Abstract
Transgenesis through biolistic of immature embryos is the most convenient way to introduce artificially new genes in bread wheat (Triticum aestivum L.). However, only a few genotypes can be efficiently transformed. To improve the transformation of wheat varieties, we stored immature seeds at room temperature or 4 °C during 4 or 7 days and extracted immature embryos prior to transformation. Shelling stops the embryo’s growth and almost all the embryos formed a callus on selective media when stored at 4 °C for 4 or 7 days (respectively 87% and 99%). We also used hybrid immature embryos derived from a cross between a transformable line (Courtot) and a non-transformable line (Chinese Spring) for biolistic transformation. Hybrid embryos showed the same response to biolistic than the responsive parent. All together, these results improve significantly the biolistic protocol for wheat transformation by reducing the number of mother plants in the greenhouse, and improve the transformation of additional genotypes through hybrid transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bread wheat (Triticum aestivum L.; 2n = 6x = 42; AABBDD) is one of the most cultivated crops in the world and represents almost 20% of protein sources for human nutrition (FAO STAT). To face the increase in world population, the improvement of bread wheat performances is a major agricultural challenge, especially within the context of sustainable agriculture with reduced uses of fertilizers, water and pesticides. One way to face this challenge is to use the huge reservoir of the exotic or related genetic resources to find new original and powerful alleles for the genes of agronomical interest. These new alleles can then be introduced into elite material through classical crosses and recombination between the appropriate segments of the genome. However, the frequency of meiotic recombination in wheat is low (average 0.2 cM/Mb; Choulet et al. 2014) and almost restricted to ~ 20% of the distal regions of the chromosomes (Saintenac et al. 2009; Darrier et al. 2017). As a consequence, low recombination rate results in massive undesirable linkage drag, if ones wish to introduce, in elite material, the desirable new alleles originating from exotic cultivars or wild species. To avoid this drawback, new alleles can be introduced through genetic transformation and/or genome-editing biotechnologies (Jones 2005; Wang et al. 2014; Zong et al. 2017; Kim et al. 2018; Liang et al. 2018; Sánchez-León et al. 2018).
Agrobacterium mediated transformation (Cheng et al. 1997; Carlos Popelka and Altpeter 2003; Przetakiewicz et al. 2004) and Biolistic® (Vasil et al. 1992; Wang et al. 2014) are successfully used for transformation of a wide range of European (Rasco-Gaunt et al. 2001) and Chinese (Zhang et al. 2015) elite varieties. The performance of Biolistic (or particle bombardment) mainly limited by the lack of plant regeneration depends on several factors: particle type, size, quantity and projection speed, the amount, structure and quality of DNA but also on the tissue type and pretreatment (for review see Altpeter et al. 2016). Traditionally, tissues with totipotent cells (callus, somatic embryos, etc.) and treated with growth regulators (auxins and cytokinins) are best transformed. Stresses of different nature (dark, protoplasting) also seems to play an important role in plant regeneration (Florentin et al. 2013; Fehér 2015; Grafi and Barak 2015).
Even though biolistic has been improved and made flexible, some wheat genotypes remain difficult to transform (recalcitrant) whereas others are responsive (Shrawat and Lörz 2006; Hiei et al. 2014). For example, the reference cultivar for in vitro culture, Bobwhite, has a transformation efficiency ranging from 2.5 to 3–6% (Tassy et al. 2014) while Courtot, a French winter wheat that is responsive to biolistic, has a transformation efficiency of only 1.47% (49 transformed plants over 3326 shooted immature embryos, Tassy and Barret 2017). Chinese Spring, a spring wheat used as a reference cultivar for genome sequencing (Choulet et al. 2014; International Wheat Genome Sequencing Consortium (IWGSC) 2014; International Wheat Genome Sequencing Consortium (IWGSC) 2018) is a recalcitrant genotype for biolistic that cannot be transformed (0 transformed plants over at least 3000 shooted immature embryos; our unpublished results). On the contrary, Chinese Spring is considered to have a high regeneration efficiency when not submitted to biolistic (Machii et al. 1998).
