Abstract
Indirect somatic embryogenesis (ISE) is required for plant propagation and a prerequisite for applications that may provide new germplasms. Genetic, epigenetic and physiological features of the explant donor are barriers for ISE establishment, hindering its wide use. Despite the identification and/or expression analysis of genes during ISE, no approach to establish the karyotype aspects has been performed so far. So, this study aims to establish the ISE and compare the in vitro responses between diploid (Coffea canephora and Coffea eugenioides), allotriploid (“Híbrido de Timor”—HT) and true allotetraploid (Coffea arabica) Coffea in a taxonomic and evolutive scenario. Under the same in vitro conditions, the four Coffea differed from each other during ISE. Leaf explants of the true allopolyploids yielded the highest mean number of friable calli (FC) in relative short time and visually exhibiting more pronounced length. FC of the allotetraploid C. arabica presented the highest mean number of mature cotyledonary somatic embryos (MCSE), which were also recovered faster in this species. However, MCSE mean number in HT was the same or lower than diploid Coffea. Besides, intraspecific variation related to the ISE responses was observed in each Coffea, mainly the mean number of FC obtained from ex vitro and in vitro C. arabica and C. eugenioides explants. So, epigenetic and physiologic features may also have influenced the ISE responses. The findings provide the basis for performing other approaches considering the ploidy level, epigenetic and physiological backgrounds. Besides, the data also contributed for understanding about the consequences of polyploidy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indirect somatic embryogenesis (ISE), an approach in which somatic embryos originate from calli (Williams and Maheswaran 1986), represents the basis for the development of tissue culture applications, for instance: in vitro propagation (Staritsky 1970; van Boxtel and Berthouly 1996; Samson et al. 2006), germplasm conservation (Etienne et al. 2002), androgenesis (Herrera et al. 2002), genetic transformation (Gatica-Arias et al. 2008), production of secondary metabolites (Karuppusamy 2009), and chromosome set doubling (Murashige and Nakano 1966; Sanglard et al. 2017). Experimental conditions to achieve an ISE response, from cell dedifferentiation to plantlet regeneration, are specific to the genetic, epigenetic and/or physiological features of the explant (Fehér 2015). For this reason, ISE has been established mainly in phylogenetically close taxa, such as within the Coffea genus (Staritsky 1970; van Boxtel and Berthouly 1996; Samson et al. 2006).
ISE in Coffea emerged in the 1970s (Staritsky 1970) as a strategy to propagate the species with agronomic relevance, such as Coffea canephora Pierre ex Froehner and Coffea arabica L. For these Coffea (van Boxtel and Berthouly 1996; Samson et al. 2006; Almeida et al. 2008; Ibrahim et al. 2015), in vitro conditions have differed in relation to physical (photoperiod—light/dark ratio) and chemical aspects (mainly concentration of macro- and micro-nutrients, as well as type, combination and concentration of growth regulators) for induction of friable calli (FC) from leaf explants, and for regeneration of somatic embryos (SE) and plantlets.
Similarly to chemical and physical tissue culture conditions, genetic features also influence the ISE responses (Fehér 2015). Substantial differences between in vitro responses (i.e. time and rate) associated with FC induction and/or SE and plantlet recovery have been reported for the diploid C. canephora, the true allotetraploid C. arabica (Staritsky 1970; van Boxtel and Berthouly 1996; Samson et al. 2006), the diploids Coffea heterocalyx Stoffelen and Coffea sp. Moloundou (Samson et al. 2006), the allopolyploid ‘Arabusta’ (C. arabica × C. canephora), and the homoploid ‘Congusta’ (Coffea congensis Fr. × C. canephora) (van Boxtel and Berthouly 1996). The genomic origin of each Coffea was defined here according to cytogenetic concepts associated to data reported by van Boxtel and Berthouly (1996) and basic chromosome number in Coffea (x = 11). van Boxtel and Berthouly (1996), who proposed the tissue culture condition most applied in Coffea, considered the leaf explants of the diploid C. canephora, allopolyploid ‘Arabusta’ and homoploid ‘Congusta’ to be more responsive toward FC formation than the true allotetraploid C. arabica. The time for plantlet regeneration also varied among these Coffea, comprising 7–8 months for C. canephora and ‘Arabusta’ and 9–10 for C. arabica.
