Abstract
Anthocyanins are a type of natural pigment that have high potential for development and utilization in regions like food, pharmaceutical, and cosmetic industries, with nitrogen and phosphorus starvation possibly promoting their accumulation in grapes. However, it remains unclear whether such starvation impacts the grape callus, or how the co-starvation of nitrogen and phosphorus affects the biosynthesis of anthocyanins. Here, we investigated how nitrogen starvation, phosphorus starvation, and the co-starvation of these two elements affects the synthesis of anthocyanins in the callus of grape skin. Results showed that separate starvation of nitrogen and phosphorus, as well as nitrogen and phosphorus co-starvation, inhibited callus growth, while significantly promoting the accumulation of anthocyanins. However, co-starvation did not facilitate anthocyanin biosynthesis during the later stages of callus growth. qRT-PCR analysis showed that the expression of VvUFGT and VvMYBA1 was closely related to anthocyanin accumulation in the callus under nitrogen and phosphorus starvation. Besides, we also confirmed that the abscisic acid signaling pathway was involved in anthocyanin accumulation as well as callus resistance under adverse conditions. This study provides a basis for investigating the regulatory mechanisms of anthocyanin synthesis in grapes, as well as theoretical support for the production of anthocyanins by callus culture.
Key message
Even though nitrogen and phosphorus deficiencies have been reported to promote anthocyanin biosynthesis in grapevine berries, whether the same deficiencies also induce anthocyanin accumulation in grape callus and whether nitrogen and phosphorus co-deficiency enhances this induction have yet to be reported. Therefore, the present study to investigate the effects of nitrogen starvation, phosphorus starvation, and nitrogen and phosphorus co-starvation on anthocyanin accumulation and ABA signaling in grape callus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grapes (Vitis vinifera L.) are cultivated in a variety of climates on six of the seven continents. According to the International Organisation of Vine and Wine (OIV), the world's wine-growing area in 2018 covered 7.4 kha, with global grape production of 78 mt. Thus, grapes have become the most widely grown fruit in the world (Schultz 2016; Gaiotti et al. 2018; Roca 2019).
Anthocyanins are a subgroup of the flavonoids, and are secondary metabolites (Bueno et al. 2012) that contribute to various biological functions in both plants and humans. For plants, anthocyanins attract pollinators and plant seed dispersers, in addition to protecting them from abiotic and biotic stresses (Petroni and Tonelli 2011). In parallel, anthocyanins exhibit multiple benefits to human health such as anti-inflammatory, anti-cancer, alleviate oxidative stress, prevent cardiovascular disease, control obesity and alleviate diabetes (He and Giusti 2010). As natural pigments, anthocyanins are already used in the food, pharmaceutical, and cosmetic industries (Santos-Buelga et al. 2014). Thus, anthocyanins have long attracted the interest of researchers and, more recently, have become a popular research focus. Both the skins and pomace extract of grapes are rich in polyphenols, especially anthocyanins (de Sales et al. 2018); thus, the prospects for the development and application of grape anthocyanins are considerable.
Both nitrogen and phosphorus are macronutrients that are essential for plant growth and development (Jiang et al. 2007; Nacry et al. 2013). Many researchers have reported that nitrogen and phosphorus starvation promote the biosynthesis of anthocyanin in several crops and model species. For example, anthocyanin was observed to accumulate in the callus cultures of ‘Fuji’ apple when 10 mM potassium phosphate was applied, but then decreased with an increasing concentration of potassium phosphate. Accumulation was also observed at 10 mM nitrogen but decreased at 50 mM nitrogen (Li et al. 2004). In addition, when a novel P-deficiency mutant of Arabidopsis thaliana was cultured on medium with no added phosphorus, the growth of the pho3 mutant declined, and the anthocyanin content was enhanced 100 fold as compared to the levels in the wild-type (Zakhleniuk et al. 2001). Yin et al. (2012) reported that, when compared to cells cultured in Murashige and Skoog (MS) medium containing 1.25 mM phosphate, cells cultured without phosphate accumulated significantly higher levels of anthocyanins. In the process of treating callus with varied concentrations of total nitrogen and light intensity, Irshad et al. (2018) found that higher concentration of total nitrogen favored biomass accumulation, while it unsupported anthocyanin accumulation. Furthermore, the clearly enhanced reddish anthocyanin pigmentation was detected in callus grown on the medium with low nitrogen concentration (40 and 50 mM) under medium light intensity. It was also found that auxin, such as 2,4-dichlorophenoxyacetic acid, could significantly inhibit anthocyanin accumulation in callus cultures of red-fleshed apple. However, this inhibition could be reversed by nitrogen deficiency (Ji et al. 2015). Such responses to nitrogen and phosphorus stress could also be applied to stimulate anthocyanin biosynthesis in grape production. Indeed, Jezek et al. (2018) reported that an appropriate reduction in nitrogen or phosphorus fertilization could be used to enrich the anthocyanin concentration of grape berries and, consequently, to improve the quality of the resulting wine. Lillo et al. (2008) reported that both late flavonoid pathway genes (DFR and ANS) and common MYB transcription factor genes (PAP1 and PAP2) are induced by deficiencies in the availability of certain nutrients, including nitrogen and phosphorus, thus enhancing the synthesis of flavonoids. Therefore, changes to gene expression might contribute to the accumulation of anthocyanin.
Anthocyanins biosynthesis is regulated by a variety of internal and external factors, including genetic regulation, plant hormone levels, non-hormone chemicals, and cultivation conditions (He et al. 2010; Meng et al. 2013). In addition, the long phenological stages (Jones 2003) further complicate the study and development of grape anthocyanins. Therefore, a feasible method for analyzing the formation and regulatory mechanisms of secondary metabolites, such as anthocyanins, at the cellular level is required, and could potentially be provided by culturing the callus.