Since fertile transgenic wheat plants regenerate only from a few target cell-types, the choice of the explant for genetic transformation is a critical step. Three types of tissues are usually used for transgenic plant production: immature inflorescence and scutellum of mature or immature zygotic embryos. Quality of mature embryos depends on the maturation conditions of the plant. Stresses such as drought, heat chock or pathogen aggression during maturation of the seed can lead to weak embryos (Samarah 2005) and to regeneration failures. Furthermore, extraction of mature embryos is quite difficult because they adhere to the endosperm and external seed layers, which results in their deterioration while removing them from the seeds whereas immature embryos are free inside the immature seed so they can be removed easily. However, transformation efficiency between mature and immature embryos fluctuate between wheat genotypes (Özgen et al. 1998).
In wheat, production of hybrid immature embryos is tedious and encounters a problem of synchronization (material impossibility to obtain enough embryos at the right stage at the same time to achieve the genetic transformation). There is a short period of optimal collection (around 14 days after pollination, dap; Pastori et al. 2001; Tassy et al. 2014) and it is an arduous task to harvest enough immature embryos at the right stage (around 1 mm long) for transformation. Moreover, we experienced fluctuation in the success rate of crosses and found that production of a required amount of seed can represent three times the amount of work expected (Online Resource 1). Li et al. (2003) used cold temperature (4 °C) to store immature spike collected few days (10–16) post anthesis to enhanced somatic embryogenesis. Cold was usually used for vernalization of winter crops such as winter wheats and it reduces or even stops plant growth and induces its flowering (Dennis and Peacock 2009). Li et al. (2003) also suggested that a long period of cold storage of spikes is necessary for efficient somatic embryogenesis.
In the present study, we report the possibility of stopping embryo growth by shelling and keeping immature seeds at cold temperature (4 °C) for a few days (4–7 days), and show that the transformation rate obtained for a hybrid line is similar to that of the most responsive parent.
Materials and methods
An add-on to this section is given in Online Resource 2.
Plant culture and embryo production
Three bread wheat genotypes were used for this experiment: Bobwhite (BW; cultivar BWS26), Chinese Spring (CS) and Courtot (Ct). Donor plants of immature embryos were grown in a dedicated greenhouse under controlled climatic conditions with a 16 h/8h and 22 °C/18 °C day/night cycle. The resulting T0 (original transgenic generation) transgenic plants were cultivated in a dedicated GMO greenhouse under the same conditions. Hybrid seeds were produced using Ct as male and CS as female. As many flowers as possible per spike were emasculated and fertilized with Ct fresh pollen 2–3 days after castration. Around eight spikes a day, 3 days a week were emasculated, to match Ct pollen availability.
Cold conservation and embryo measurement
At 11–12 days after pollination, depending on the culture conditions, we harvested immature seeds (IS) and sterilized them in 70% ethanol for 1 min. Then the IS are rinsed with autoclaved pure water and allowed to dry on sterile absorbent paper. We stored the sterilized IS in a 50 mL plastic tube either at 4 °C in a cold chamber or on the bench at room temperature (RT). For measurement, immature embryos (IE) are extracted from IS and placed on plasmolysis medium (Tassy et al. 2014). To measure the IE sizes, a picture of each set of IE is taken on millimeter paper using a stereomicroscope. Each picture is analyzed with the imageJ Measure tool (https://imagej.nih.gov/ij/docs/guide/146.html, subsection 30.1) to determine IE sizes that are given in pixels and translated into millimeters using the references on the millimeter paper.