Thus, there are differences among the ISE responses obtained in Coffea (van Boxtel and Berthouly 1996; Samson et al. 2006) with pronounced karyotype attributes. C. canephora, differentiated ~ 4.2 mya (million years ago) (Yu et al. 2011), presents 2C = 1.41 picograms (pg), 2n = 2x = 22 chromosomes, two sites of 5S rDNA (Hamon et al. 2009), and one chromosome (6) with secondary constriction (SC) (Clarindo and Carvalho 2006) corresponding to one 45S rDNA site (Hamon et al. 2009). C. arabica, a true allotetraploid (C. canephora × Coffea eugenioides Moore; Lashermes et al. 1999; Yu et al. 2011; Hamon et al. 2015), originated ~ 0.665 mya (Yu et al. 2011), possesses 2C = 2.62 pg, 2n = 4x = 44 chromosomes (Clarindo and Carvalho 2009), two sites of 5S rDNA (Hamon et al. 2009), and three chromosomes (14, 20, 21) with SC (Pinto-Maglio and Da Cruz 1998) relative to three 45S rDNA sites (Hamon et al. 2009).
Considering all the above, the present study aimed to revisit, in a taxonomic and evolutive scenario, the ISE response in Coffea with marked karyotype features. For this purpose, C. eugenioides, as a possible progenitor of C. arabica (Lashermes et al. 1999; Hamon et al. 2015), was one of the species included in the analyses. C. eugenioides presents 2C = 1.36 pg (Noirot et al. 2003), 2n = 2x = 22 chromosomes, one 5S rDNA site, and two 45S rDNA sites (Hamon et al. 2009). However, since no in vitro tissue culture study has been conducted in this species so far, at first ISE should be established for C. eugenioides in order to compare its morphogenic responses with those of other Coffea.
“Híbrido de Timor” (HT) (C. arabica × C. canephora) is an allopolyploid Coffea relevant to clarify the matter at hand, owing to its ancestry and evolutionary origin. This natural allopolyploid originated ~ 100 years ago on the Timor Island (Bettencourt 1973). HT ‘CIFC 4106’, the probable first HT plant, is an allotriploid (anorthoploid—odd number of chromosome sets) with 1C = 2.10 pg and 2n = 3x = 33 chromosomes (Clarindo et al. 2013), and has been recently propagated via direct somatic embryogenesis (Sattler et al. 2016) and ISE (Sanglard et al. 2017).
Considering the divergences observed in the ISE responses among Coffea germplasms (van Boxtel and Berthouly 1996; Samson et al. 2006), the following aims were pursued for C. eugenioides, C. canephora, C. arabica and HT ‘CIFC 4106’: (a) to establish the ISE; (b) to confirm the chromosome number, ploidy level, and nuclear DNA content; and (c) to compare the four Coffea in relation to ISE responses. Besides, this study also showed variation in the ISE responses obtained from explants collected from ex vitro and in vitro donor plants.
Materials and methods
Biological material
Leaves were collected from three individuals of C. canephora, C. eugenioides, HT ‘CIFC 4106’ and C. arabica (explant donors). C. eugenioides and C. arabica ‘Catuaí Vermelho’ had been grown in greenhouse of the Coffea germplasm bank of the Universidade Federal de Viçosa (Minas Gerais, Brazil, 20°45′S, 42°52′W) under controlled phytosanitary and environmental conditions. C. canephora and HT ‘CIFC 4106’ plantlets were obtained via direct somatic embryogenesis and had been propagated in vitro (Universidade Federal do Espírito Santo, Espírito Santo, Brazil) in medium consisting of MS (Murashige and Skoog 1962) salts, 10 mL L−1 Gamborg’s B5 vitamins (Gamborg et al. 1968), 30 g L−1 sucrose, and 7.0 g L−1 Agar (Sigma®) (Sattler et al. 2016). Collected leaves from plants cultivated in greenhouse (C. eugenioides and C. arabica) and in vitro culture (C. canephora and HT ‘CIFC 4106’) were used as explant sources for the first ISE experiment, as well as for nuclear DNA content measurement and DNA ploidy level determination. After regeneration of the four Coffea, a second and a third ISE experiments were established from leaf explants collected of in vitro propagated plantlets.