The plant callus is a mass of plant cells that originate from almost any part of the plant. The callus can be maintained indefinitely in vitro by periodically passing cells and can be differentiated into complete plantlets under appropriate conditions. Callus cultures allow explants to be grown under artificially controlled conditions, where uncontrollable external conditions (such as seasonal, climate, and geographical factors) are absent. This technology has been used to produce agricultural plants, horticultural plants, therapeutic antibodies, and secondary metabolites. It facilitates the production of consistently high-quality secondary metabolites under controlled conditions. Thus, now, it is necessary to develop and market more callus culture-based products (Efferth 2019). The callus has become an important experimental material for biotechnology applications, such as the use of callus culture in models for studying interactions between plants and environmental factors. Thus, the present study used callus culture to investigate the regulatory mechanisms of anthocyanin accumulation at the cellular level to elucidate how environmental stressors affect the biosynthesis of anthocyanins in vivo. Recent studies have reported that nitrogen and phosphorus starvation effectively promote the synthesis of anthocyanins in several crops and model plant species, including grapes. However, the mechanisms underlying this synthesis remain unclear. Fortunately, callus culture provides a unique opportunity to study this phenomenon under controlled experimental conditions. However, it remains unclear whether nitrogen and phosphorus starvation also induce the accumulation of anthocyanins in grape callus, or whether nitrogen and phosphorus co-starvation is required to enhance this process.
This study investigated the effects of nitrogen starvation, phosphorus starvation, and their co-starvation on the accumulation of anthocyanin and the expression of abscisic acid (ABA) signaling pathway genes in the callus derived from the skin of Cabernet Sauvignon (Vitis vinifera). Through this study, we expect to establish a theoretical foundation for investigating the regulatory mechanisms underlying anthocyanin synthesis in grapes and for the production of anthocyanins using callus cultures.
Materials and methods
Plant materials
The callus of grape berry skin used in the present study were kindly provided by Prof. Jicheng Zhan from the College of Food Science and Nutritional Engineering, China Agricultural University, Beijing, China. The callus was induced from the skins of Vitis vinifera cv. ‘Cabernet Sauvignon’ grapes (Wang et al. 2015) and then transferred onto a subculture medium consisting of the basal MS medium (Murashige and Skoog 1962) supplemented with 1.126 mg·L−1 6-BA (6-benzylaminopurine) and 0.55 mg·L−1 NOA (naphthoxyacetic acid). Solid MS medium with 0.3% phytagel, 3% sucrose, 0.1% inositol and 0.03% KNO3 was used as basal medium. The pH was adjusted to 5.8–5.9, then the media were autoclaved for 20 min at 121 °C (Shanghai Bo Xun Medical Biological Instrument Co., Ltd., Shanghai, China). All the callus was sub-cultured every 20 days under dark condition.
Callus culture methods
Those well-grown and uniform callus was then sub-cultured on complete (A: N+P+), nitrogen-deficient (B: N−P+), phosphorus-deficient (C: N+P−), and co-deficient (D: N−P−) MS medium. All media were supplemented with 3% sucrose, 0.3% phytagel, 0.1% inositol, 0.03% KNO3, 1.126 mg L−1 6-BA, and 0.55 mg L−1 NOA. The pH of the media was adjusted to 5.8–5.9 before autoclaving for 20 min at 121 °C. In total, 2.7 g callus was inoculated in each medium, then transferred to a tissue culture room, where it was maintained at 25 ± 1 °C under a 16 h light photoperiod (2000–2400 Lx) and relative humidity of 60–70%.
The deficiency of nitrogen and phosphorus in MS medium was established based on the conservation of elements. For nitrogen-deficient medium, the chemicals of NH4NO3 and KNO3 were replaced with the same moles of KCl. For phosphorus-deficient medium, KH2PO4 was replaced with the same moles of KCl. Complete mineral composition of each medium was listed in Supplementary Table 1.
The callus cultures were harvested on days 0, 5, 10, 15, 20, and 25 days after inoculation. Then they were frozen immediately in liquid nitrogen and transferred into a 15 mL of chilled stainless steel tube with chilled beads, then ground to a fine powder in the tissue lyser (Absolute 1100, Monad Biotech Co., Ltd, Suzhou, China), and then stored at − 80 °C for further use.
Growth measurement
Six plates of callus were harvested from the four groups on days 0, 5, 10, 15, 20, and 25 after inoculation, and then divided them into three portions. During each sampling event, the fresh weight of the callus in each plate was first measured. The callus was then quickly frozen using liquid nitrogen, and stored at − 80 °C until analysis.
Determination of total anthocyanin
Each resulting powder sample (0.5 g) was dissolved in 10 mL 60% methanol extract, which contained 0.1% HCl. The supernatant was collected after ultrasound extraction at 40 W for 30 min at 30 °C (KQ-300DE, Kunshan Ultrasonic Instruments CO., Ltd. Kunshan, Jiangsu, China). It was then centrifuged at 8000×g for 10 min at 4 °C (Centrifuge 5804R, Eppendorf, Hamburg, Germany). The remaining residue was subjected to another 10 mL extract for two additional rounds of extraction (Liu et al. 2018). The anthocyanin concentration of the pooled extract solutions was estimated using the pH differential method and expressed as the malvidin-3-O-glucoside described by Lee et al. (2005).
Identification and quantification of anthocyanins
About 3.0 g callus powder was freeze-dried using a lyophilizer (Biocoll, Beijing, China). Dry powder (0.25 g) were transferred to separate 50 mL centrifuge tubes and mixed with 5 mL of a 98:2 (v:v) mixture of methanol and formic acid. The resulting supernatant was collected after the samples were subject to ultrasound extraction for 10 min (20 °C, 100 W) and then placed on a shaker (Zhicheng, Shanghai, China) for 30 min (25 °C, 130 rpm), followed by centrifugation for 10 min at 8000×g. For each sample, the precipitate was re-extracted with another 5 mL extract for three additional rounds of extraction. Finally, the pooled extraction supernatants were subjected to rotary evaporation at 30 °C (Shanghai Shensheng Technology Co., Ltd. Shanghai, China) until they were dry. The final sediment was diluted to 5 mL with a mixture containing nine volumes of mobile phase A (water:formic acid:acetonitrile = 92:2:6, v:v:v), and one volume of mobile phase B (water:formic acid:acetonitrile = 44:2:54, v:v:v). The mixture was stored at − 80 °C for subsequent analysis (Wang et al. 2008).