Preparation of immature embryos (IE) or hybrid immature embryos (HIE), shooting and in vitro culture
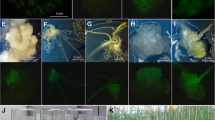
Preparation of IE (or HIE) and shooting are performed as described in Tassy et al. (2014). IE (or HIE) were extracted and their radicula removed. IE (or HIE) prepared according to the Tassy et al. (2014) protocol are thereafter still designated as IE (or HIE). Some modifications were made to the Tassy et al. (2014) shooting protocol: for 10 shootings, 200–250 ng of DNA were coated to gold micro particles. In case of co-transformation with two DNA fragments, both fragments are added in equimolar ratio according to the size of each fragment. DNA fragments for shooting were prepared as described in Tassy et al. (2014), using gene cassettes (Breitler et al. 2002; Yao et al. 2007). In vitro culture was performed as described in Tassy et al. (2014), on Phospho-Mannose Isomerase (PMI) selective media (Fig. 1, Wright et al. 2001; Stoykova and Stoeva-Popova 2011). Briefly, after one night at 30 °C, freshly transformed IE (or HIE) are put on callogenesis medium for 2 weeks. Generated calli are transferred to two consecutives selective media for organogenesis (leaves) for 2 weeks each. The second medium is less selective than the first one to let the regenerating plantlets grow properly. Regenerated plantlets are then transferred to a third selective medium for final selection of transformed ones. This step avoids escape events (plants that survive selection but that are not transformed). In the end, after 2 weeks, plantlets selected on the third medium are transferred to a fourth selective medium for root organogenesis. Once the plantlets have developed roots and leaves in sterile IVC containers, each of them are transferred individually in small pots with germination soil and placed in an acclimation cabinet for 2 weeks with a 16 h/8 h and 22 °C/18 °C day/night cycle and 60% of moisture, awaiting for PCR analysis.
IVC steps following biolistic protocol from IE (or HIE) to T1 (original transgenic generation’s first progeny) seeds. a Embryos just after transformation. b Callogenesis step. c First organogenesis step (leaves) on the first selective medium. d, e Leaf development on the second selective medium. f Leaf development on the third selective medium. g Second organogenesis step (roots) and leaf development just before transfer to greenhouse. h Plantlets transferred in small pots in acclimation chamber. At this stage, plantlets are not big enough for DNA sampling (PCR analysis). 1 or 2 weeks are needed to allow the plant to grow in acclimation chamber. i After 2 weeks in acclimation chamber (with additional 2 months at 6 °C for winter genotypes only), T0 transgenic plants (positives to PCR) are transferred to 4L pots with cultivation soil in a dedicated place. j Flowering T0 transgenic plants. Each spike is isolated in a moisture permeable plastic bag for auto-pollination. k Fully fertile T0 transgenic spikes. l T1 seeds collected from T0 transgenic spikes, each plastic bag represent T1 seeds collected from one T0 transgenic plant
Analysis of transgenic plants
Transgenic plant DNA was extracted using Oktopure automated DNA extraction system and sbeadex™ plant DNA extraction kit (LGC Limited©). Transgenic plants were detected by PCR using specific primers for the DNA fragments inserted and Amplitaq Gold® 360 Master Mix and protocol (Applied Biosystems™). Transformation was assessed by PCR on transgene (here PMI gene with primers PMI1 and PMI2, Online Resource 3) with, if possible, a maximum of three leaves samples from three different stems of the same plant. To avoid chimeras, plants were considered as transformed when all samples of the same plant are positive to PCR. Microsatellite specific primers were used on each sample (WMS257, Online Resource 3) as a PCR control. Melting temperatures are specified in Online Resource 3.
Calculations and statistics
Regeneration and transformation rates were determined using the initial number of embryos and the final numbers of respectively regenerated and transformed plants. To generate the violin plots for embryo sizes repartition, R ggplot function was used in association with geom_violin and geom_boxplot functions from R package ggplot2. To assess the significance between the results obtained and the control, R wilcox.test function (for embryo size repartitions comparison) and R t-test function (for regeneration capacity comparison) were applied with a threshold of α = 0.05. R software (version 3.4.3) was exploited with Rstudio (version 1.1.423).
Results
Shelling stops the growth of the IE and cold storage preserves its embryogenetic capacity
To analyze the impact of shelling and cold storage on wheat IE, we collected 796 IS at 11 days after pollination from thirty different spikes of BW, each IS giving one IE. IS from ten spikes were attributed to the control set (BW0, 192 IS). IS from the twenty spikes left were divided into four test sets (IS from five spikes each). To test the impact of shelling, two sets were kept at RT during 4 days (155 IS, BW4RT) and 7 days (161 IS, BW7RT). We then extracted the IE of each IS and measured their size, in comparison to BW0’s IE extracted right after collection as described in “Materials and methods" (Fig. 2). After 4 days at RT (BW4RT), the mean size of the IE (0.85 ± 0.19 cm) was not significantly different from the control (BW0: 0.89 ± 0.22 mm; p-value = 0.06676). On the contrary, after 7 days at RT (BW7RT), the mean size of the IE was 0.77 ± 0.15 mm, significantly lower than the control (p-value = 1.921e−08). Then, to test the impact of cold storage, we conducted the same experiment on the other two sets by storing the IS at 4 °C for 4 and 7 days (BW4C, BW7C) and the mean size of the IE was 0.87 ± 0.15 mm and 0.92 ± 0.17 mm, respectively. This is not significantly different from the control (BW0 0.89 ± 0.22 mm; p-value = 0.4496 and 0.1804, respectively).
