery Vehicles. Trends Biotechnol 34(2):124–136. https://doi.org/10.1016/j.tibtech.2015.11.007
Rosales-Mendoza S, Sández-Robledo C, Bañuelos-Hernández B, Angulo C (2017) Corn-based vaccines: current status and prospects. Planta 245(5):875–888. https://doi.org/10.1007/s00425-017-2680-1
Rubio-Infante N, Govea-Alonso DO, Romero-Maldonado A, García-Hernández AL, Ilhuicatzi-Alvarado D, Salazar-González JA, Moreno-Fierros L (2015) A plant-derived multi-HIV antigen induces broad immune responses in orally immunized mice. Mol Biotechnol 57(7):662–674. https://doi.org/10.1007/s12033-015-9856-3
Rubio-Infante N, Moreno‐Fierros L (2016) An overview of the safety and biological effects of Bacillus thuringiensis cry toxins in mammals. J Appl Toxicol 36(5):630–648. https://doi.org/10.1002/jat.3252
Rueda F, Eich C, Cordobilla B, Domingo P, Acosta G, Albericio F, Cruz LJ, Domingo JC (2017) Effect of TLR ligands co-encapsulated with multiepitopic antigen in nanoliposomes targeted to human DCs via fc receptor for cancer vaccines. Immunobiology 222(11):989–997. https://doi.org/10.1016/j.imbio.2017.06.002
Ruan X, Sack DA, Zhang W (2015) Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusión antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS ONE 10(3), e0121623. https://doi.org/10.1371/journal.pone.0121623
Rawat P, Singh AK, Ray K, Chaudhary B, Kumar S, Gautam T, Burma PK (2011) Detrimental effect of expression of Bt endotoxin Cry1Ac on in vitro regeneration, in vivo growth and development of tobacco and cotton transgenics. J Biosci 36(2):363–376. https://doi.org/10.1007/s12038-011-9074-5
Sambrook J, Russell DW (2006) The condensed protocols from molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press. https://katalog.ub.uni-heidelberg.de/titel/66895028
Soria-Guerra RE, Rosales-Mendoza S, Márquez-Mercado C, Lopez-Revilla R, Castillo-Collazo R, Alpuche-Solís ÁG (2007) Transgenic tomatoes express an antigenic polypeptide containing epitopes of the diphtheria, pertussis and tetanus exotoxins, encoded by a synthetic gene. Plant Cell Rep 26(7):961–968. https://doi.org/10.1007/s00299-007-0306-y
Trujillo E, Rosales-Mendoza S, Angulo C (2020) A multi-epitope plant-made chimeric protein (LTBentero) targeting common enteric pathogens is immunogenic in mice. Plant Mol Biol 102:159–169. https://doi.org/10.1007/s11103-019-00938-3
WHO (2017) Diarrhoeal disease: http://www.who.int/news-room/factsheets/detail/diarrhoeal-disease. Accessed 29 April 2020
Zha Z, Li C, Li W, Ye Z, Pan J (2016) LptD is a promising vaccine antigen and potential immunotherapeutic target for protection against Vibrio species infection. Nat Sci Rep 6:38577. https://doi.org/10.1038/srep38577
Funding
Investigations from the group are supported by Consejo Nacional de Ciencia y Tecnología (CONACYT): grants INFR-2016-271182 and CB-256063 to Sergio Rosales Mendoza and CB-2010-01-151818 and INFR- 2014-01-225924 to Carlos Angulo.
Author information
Authors and Affiliations
Contributions
ET: Investigation, methodology and formal analysis. DOGA, ARM and ET: Resources and methodology. CA and SRM: Writing-original draft preparation. CA, ET and SRM: Writing-review and editing the manuscript. CA and SRM: Conceptualization, supervision and funding acquisition. All authors discuss the results, read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Vijay Kumar.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trujillo, E., Govea-Alonso, D.O., Romero-Maldonado, A. et al. Production and immunogenicity assessment of a ToxA-based multiepitope plant-made protein targeting enteric pathogens. Plant Cell Tiss Organ Cult 154, 645–656 (2023). https://doi.org/10.1007/s11240-023-02539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02539-x