Aliquots (30 μL) of each diluted extract were analyzed three times by HPLC–ESI–MS using an Agilent 1100 series LC-MSD trap VL (Agilent Corporation, Santa Clara, CA, USA), which was equipped with a de-gasser (G1379A), quaternary pump (G1311A), ALS autosampler (G1313A), photodiode array detector (G1315B), and reversed-phase column (Kromasil C18, 250 × 4.6 mm i.d., 5 μm). The mobile phase contained: phase A, water:formic acid:acetonitrile (92:2:6, v:v:v); phase B, water:formic acid:acetonitrile (44:2:54, v:v:v). The column was maintained at 50 °C, and the gradient elution had the following proportions (v/v) of solvent B: 0–1 min, 10%; 1–18 min, 10–25%; 18–20 min, 25%; 20–30 min, 25–40%; 30–35 min, 40–70%; 35–40 min, 70–100%; 40–45 min, and 100–10%, with a flow rate of 1.0 mL min−1. Quantification was achieved by peak area measurement at 525 nm. The MS (mass spectrometry) conditions were: electrospray ionization interface, positive ion model, nebulizer, 35 psi, 10 mL min−1 dry gas (325 °C) flow rate, and scans between 100–1000 m z−1 (Li et al. 2011).
Monomeric anthocyanin was identified by comparing their molecular ions, product ions, and the elution orders with those available in the published literature (De Villiers et al. 2004; Wu and Prior 2005; Pati et al. 2009). The quantification of phenolic compounds was obtained by the use of external standards. The relative content of each anthocyanin was obtained as the equivalent of malvidin-3-O-glucoside using HPLC peak areas at the detection wavelength. The final results were multiplied by the water content of callus and expressed as mg·kg−1 FW.
The standards of malvidin-3-O-glucoside were supplied by Sigma-Aldrich (St. Louis, MO, USA) and its purity was > 97%. Methanol, formic acid and acetonitrile (HPLC grade) were obtained from Fisher Co. (Fairlawn, NJ, USA). All other chemicals used were analytical grade.
Gene expression analysis
Total RNA was isolated from callus samples collected on day 0, 5, 10, 15, 20, and 25 after inoculation using the Universal Plant Total RNA Extraction Kit (Bio Keke, Beijing, China). A quantity of 500 ng RNA was used to synthesize cDNA through HiScript II Q Select RT SuperMix for qPCR Kit (Vazyme, Nanjing, China). The cDNA products were diluted 20 times and stored at − 20 °C. Finally, quantitative real-time polymerase chain reaction (qRT-PCR) analyses were conducted using ChamQ SYBR qPCR Master Mix Kit (Vazyme, Nanjing, China), with 2 μL template cDNA and 0.4 μL of each gene-specific primer (Table 1). We designed primers using the NCBI tool Primer BLAST as query and the parameters suggested by NCBI. The primers were designed to span an exon-exon junction or separated by at least a large intron on the corresponding genomic DNA to reduce the risk of false positives from amplification of any contaminating genomic DNA. The investigated anthocyanin biosynthesis genes included nine structural genes (VvCHS1, VvCHS2, VvCHS3, VvCHI, VvF3H1, VvF3H2, VvDFR, VvLDOX, and VvUFGT) and a regulatory gene (VvMYBA1)). Abscisic acid signaling pathway genes included VvNCED1 (a key gene in ABA synthesis), VvHYD2 (involved in ABA hydroxylation), VvGT1 (involved in ABA conjugation), VvBG1 (involved in ABA de-conjugation), and VvABCG40 (a plant transporter involved in ABA absorbance). VvActin was used as the reference gene. Three biological and technical replicates were performed for each template cDNA. The relative expression levels of the target genes were determined using the 2−∆∆CT method, as described by Livak and Schmittgen (2001).
Nitrogen and phosphorus measurement
Total nitrogen was determined by micro-Kjeldahl digestion (KDN-102C, Shanghai Xianjian Instruments Co., Ltd, Shanghai, China) according to the Standard Methods (China Food and Drug Administration 2016a). Total phosphorus was quantified using molybdenum blue colorimetric analysis according to the Standard Methods (China Food and Drug Administration 2016b).
Statistical analysis
All the experimental data were analyzed using SPSS.21 (SPSS Inc., Chicago, USA). The significance of differences between group means was assessed at the p < 0.05 level (Duncan’s multiple).
Results
Callus growth
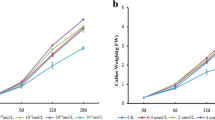
The size and color of the callus cultures (Fig. 1) were consistent with callus growth and anthocyanin concentration. Different growth patterns were observed in the four treatment groups (Fig. 2). There were no significant differences among the four groups after 5 or 10 days, but significant differences were observed thereafter. Group A exhibited optimum callus growth, and the growth curve followed an S-shaped pattern. After 25 days, the fresh weight of Group A reached 10.11 g, which was 3.74 times that of the initial callus weight. Although the effect of nitrogen starvation (Group B) on callus growth was not as significant as that observed in Group A, it was 0.53 times that of Group A after 25 days. However, the callus growth was relatively stable in Group B. The fresh weights of Groups C and D were significantly lower than those of Group A. The co-starvation treatment had the most obvious inhibiting effect, with negative growth (− 0.14 and − 0.13 g) occurring after 20 and 25 days, respectively.