Distribution of Bobwhite (BW) IE sizes in millimeters for IE stored at RT for 4 and 7 days (BW4RT and BW7RT) and at 4 °C (C) during 4 or 7 days after collection (BW4C, BW7C). BW0 is the control. BW IE were extracted from IS and then measured as described in “Materials and methods”. Violin plots: distribution of all embryo sizes for each set. Boxplots: white boxes: lower and upper quartile for each set, dashed lines: Median size of embryos for each set, circles: mean size of embryos for each set, black dots: outliers
Next, to test the embryogenic capacity and the transformability of the IE stored at RT or 4 °C, we conducted a transformation experiment for the PMI gene on the previous five IE sets with Biolistic® and placed them on successive mannose selective media (Tassy et al. 2014). For the control set, 0% of the IE aborted, 100% formed a callus, 9% generated a plantlet and 4% generated a transformed plantlet (Fig. 3). In contrast, for RT-stored sets, we observed that 76% and 100% of the IE aborted after 4 and 7 days, respectively. Only 2% of the total IE generated a plantlet after 4 days at RT, which is significantly lower than the control (p-value = 0.002, α = 0.05) and 1% of the total embryos generated a plantlet that were transformed with PMI gene, which is not significantly different from control (p-value = 0.15, α = 0.05). However, callus that formed a plantlet represents 8% of the callus formed after 4 days at RT, which is not significantly different from the control (p-value = 0.80, α = 0.05) and callus that formed a transformed plantlet represents 5% of the callus formed after 4 days at RT, which is not significantly different from the control (p-value = 0.66, α = 0.05). We then observed that after 4 or 7 days at 4 °C, respectively 87% and 99% of the embryos formed a callus and respectively 13% and 16% of the callus generated a plantlet, which is not significantly different from the control (p-value = 0.35 and 0.06, respectively, α = 0.05). Callus that generated a transformed plantlet for BW4C et BW7C represent 1% and 3% of the total embryo set, which is not significantly different from the control (p-value = 0.06 and 0.91, α = 0.05).
Regeneration capacity and transformability of embryos kept at RT during 4 or 7 days after collection (BW4RT and BW7RT) and at 4 °C during 4 or 7 days after collection (BW4C, BW7C). BW0 is the control. White: aborted embryos (IE that did not form a callus on the cell multiplication media). Light grey: non-embryogenic callus (IE that formed a callus on selective media but that did not generate a plantlet). Grey: embryogenic callus (IE that formed a callus and generated a plantlet). Dark grey: transformed plantlets (plantlets generated from embryogenic callus and positives to PMI PCR)
The number of embryos that formed a callus and further developed a plantlet represents respectively 15% and 17% of the callus formed for BW4C and BW7C, which is not significantly different from the control (p-value = 0.18 and 0.06, respectively, α = 0.05). Then the number of embryos that formed a callus and further developed a transformed plantlet represents respectively 1% and 4% of the callus formed for BW4C and BW7C, which is not significantly different from the control (p-value = 0.07 and 0.92, respectively, α = 0.05).
Hybrid embryos transform as the best transformable parent
We performed Biolistic transformation as described in “Materials and methods” using HIE derived from the cross between Chinese Spring (CS female) and Courtot (Ct male; CS × Ct) kept at 4 °C for 3–5 days. Ct and CS exhibit a transformation efficiency of 1.5% (Tassy and Barret 2017) and 0% (our unpublished results), respectively. For the first set of 535 HIE, we obtained 10 PCR-validated transformed plants. For the second and the third sets of 615 and 1901 HIE respectively, we obtained 14 and 29 PCR validated transformed plants, respectively. This represents 1.9%, 2.3% and 1.5%, for HIE sets 1, 2 and 3, respectively. This is not significantly different from the results obtained with Ct alone (Table 1), indicating that the transformation efficiency of Ct is dominant compared to that of CS. Fertility level of resulting transgenic plants is normal with a mean of 18 ± 5 seeds per spike (on 7 independent transformation events).