Effect of nutrient starvation on callus growth. Data are expressed as mean value per plate ± standard deviation (n = 6). Lowercase letters indicate significant differences between treatment groups (p < 0.05, Duncan’s multiple). N+ nitrogen, N− nitrogen starvation, P+ phosphorus, P− phosphorus starvation
Anthocyanin concentration
The anthocyanin concentration of the callus was significantly affected by nitrogen and phosphorus availability (Fig. 3), which was shown by the callus color. The anthocyanin concentration of Groups A, B, and C increased with culture duration, whereas that of Group D first increased and then decreased. When compared to the other three treatment groups, the anthocyanin concentration of Group A was consistently the lowest, except after 25 days. Groups B and C exhibited noticeably enhanced levels of anthocyanin accumulation after 5 days. However, even though the anthocyanin concentration of both Groups B and C increased rapidly during early culture, the rate of accumulation in Group C slowed during later culture. Despite this, after 25 days, Group C had the highest anthocyanin concentration, which was 3.04, 1.09, and 3.9 times greater than the anthocyanin levels of Groups A, B, and D, respectively. Interestingly, the anthocyanin concentration of Group D was significantly higher than that of the other three treatment groups after 5 and 10 days, which were 6.97 and 3.19 times higher than those of Group A, respectively. However, the positive effect of co-starvation on anthocyanin accumulation gradually weakened. After 25 days, the anthocyanin concentration of Group D was the lowest of all four treatment groups, and only 78% that of Group A.
Effect of nutrient starvation on callus anthocyanin concentration. Anthocyanin levels were calculated using the pH differential method. Data are expressed as mean value per plate ± standard deviation (n = 6). Lowercase letters indicate significant differences between treatment groups (p < 0.05, Duncan’s multiple). N+ nitrogen, N− nitrogen starvation, P+ phosphorus, P− phosphorus starvation
Anthocyanin distribution
Eleven monomeric anthocyanins were detected, identified, and quantified by HPLC–ESI–MS (High-performance liquid chromatography-electrospray ionization tandem-mass spectrometry) in the callus (Table 2). The most abundant anthocyanin was peonidin-3-O-(trans-6-O-coumaroyl)-glucoside, which reached 25.30 mg·kg−1 in Group C, followed by malvidin-3-O-(6-O-caffeoyl)-glucoside, peonidin-3-O-glucoside, and cyanidin-3-O-glucoside, respectively. Among the monomeric anthocyanins that were present in Groups A, B, and C, the concentration in Groups B and C were significantly higher than those in Group A. For example, the peonidin-3-O-glucoside concentration of Groups B and C were 7.78 and 9.40 times more than that of Group A, respectively, confirming that nitrogen and phosphorus starvation (but not co-starvation) promote the accumulation of anthocyanins. Group C had the greatest number of detectable monomeric anthocyanins (n = 11), which was four and three more than the number detected in Groups A and B, respectively. Petunidin-3-O-glucoside and petunidin-3-O-(6-O-coumaroyl)-glucoside were only detected in Group C.
Nitrogen and phosphorus levels
The nitrogen contents of Groups A and C were increased by the callus culture; however, the nitrogen content of Groups B and D (which were nitrogen-starvation treatment) declined (Fig. 4). The effects of phosphorus starvation on the total phosphorus contents of the callus were inconsistent; however, the total phosphorus levels of Groups B, C, and D were lower than those of Group A throughout most of the culture period. Thus, phosphorus starvation affected the absorption of phosphorus by the callus. Thus, both nitrogen and phosphorus starvation influence the total nitrogen and phosphorus contents of the callus.
Effect of nutrient starvation on the nutrient content of the callus. a total nitrogen content; b total phosphorus content. Values indicate means (n = 3). Statistical analysis indicated that there were no significant differences among replicates. N+ nitrogen, N− nitrogen starvation, P+ phosphorus, P− phosphorus starvation
Expression of anthocyanin biosynthesis genes
The relative expression of anthocyanin synthesis and regulation-related genes was measured over 25 days. The relative expression of anthocyanin synthesis-related genes was greater in Groups B and C than in Group A throughout the culture process. In general, the expression of most genes in Group C was much higher than that in Group A during the first 20 days, but was more similar after 25 days (Fig. 5).
Effect of nutrient starvation on the relative expression levels of anthocyanin biosynthesis-related genes; (a–i) structural genes in the anthocyanin biosynthesis pathway; j regulated gene in the anthocyanin biosynthesis pathway. Gene expression levels were normalized to the transcript level of VvActin and the data were expressed as mean value per plate ± standard deviation (n = 6). N+ nitrogen, N− nitrogen starvation, P+ phosphorus, P− phosphorus starvation
The relative expression of most genes in Group D was significantly different to that in A. The changes in the expression of each gene were relatively consistent; they first increased and then decreased, with maximum gene expression occurring at 10 days. Until 20 days after inoculation, the relative expression of VvCHS1, VvCHS2, VvCHI, VvF3H1, VvF3H2, VvDFR, and VvLDOX in Group D was lower than that in Group A. Therefore, when compared to Group A, the co-starvation treatment actually upregulated the expression of anthocyanin biosynthesis-related genes during the first 10 days; however, the upregulation was subsequently weakened or, even, inhibited, which was coincident with the decline in anthocyanin concentration during the late-stage culture.
Expression of ABA signaling pathway genes
Separate nitrogen and phosphorus starvation had similar effects on the expression of ABA signaling pathway genes (Fig. 6). The expression of the ABA-synthesis gene VvNCED1 was significantly upregulated, whereas the two ABA-degrading genes, VvHYD2 and VvGT1, were downregulated. Even though the expression of VvNCED1 in Group D was upregulated throughout the experiment, VvHYD2 was also significantly upregulated 20 days after inoculation, at which point the expression of VvHYD2 in Group D was 14.70 times more than that in Group A. The expression of VvGT1 started decreasing 10 days after inoculation, but remained higher than that of Group A.