Discussion
The construction of transgenic plants is a valuable approach for candidate gene validation and genome editing but is limited by two main factors: the transformation efficiency that remains relatively low and genotype dependent (< 5% for the best genotypes; Tassy et al. 2014), and the availability of sufficient amount of appropriate material (here, IE).
Shelling stops the growth of the embryos
Here, we observed no difference between sizes of the fresh IE and those stored at RT for 4 days or kept at 4 °C for 4 or 7 days. Thus, our results are in accordance with data obtained in carrot (Daucus carota L.; Geard et al. 2007), where the maximum seed size is achieved 40–60 days after full blooming while embryo development continues up 30–50 days after. The results suggested that early shelling blocks the development of embryos in carrot leading to poor seed quality. However, the embryos kept at RT for 7 days are significantly smaller than fresh embryos. Between 10 and 15 days post anthesis, the embryo is in an exponential growth phase (Golovina et al. 2001; Fábián et al. 2011) with a growth of 0.5 mm per day. In wheat, the difference between the control and BW7RT is of 0.08 mm, representing approximatively 4 h during the exponential growth phase. BW7RT may have been sampled earlier than the control BW0, regarding the days post-anthesis, and this may justify the small difference in size.
Cold storage preserves embryogenetic capacity of immature embryos
Previous studies described the positive effect of cold treatment on somatic embryogenesis in Maize (Zea mais, Pescitelli et al. 1990), Oat (Avena sativa, Kiviharju and Pehu 1998), Prairie Milkvetch (Astragalus adsurgens, Luo et al. 2003), Patula Pine (Pinus patula, Malabadi and Staden 2006), Oilseed Rape (Brassica napus, Gu et al. 2004), and Monterey Pine (Pinus radiata, Montalbán et al. 2015). In bread wheat (Triticum aestivum L.), the storage of spikes collected 11–12 days post anthesis (DPA) during 13 to 16 days at 4 °C was found to significantly affect the percentage of embryo’s scutella producing somatic embryos (Li et al. 2003). They reported that a long period of spike storage at 4 °C is necessary for efficient somatic embryogenesis. In our experiments, we see any significant difference neither in regeneration efficiency nor in transformability compared to the control and we found no effect of short cold-storage period on somatic embryogenesis. This is in total accordance with their results, as we did not perform a long period of storage at 4 °C.
On the contrary, storage at RT abolishes callus formation upon 7 days conservation. Lublin and Sela (2008) showed that when stored at RT, pathogens present at the surface of an egg are capable of multiplication to large numbers. However, at low temperature (6 °C), even if the pathogen can survive up to 6 weeks, it is not able to multiply and infect the egg. Nevertheless, we performed only a surface treatment on the IS and bacteria could have been introduce inside the IS through stinging insects (Thysanoptera, Bailey 1935). As bacterial infection is likely to happen at RT, IS stored at RT can develop internal bacterial infection affecting the IE, making it unsuitable for genetic transformation. In contrast, cold storage is likely to reduce bacterial spreading, preventing infection of the IE and preserving its integrity and embryogenic ability.
Hybrid transformation
Like in maize (Hodges et al. 1986), we found that wheat hybrid embryos derived from a cross between Chinese Spring (recalcitrant parent) and Courtot (responsive parent) retain an equivalent embryogenic capacity than the embryos of the best transformable parent. It could be due to the heterosis effect (Birchler et al. 2010) but as we found no evidence of a such mecanism in in vitro tissue cultures, we conclude that the transformation capacity could be a heritable trait dominant in Courtot compared to Chinese Spring. Transformability and more generally tissue-culture response is a trait that is genetically controlled and several QTLs have been identified (Amer et al. 1997; Bolibok and Rakoczy-Trojanowska 2006; Jia et al. 2007). Gene transfer in wheat has also long been limited to highly responsive genotypes such as Bobwhite and Florida, selected for their good response in tissue culture and amenability to transformation. However, transformation of elite breeding lines remains difficult, mainly ranging between 1–5% (Rasco-Gaunt et al. 2001; Tassy et al. 2014). Only a few elite genotypes (15%) exhibited percentages of ~ 6–8% (Rasco-Gaunt et al. 2001) slightly higher than the reference cultivar Bobwhite (~ 3–5%; Tassy et al. 2014).
Conclusion
Here we reported that shelling associated to cold conservation provides the possibility to store multiple sets of IS up to 7 days while keeping the optimal embryogenic ability of the IE until transformation. This reduces the number of plants that is necessary to cultivate and so the cost of a transformation experiment. We also showed that the best transformation capacity of a parental line is retain in hybrid embryos. Altogether, these results optimize the wheat biolistic protocol for genome engineering and transgenic applications.
References
Altpeter F, Springer NM, Bartley LE et al (2016) Advancing crop transformation in the era of genome editing. Plant Cell 28:1510–1520. https://doi.org/10.1105/tpc.16.00196
Amer IB, Worland AJ, Korzun V, Börner A (1997) Genetic mapping of QTL controlling tissue-culture response on chromosome 2B of wheat (Triticum aestivum L.) in relation to major genes and RFLP markers. TAG Theor Appl Genet 94:1047–1052. https://doi.org/10.1007/s001220050513
Bailey SF (1935) Thrips as vectors of plant disease. J Econ Entomol 28:856–863. https://doi.org/10.1093/jee/28.6.856
Birchler JA, Yao H, Chudalayandi S et al (2010) Heterosis. Plant Cell 22:2105–2112. https://doi.org/10.1105/tpc.110.076133
Bolibok H, Rakoczy-Trojanowska M (2006) Genetic mapping of QTLs for tissue-culture response in plants. Euphytica 149:73–83. https://doi.org/10.1007/s10681-005-9055-6
Breitler J-C, Labeyrie A, Meynard D et al (2002) Efficient microprojectile bombardment-mediated transformation of rice using gene cassettes. TAG Theor Appl Genet 104:709–719. https://doi.org/10.1007/s00122-001-0786-z
Carlos Popelka J, Altpeter F (2003) Agrobacterium tumefaciens-mediated genetic transformation of rye (Secale cereale L.). Mol Breed 11:203–211. https://doi.org/10.1023/A:1022876318276
Cheng M, Fry JE, Pang S et al (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980. https://doi.org/10.1104/pp.115.3.971
Choulet F, Alberti A, Theil S et al (2014) Structural and functional partitioning of bread wheat chromosome 3B. Science 345:1249721. https://doi.org/10.1126/science.1249721
Darrier B, Rimbert H, Balfourier F et al (2017) High-resolution mapping of crossover events in the hexaploid wheat genome suggests a universal recombination mechanism. Genetics 206:1373–1388. https://doi.org/10.1534/genetics.116.196014
Dennis ES, Peacock WJ (2009) Vernalization in cereals. J Biol 8:57. https://doi.org/10.1186/jbiol156
Fábián A, Jäger K, Rakszegi M, Barnabás B (2011) Embryo and endosperm development in wheat (Triticum aestivum L.) kernels subjected to drought stress. Plant Cell Rep 30:551–563. https://doi.org/10.1007/s00299-010-0966-x
Fehér A (2015) Somatic embryogenesis—stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849:385–402. https://doi.org/10.1016/j.bbagrm.2014.07.005
Florentin A, Damri M, Grafi G (2013) Stress induces plant somatic cells to acquire some features of stem cells accompanied by selective chromatin reorganization. Dev Dyn 242:1121–1133. https://doi.org/10.1002/dvdy.24003
Geard A, Spurr CJ, Brown PH (2007) Embryo development and time of cutting in cool temperate carrot seed crops. In: Adkins SW, Ashmore S, Navie SC (eds) Seeds: biology, development and ecology. Proceedings of the eighth international workshop on seeds. CABI, Wallingford, pp 120–129
Golovina EA, Hoekstra FA, Van Aelst AC (2001) The competence to acquire cellular desiccation tolerance is independent of seed morphological development. J Exp Bot 52:1015–1027. https://doi.org/10.1093/jexbot/52.358.1015
Grafi G, Barak S (2015) Stress induces cell dedifferentiation in plants. Biochim Biophys Acta 1849:378–384. https://doi.org/10.1016/j.bbagrm.2014.07.015