Effect of nutrient starvation on the relative expression levels of ABA signaling pathway-related genes. Gene expression levels were normalized to the transcript level of VvActin and the data were expressed as mean value per plate ± standard deviation (n = 6). N+ nitrogen, N− nitrogen starvation, P+ phosphorus, P− phosphorus starvation
Discussion
Previous studies have confirmed that nitrogen and phosphorus enhance the biosynthesis of anthocyanin in the field. For example, Soubeyrand et al. (2014) reported that the total anthocyanin content of field-grown grapes cultivated under low-nitrogen conditions was significantly greater than that of grapes cultivated under high-nitrogen conditions. This phenomenon occurred because nitrogen starvation upregulates both the structural and regulatory genes involved in anthocyanin synthesis. Therefore, the appropriate reduction in nitrogen or phosphorus fertilization under culture conditions could increase anthocyanin content and, consequently, enhance fruit quality. However, it remains unclear whether nitrogen and phosphorus starvation have the same effect on grape callus. Therefore, the present study investigated how nitrogen and phosphorus starvation affect anthocyanin biosynthesis and the ABA signaling pathway of the grape callus, along with the underlying genetic mechanisms that regulate changes in the anthocyanin concentration.
Isah (2019) demonstrated that greater productions of secondary compounds in plants prevents free radicals from causing damage associated with nutrition-based stress. As an important secondary metabolite, anthocyanin has several functional roles in plant-environment interactions, and it hinders the progression of senescence under mineral deficiencies, such as deficiencies in nitrogen or phosphorus (Landi et al. 2015). Liang and He (2018) recently reported that nitrogen-starved Arabidopsis plants exhibit retarded growth and enhanced anthocyanin accumulation; thus, anthocyanins likely contribute to the tolerance of plants to low-nitrogen stress. Besides, recent research has found that MdLBD13 is an important regulatory component in anthocyanin biosynthesis and nitrate signaling pathway. It can strongly inhibit the expression of MYB and bHLH genes, acting as a negative regulator in anthocyanin biosynthesis, and could be rapidly induced by nitrate (Li et al. 2017). This, on the other hand, confirms the positive effect of nitrogen deficiency on anthocyanin accumulation. In the present study, nitrogen and phosphorus starvation inhibited callus growth, but induced anthocyanin accumulation. In addition, separate nitrogen and phosphorus starvation significantly upregulated the expression of almost all the tested structural and regulatory genes involved in anthocyanin synthesis, supporting existing research (Wolf-Rüdiger et al. 2004; Yin et al. 2012; Liang and He 2018). Therefore, the results of the present study support that primary metabolism (mainly callus growth) is dominant when nitrogen and phosphorus are readily available. Thus, sufficient nitrogen and phosphorus levels are vital for callus growth. The accumulation of anthocyanins showed that secondary metabolite systems are stimulated by nitrogen or phosphorus starvation, and that anthocyanin biosynthesis might serve as a defense mechanism against environmental stress. Combined with the growth and accumulation of anthocyanins of the callus, we showed that the nitrogen starvation treatment represents an appropriate model to produce anthocyanin, because it promoted steady callus growth and anthocyanin accumulation throughout the culture period.
Up to 11 anthocyanins were detected in the callus in our study, with previous studies detecting 19 anthocyanins in the skin of ‘Cabernet Sauvignon’ berries (Ali et al. 2011). Thus, the biosynthesis of anthocyanins appears to be somewhat restricted in the callus. Furthermore, the main anthocyanins in grape berry of Cabernet Sauvignon were anthocyanin monoglucosides, especially malvidin-3-O-glucoside according to the previous research (Dimitrovska et al. 2011). However, some coumaroylated derivatives, especially peonidin-3-O-(trans-6-O-coumaroyl)-glucoside, were identified as main anthocyanins in ‘Cabernet Sauvignon’ grape callus in this study. Recently, the study from Yu et al. (2020) also showed that peonidin-3-O-(trans-6-O-coumaroyl)-glucoside are the main anthocyanins in ‘Gamay’ grape callus, suggesting that peonidin-3-O-(trans-6-O-coumaroyl)-glucoside might be the main anthocyanin in grape callus. The most likely explanation could be that the anthocyanins profiles are often regulated in tissue-specific manner. In addition, light could also significantly induce the phenolic acids accumulation in grape callus, such as chlorogenic acid, coumaric acid (Liu et al. 2015), which provide critical substrate for the biosynthesis of acylated anthocyanins. Moreover, total concentration of anthocyanins was different from pH differential method to HPLC–ESI–MS method, but they showed a similar result tendency on twenty-five-day-old callus samples. For group D, combining with callus color faded and total anthocyanins concentration dropped sharply after 20 days, undetected monomeric anthocyanins might due to their bitterly low concentration.
In contrast to what was observed during separate starvation, nitrogen and phosphorus co-starvation initially enhanced anthocyanin accumulation, but later inhibited it. Indeed, the relative expression of genes under this treatment also increased initially, and then decreased after peaking at 10 days after inoculation. Peng et al. (2008) showed that, in contrast to wild-type plants, the Arabidopsis nla (nitrogen limitation adaptation) mutant could not control nitrogen limitation-induced anthocyanin synthesis; consequently, it exhibits early senescence. However, under co-starvation conditions, the mutant accumulated anthocyanins, allowing it to adapt to adverse conditions. Therefore, interactions between nitrogen and phosphorus might affect how plants respond to adversity; in fact, such interactions might have driven the pattern of anthocyanin accumulation detected under co-starvation conditions in the present study. Finally, due to the absence of nitrogen and phosphorus in the co-starvation treatment, the original nutritional elements of the media could not support callus growth. Specifically, the physiological activity of the callus, including its resistance to nutrient starvation, decreased with culture duration, thus limiting anthocyanin biosynthesis. In addition, at least until 25 days after inoculation, the callus appeared to have been inactivated, making it difficult to extract RNA for analysis. Overall, additional research is needed to elucidate the interactions between co-occurring nitrogen and phosphorus starvation.