Gu HH, Hagberg P, Zhou WJ (2004) Cold pretreatment enhances microspore embryogenesis in oilseed rape (Brassica napus L.). Plant Growth Regul 42:137–143. https://doi.org/10.1023/B:GROW.0000017488.29181.fa
Hiei Y, Ishida Y, Komari T (2014) Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front Plant Sci 5:628. https://doi.org/10.3389/fpls.2014.00628
Hodges TK, Kamo KK, Imbrie CW, Becwar MR (1986) Genotype specificity of somatic embryogenesis and regeneration in maize. Nat Biotechnol 4:219–223. https://doi.org/10.1038/nbt0386-219
International Wheat Genome Sequencing Consortium (IWGSC) (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788. https://doi.org/10.1126/science.1251788
International Wheat Genome Sequencing Consortium (IWGSC) (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:7191. https://doi.org/10.1126/science.aar7191
Jia H, Yi D, Yu J et al (2007) Mapping QTLs for tissue culture response of mature wheat embryos. Mol Cells 23:323–330
Jones HD (2005) Wheat transformation: current technology and applications to grain development and composition. J Cereal Sci 41:137–147. https://doi.org/10.1016/j.jcs.2004.08.009
Kim D, Alptekin B, Budak H (2018) CRISPR/Cas9 genome editing in wheat. Funct Integr Genomics 18:31–41. https://doi.org/10.1007/s10142-017-0572-x
Kiviharju E, Pehu E (1998) The effect of cold and heat pretreatments on anther culture response of Avena sativa and A. sterilis. Plant Cell Tissue Organ Cult 54:97–104. https://doi.org/10.1023/A:1006167306638
Li B, Caswell K, Leung N, Chibbar RN (2003) Wheat (Triticum aestivum L.) somatic embryogenesis from isolated scutellum: days post anthesis, days of spike storage, and sucrose concentration affect efficiency. In Vitro Cell Dev Biol 39:20–23. https://doi.org/10.1079/IVP2002356
Liang Z, Chen K, Zhang Y et al (2018) Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat Protoc 13:413–430. https://doi.org/10.1038/nprot.2017.145
Lublin A, Sela S (2008) The impact of temperature during the storage of table eggs on the viability of Salmonella enterica serovars enteritidis and virchow in the eggs. Poult Sci 87:2208–2214. https://doi.org/10.3382/ps.2008-00153
Luo J, Jiang S, Pan L (2003) Cold-enhanced somatic embryogenesis in cell suspension cultures of Astragalus adsurgens Pall.: relationship with exogenous calcium during cold pretreatment. Plant Growth Regul 40:171–177. https://doi.org/10.1023/A:1024295901808
Machii H, Mizuno H, Hirabayashi T et al (1998) Screening wheat genotypes for high callus induction and regeneration capability from anther and immature embryo cultures. Plant Cell Tissue Organ Cult 53:67–74. https://doi.org/10.1023/A:1006023725640
Malabadi RB, van Staden J (2006) Cold-enhanced somatic embryogenesis in Pinus patula is mediated by calcium. S Afr J Bot 72:613–618. https://doi.org/10.1016/j.sajb.2006.04.001
Montalbán IA, García-Mendiguren O, Goicoa T et al (2015) Cold storage of initial plant material affects positively somatic embryogenesis in Pinus radiata. New Forest 46:309–317. https://doi.org/10.1007/s11056-014-9457-1
Özgen M, Türet M, Altınok S, Sancak C (1998) Efficient callus induction and plant regeneration from mature embryo culture of winter wheat (Triticum aestivum L.) genotypes. Plant Cell Rep 18:331–335. https://doi.org/10.1007/s002990050581
Pastori GM, Wilkinson MD, Steele SH et al (2001) Age-dependent transformation frequency in elite wheat varieties. J Exp Bot 52:857–863. https://doi.org/10.1093/jexbot/52.357.857
Pescitelli SM, Johnson CD, Petolino JF (1990) Isolated microspore culture of maize: effects of isolation technique, reduced temperature, and sucrose level. Plant Cell Rep 8:628–631. https://doi.org/10.1007/BF00270070