In the present study, changes of anthocyanin concentration were shown both in callus color and the level of anthocyanin biosynthesis-related genes transcripts. VvCHS1, VvCHS2, VvCHS3, VvF3H1, and VvF3H2 might be the key genes for the accumulation of anthocyanins under nitrogen starvation, whereas VvCHS1, VvCHS2, VvCHS3, VvF3H1, and VvLDOX might be the key genes under phosphorus starvation. The final step in the flavonoid pathway is the transformation of anthocyanidins to water-soluble anthocyanins, which are more stable and deeper in color. This process is under the regulation of UFGT, which is controlled by VvMYBA1. Accordingly, the VvMYBA1 factor is considered as a major gene that determines anthocyanin synthesis and the color of grape skin (Azuma et al. 2008; Cutanda-Perez et al. 2009; Enoki et al. 2017). In the present study, the relative expression of VvUFGT and VvMYBA1 expression in the three starvation treatments was significantly upregulated during the early stages of culture, but was relatively unaffected (i.e., similar to the control group) during later stages. Thus, nitrogen and phosphorus starvation likely promote the pre-expression of these two genes. Therefore, the early expression of VvUFGT and VvMYBA1 might be associated with anthocyanin accumulation under nitrogen and phosphorus starvation in the callus.
Many researchers have investigated the regulatory effects of nitrogen and phosphorus starvation on anthocyanin accumulation. For example, Jiang et al. (2007) reported that phosphorus starvation reduces the level of bioactive gibberellin in Arabidopsis, thus promoting DELLA accumulation (core components of the gibberellin-signaling pathway). As a consequence, several adaptively significant plant phosphorous-starvation responses are modulated, including the inhibition of growth, accumulation of anthocyanins, and elongation of root hairs. Using both molecular and genetic approaches, Lei et al. (2011) demonstrated that ethylene signaling upregulates phosphorus starvation-induced genes and acid phosphatase, while negatively regulating the accumulation of anthocyanin. Thus, plant hormones are clearly related to anthocyanin production under nutrient-starvation conditions. The current study investigated the relationship between ABA and anthocyanin production under nitrogen and phosphorus starvation. ABA is a key component of plant growth and development associated with the environment. As a sesquiterpene plant hormone that regulates a variety of plant processes, ABA helps plants to adapt to abiotic and biotic stresses (Nambara and Kuchitsu 2011), thus contributing to the responses of plants to adverse environmental conditions. Several studies reported that exogenous ABA application promotes the synthesis of anthocyanins in grapes (Ban et al. 2003; Sandhu et al. 2011; Jia et al. 2018). In particular, Jia et al. (2018) reported that VvMYBA2 and VvUFGT are significantly upregulated in ABA-treated berries, contributing to elevated anthocyanin content. The present study evaluated the expression levels of five genes related to the abscisic acid signaling pathway based on Ferrero et al. (2018). Our results showed that separate nitrogen and phosphorus starvation upregulated the key gene of the ABA-synthesis pathway (VvNCED1), and downregulated the ABA-degradation genes (VvHYD2 and VvGT1). Furthermore, the levels of anthocyanins that accumulated under these two treatments were significantly elevated. The expression of VvNCED1 was partly upregulated, whereas the expression of VvHYD2 significantly increased during late stages of culture. Compared to Group A, the expression of VvGT1 was consistently higher under nitrogen and phosphorus co-starvation.
Therefore, the callus might synthesize ABA by upregulating ABA-synthesis genes and downregulating ABA-degrading genes when exposed to separate nitrogen and phosphorus starvation. This phenomenon might promote the biosynthesis of anthocyanins and improve the ability of the callus to adjust to nutrient-deficient environments. The downregulation of ABA-synthesis genes and upregulation of ABA-degrading genes during the late stage of co-starvation treatment might be related to the downregulation of anthocyanin biosynthetic genes. Consequently, this phenomenon might reduce anthocyanin concentration in the callus.
Our study demonstrated that grape callus culture could be an appropriate system for investigating how nitrogen and phosphorus starvation affect anthocyanin synthesis in grapes. Results showed separate nitrogen and phosphorus starvation inhibited callus growth but promoted anthocyanin biosynthesis. This phenomenon was attributed to changes in the expression of certain genes (e.g., VvUFGT and VvMYBA1), which are related to ABA-mediated anthocyanin biosynthesis. The upregulation and downregulation of ABA-degrading and synthetic genes, respectively, might inhibit the accumulation of anthocyanins and lower the tolerance of the callus to stress caused by nitrogen and phosphorus co-starvation. This phenomenon ultimately causes the inactivation of the callus.
Abbreviations
- MS:
-
Murashige and Skoog medium
- ABA:
-
Abscisic acid
- HPLC–ESI–MS:
-
High performance liquid chromatography-electrospray ionization-mass spectrometry
- 6-BA:
-
6-Benzylaminopurine
- NOA:
-
Naphthoxyacetic acid
- FW:
-
Fresh weight
References
Ali MB, Howard S, Shangwu C, Yechun W, Oliver Y, Kovacs LG, Wenping Q (2011) Berry skin development in Norton grape: distinct patterns of transcriptional regulation and flavonoid biosynthesis. BMC Plant Biol 11:1–23. https://doi.org/10.1186/1471-2229-11-7
Azuma A, Kobayashi S, Mitani N et al (2008) Genomic and genetic analysis of Myb-related genes that regulate anthocyanin biosynthesis in grape berry skin. Theor Appl Genet 117:1009–1019. https://doi.org/10.1007/s00122-008-0840-1
Ban T, Ishimaru M, Kobayashi S, Goto-Yamamoto N, Horiuchi S (2003) Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. J Horticult Sci Biotechnol 78:586–589. https://doi.org/10.1080/14620316.2003.11511668
Bueno JM, Sáez-Plaza P, Ramos-Escudero F, Jiménez AM, Fett R, Asuero AG (2012) Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit Rev Anal Chem 42:126–151. https://doi.org/10.1080/10408347.2011.632314
China Food and Drug Administration (2016a) Determination of proteins in food GB 5009.5–2016. China
China Food and Drug Administration (2016b) Determination of phosphorus in food GB 5009.87–2016. China
Cutanda-Perez M-C, Ageorges A, Gomez C, Vialet S, Terrier N, Romieu C, Torregrosa L (2009) Ectopic expression of VlmybA1 in grapevine activates a narrow set of genes involved in anthocyanin synthesis and transport. Plant Mol Biol 69:633–648. https://doi.org/10.1007/s11103-008-9446-x
De Sales NFF, Silva da Costa L, Carneiro TIA, Minuzzo DA, Oliveira FL, Cabral LMC, Torres AG, El-Bacha T (2018) Anthocyanin-rich grape pomace extract (Vitis vinifera L.) from wine Industry affects mitochondrial mioenergetics and glucose metabolism in human hepatocarcinoma HepG2 cells. Molecules 23:611. https://doi.org/10.3390/molecules23030611
De Villiers A, Vanhoenacker G, Majek P, Sandra P (2004) Determinaton of anthocyanins in wine by direct injection liquid chromatography-diode array detection-mass spectrometry and classification of wines using discriminant analysis. J Chromatogr A 1054:195–204. https://doi.org/10.1016/s0021-9673(04)01291-9
Dimitrovska M, Bocevska M, Dimitrovski D, Murkovic M (2011) Anthocyanin composition of Vranec, Cabernet Sauvignon, Merlot and Pinot Noir grapes as indicator of their varietal differentiation. Eur Food Res Technol 232:591–600. https://doi.org/10.1007/s00217-011-1425-9
Efferth T (2019) Biotechnology applications of plant callus cultures. Engineering 5:50–59. https://doi.org/10.1016/j.eng.2018.11.006
Enoki S, Hattori T, Ishiai S et al (2017) Vanillylacetone up-regulates anthocyanin accumulation and expression of anthocyanin biosynthetic genes by inducing endogenous abscisic acid in grapevine tissues. J Plant Physiol 219:22–27. https://doi.org/10.1016/j.jplph.2017.09.005
Ferrero M, Pagliarani C, Novak O, Ferrandino A, Cardinale F, Visentin I, Schubert A (2018) Exogenous strigolactone interacts with abscisic acid-mediated accumulation of anthocyanins in grapevine berries. J Exp Bot 69:2391–2402. https://doi.org/10.1093/jxb/ery033
Gaiotti F, Pastore C, Filippetti I, Lovat L, Belfiore N, Tomasi D (2018) Low night temperature at veraison enhances the accumulation of anthocyanins in Corvina grapes (Vitis Vinifera L.). Sci Rep 8:8719. https://doi.org/10.1038/s41598-018-26921-4
He F, Mu L, Yan GL et al (2010) Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 15:9057–9091. https://doi.org/10.3390/molecules15129057
He J, Giusti MM (2010) Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol 1:163–187. https://doi.org/10.1146/annurev.food.080708.100754
Irshad M, Debnath B, Mitra S, Arafat Y, Li M, Sun Y, Qiu D (2018) Accumulation of anthocyanin in callus cultures of red-pod okra [Abelmoschus esculentus (L.) Hongjiao] in response to light and nitrogen levels. Plant Cell, Tissue Organ Cult 134:29–39. https://doi.org/10.1007/s11240-018-1397-6
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52:39. https://doi.org/10.1186/s40659-019-0246-3
Jezek M, Zörb C, Merkt N, Geilfus C-M (2018) Anthocyanin management in fruits by fertilization. J Agric Food Chem 66:753–764. https://doi.org/10.1021/acs.jafc.7b03813
Ji X-H, Wang Y-T, Zhang R et al (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f.niedzwetzkyana). Plant Cell, Tissue Organ Cult 120:325–337. https://doi.org/10.1007/s11240-014-0609-y
Jia H, Wang S, Lin H, Satio T, Ampa K, Todoroki Y, Kondo S (2018) Effects of abscisic acid agonist or antagonist applications on aroma volatiles and anthocyanin biosynthesis in grape berries. J Horticult Sci Biotechnol 93:392–399. https://doi.org/10.1080/14620316.2017.1379364
Jiang C, Gao X, Liao L, Harberd NP, Fu X (2007) Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol 145:1460–1470. https://doi.org/10.1104/pp.107.103788
Jones G (2003) Phenology: an integrative environmental science. In: Schwartz MD (ed) Winegrape phenology, 1st edn. Kluwer Academic Publishers, Netherlands, pp 523–539
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17. https://doi.org/10.1016/j.envexpbot.2015.05.012
Lee J, Durst RW, Wrolstad RE (2005) Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 88:1269–1278. https://doi.org/10.1093/jaoac/88.5.1269
Lei M, Zhu C, Liu Y, Karthikeyan AS, Bressan RA, Raghothama KG, Liu D (2011) Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol 189:1084–1095. https://doi.org/10.1111/j.1469-8137.2010.03555.x
Li H-H, Liu X, An J-P, Hao Y-J, Wang X-F, You C-X (2017) Cloning and elucidation of the functional role of apple MdLBD13 in anthocyanin biosynthesis and nitrate assimilation. Plant Cell, Tissue Organ Cult 130:47–59. https://doi.org/10.1007/s11240-017-1203-x
Li Z, Pan Q, Jin Z, Mu L, Duan C (2011) Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem 125:77–83. https://doi.org/10.1016/j.foodchem.2010.08.039
Li ZH, Sugaya S, Gemma H, Iwahori S (2004) The effect of calcium, nitrogen and phosphorus on anthocyanin synthesis in 'Fuji' apple callus. Acta Hortic 653:209–214. https://doi.org/10.17660/ActaHortic.2004.653.29