Przetakiewicz A, Karaś A, Orczyk W, Nadolska-Orczyk A (2004) Agrobacterium-mediated transformation of polyploid cereals. The efficiency of selection and transgene expression in wheat. Cell Mol Biol Lett 9:903–917
Rasco-Gaunt S, Riley A, Cannell M et al (2001) Procedures allowing the transformation of a range of European elite wheat (Triticum aestivum L.) varieties via particle bombardment. J Exp Bot 52:865–874. https://doi.org/10.1093/jexbot/52.357.865
Saintenac C, Falque M, Martin OC et al (2009) Detailed recombination studies along chromosome 3B provide new insights on crossover distribution in wheat (Triticum aestivum L.). Genetics 181:393–403. https://doi.org/10.1534/genetics.108.097469
Samarah NH (2005) Effects of drought stress on growth and yield of barley. Agron Sustain Dev 25:145–149. https://doi.org/10.1051/agro:2004064
Sánchez-León S, Gil-Humanes J, Ozuna CV et al (2018) Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J 16:902–910. https://doi.org/10.1111/pbi.12837
Shrawat AK, Lörz H (2006) Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol J 4:575–603. https://doi.org/10.1111/j.1467-7652.2006.00209.x
Stoykova P, Stoeva-Popova P (2011) PMI (manA) as a nonantibiotic selectable marker gene in plant biotechnology. Plant Cell Tissue Organ Cult 105:141–148. https://doi.org/10.1007/s11240-010-9858-6
Tassy C, Barret P (2017) Biolistic transformation of wheat. In: Bhalla P, Singh M (eds) Wheat biotechnology. Methods in molecular biology, vol 1679. Humana Press, New York, pp 141–152
Tassy C, Partier A, Beckert M et al (2014) Biolistic transformation of wheat: increased production of plants with simple insertions and heritable transgene expression. Plant Cell Tissue Organ Cult 119:171–181. https://doi.org/10.1007/s11240-014-0524-2
Vasil V, Castillo AM, Fromm ME, Vasil IK (1992) Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Nat Biotechnol 10:667–674. https://doi.org/10.1038/nbt0692-667
Wang Y, Cheng X, Shan Q et al (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947–951. https://doi.org/10.1038/nbt.2969
Wright M, Dawson J, Dunder E et al (2001) Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep 20:429–436. https://doi.org/10.1007/s002990100318
Yao Q, Cong L, He G et al (2007) Optimization of wheat co-transformation procedure with gene cassettes resulted in an improvement in transformation frequency. Mol Biol Rep 34:61–67. https://doi.org/10.1007/s11033-006-9016-8
Zhang K, Liu J, Zhang Y et al (2015) Biolistic genetic transformation of a wide range of Chinese elite wheat (Triticum aestivum L.) varieties. J Genet Genomics 42:39–42. https://doi.org/10.1016/j.jgg.2014.11.005
Zong Y, Wang Y, Li C et al (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat Biotechnol 35:438–440. https://doi.org/10.1038/nbt.3811
Acknowledgements
Members of the team CPCC are greatly acknowledged for taking care of the plants. Members of the team ValFon are also acknowledged for helpful discussions and for providing with all facilities for biolistic transformation. RM is funded by ANRT CIFRE Grant No. 2014/1020.
Author information
Authors and Affiliations
Contributions
RM, CT, MCD, AL and MB conducted the experiments; RM analyzed all data; RM, PB and PS conceived this work and wrote the paper. GB and AN reviewed the paper. All authors contributed in the writing of this paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Francisco de Assis Alves Mourão Filho.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michard, R., Batista, M., Debote, MC. et al. Cold-conserved hybrid immature embryos for efficient wheat transformation. Plant Cell Tiss Organ Cult 136, 365–372 (2019). https://doi.org/10.1007/s11240-018-1521-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1521-7