Liang J, He J (2018) Protective role of anthocyanins in plants under low nitrogen stress. Biochem Biophys Res Commun 498:946–953. https://doi.org/10.1016/j.bbrc.2018.03.087
Lillo C, Lea US, Ruoff P (2008) Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 31:587–601. https://doi.org/10.1111/j.1365-3040.2007.01748.x
Liu D, Wang Z, Xie S, Liu M, Liang P, Zhang Z (2018) Effect of cluster thinning during veraison on phenolic substances of Vitis vinifera L. cv. Syrah. J Northwest A&F Univ 46:124–131. https://doi.org/10.13207/j.cnki.jnwafu.2018.07.017
Liu R, Yang G, Wu Y, Rao H, Li X, Li M, Qian P (2015) Effects of light intensity on associated enzyme activity and gene expression during callus formation of Vitis vinifera. Chin J Biotechnol 31:1219–1229. https://doi.org/10.13345/j.cjb.140494
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Meng JF, Ning PF, Xu TF, Zhang ZW (2013) Effect of rain-shelter cultivation of Vitis vinifera cv. Cabernet Gernischet on the phenolic profile of berry skins and the incidence of grape diseases. Molecules 18:381–397. https://doi.org/10.3390/molecules18010381
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nacry P, Bouguyon E, Gojon A (2013) Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370:1–29. https://doi.org/10.1007/s11104-013-1645-9
Nambara E, Kuchitsu K (2011) Opening a new era of ABA research. J Plant Res 124:431–435. https://doi.org/10.1007/s10265-011-0437-7
Pati S, Liberatore MT, Gambacorta G, Antonacci D, La Notte E (2009) Rapid screening for anthocyanins and anthocyanin dimers in crude grape extracts by high performance liquid chromatography coupled with diode array detection and tandem mass spectrometry. J Chromatogr A 1216:3864–3868. https://doi.org/10.1016/j.chroma.2009.02.068
Peng M, Hudson D, Schofield A et al (2008) Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J Exp Bot 59:2933–2944. https://doi.org/10.1093/jxb/ern148
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181:219–229. https://doi.org/10.1016/j.plantsci.2011.05.009
Roca P (2019) OIV 2019 report on the world vitivinicultural situation. International Organisation of Vine and Wine. https://www.oiv.int/. Accessed 3 Aug 2019
Sandhu AK, Gray DJ, Lu J, Gu L (2011) Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem 126:982–988. https://doi.org/10.1016/j.foodchem.2010.11.105
Santos-Buelga C, Mateus N, De Freitas V (2014) Anthocyanins. Plant pigments and beyond. J Agric Food Chem 62:6879–6884. https://doi.org/10.1021/jf501950s
Schultz HR (2016) Global climate change, sustainability, and some challenges for grape and wine production. J Wine Econ 11:181–200. https://doi.org/10.1017/jwe.2015.31
Soubeyrand E, Basteau C, Hilbert G, van Leeuwen C, Delrot S, Gomès E (2014) Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv Cabernet-Sauvignon berries. Phytochemistry 103:38–49. https://doi.org/10.1016/j.phytochem.2014.03.024
Wang H, Wang W, Zhan J, Huang W, Xu H (2015) An efficient PEG-mediated transient gene expression system in grape protoplasts and its application in subcellular localization studies of flavonoids biosynthesis enzymes. Sci Hortic 191:82–89. https://doi.org/10.1016/j.scienta.2015.04.039
Wang Z, Han F, Wang Y, Qi X, Wang X, Tian Y, Zhao R (2008) Determination of anthocyanin in Granoir grape and wine with HPLC. J Agric Univ Hebei 31:59–61. https://doi.org/10.3969/j.issn.1000-1573.2008.06.014
Wolf-Rüdiger S, Rosa M, Tomasz C et al (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499. https://doi.org/10.1104/pp.104.047019
Wu X, Prior RL (2005) Systematic identification and characterization of anthocyanins by HPLC- ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem 53:2589–2599. https://doi.org/10.1021/jf048068b
Yin Y, Borges G, Sakuta M, Crozier A, Ashihara H (2012) Effect of phosphate deficiency on the content and biosynthesis of anthocyanins and the expression of related genes in suspension-cultured grape (Vitis sp.) cells. Plant Physiol Biochem 55:77–84. https://doi.org/10.1016/j.plaphy.2012.03.009
Yu M, Chen JC, Qu JZ, Liu F, Zhou M, Ma YM, Xiang SY, Pan XX, Zhang HB, Yang MZ (2020) Exposure to endophytic fungi quantitatively and compositionally alters anthocyanins in grape cells. Plant Physiol Biochem 149:144–152. https://doi.org/10.1016/j.plaphy.2020.02.006
Zakhleniuk OV, Raines CA, Lloyd JC (2001) pho3: a phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta 212:529–534. https://doi.org/10.1007/s004250000450
Acknowledgements
The authors thank Prof. Jicheng Zhan (College of Food Science and Nutritional Engineering, China Agricultural University) for kindly giving us callus of grape berry skin. The authors are also grateful to Editage (www.editage.cn) for English language editing. This work was financially supported by the National Natural Science Foundation of China (31801833 and 31801811), Special Project for Reform and Development of National Science and Technology (106001000000150012), China Postdoctoral Science Foundation (2019M653771, 2019T120953, and 2018M633589), Shaanxi Science and Technology Project (2020KJXX-035 and 2020NY-049), the Fundamental Research Funds for the Central Universities (2452020178), and China Agriculture Research System for Grape (CARS-29-zp-6).
Author information
Authors and Affiliations
Contributions
JFM and TFX designed the research and proposed the research process. HZZ, HW, SHG, MXF, and XQJ performed the experiments. YLF and ZWZ formulated the experimental platform. HZZ analyzed the data and wrote the paper. XY revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Communicated by Klaus Eimert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The paper has a registered preprint online (https://doi.org/10.21203/rs.2.17799/v1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, HZ., Wei, H., Guo, SH. et al. Nitrogen and phosphorus co-starvation inhibits anthocyanin synthesis in the callus of grape berry skin. Plant Cell Tiss Organ Cult 142, 313–325 (2020). https://doi.org/10.1007/s11240-020-01864-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01864-9