Nuclear DNA content and DNA ploidy level determination of the explant donors
Leaf fragments of each Coffea explant donor plant and of the internal standard Solanum lycopersicum L. (2C = 2.00 pg, Praça-Fontes et al. 2011) were co-chopped in nuclei extraction buffer (Otto 1990). The nuclei suspensions were processed, stained (Otto 1990; Clarindo and Carvalho 2009; Clarindo et al. 2013) and analyzed in a Partec PAS® flow cytometer (Partec® GmbH, Muenster, Germany). The 2C value was measured considering the G0/G1 nuclei peak of the Coffea samples and S. lycopersicum. From the mean 2C value, the DNA ploidy level was also confirmed for all Coffea.
ISE establishment
The leaves of C. eugenioides and C. arabica explant donor plants, cultivated in greenhouse, were disinfected prior to inoculation (Clarindo et al. 2012). Leaf explants (~ 1 cm2) of the four Coffea were excised, and five fragments were inoculated in Petri dish containing FC induction medium (M1, Table 1, van Boxtel and Berthouly 1996; Sanglard et al. 2017). In accordance with the availability of leaves from each Coffea, 51 Petri dishes (repetitions) were accomplished for C. eugenioides, 30 for C. canephora, 29 for HT ‘CIFC 4106’, and 102 for C. arabica. The Petri dishes were maintained in the dark at 25 ± 2 °C.
After 90 days, only the explants showing FC were individually transferred to Petri dishes containing SE regeneration medium (M2, Table 1, van Boxtel and Berthouly 1996; Sanglard et al. 2017). A total of 23 Petri dishes (repetitions) were prepared for C. eugenioides, 21 for C. canephora, 98 for HT ‘CIFC 4106’, and 143 for C. arabica. The culture was maintained in the dark at 25 ± 2 °C for 180 days. Subsequently, each mature cotyledonary somatic embryo (MCSE) was transferred to plantlet recovery medium (M3, Table 1) for SE germination and seedling development. The tubes were maintained at 25 °C ± 2 °C, under a 16/8 h (light/dark) regimen with 36 µmol m−2 s−1 light radiation provided by two fluorescent lamps (20 W, Osram®).
As the initial explant donors have been maintained in distinct environmental (greenhouse—C. eugenioides and C. arabica, or in vitro—C. canephora and HT ‘CIFC 4106’), a second ISE experiment was performed from leaves of the four Coffea regenerated and propagated in M3 (Table 1). For each Coffea, five leaf fragments were inoculated in 20 Petri dishes (replicates) with M1 (Table 1). Based on results of the first ISE experiment, after 60 days, each resulted FC was transferred for the M2 (Table 1). 20 Petri dishes (replicates) were performed for each Coffea, and all cultures were maintained in the dark at 25 ± 2 °C.
Besides, for all Coffea, 0.5 g of FC was transferred for Erlenmeyers containing 30 mL of liquid M1 (Table 1, van Boxtel and Berthouly 1996) without Phytagel (Sigma®). Six subcultures were performed every 15 days, and after, 0.5 g of the resulted cell aggregation suspension was transferred to Erlenmeyers containing 30 mL of liquid M2 (Table 1). Six subcultures were also performed every 15 days, during 90 days. The Erlenmeyers were maintained on shaker at 100 rpm in the dark at 25 ± 2 °C.
In March 2018, a third FC induction (M1, Table 1) was conducted for all Coffea regenerated and propagated in M3 (Table 1). Five leaf fragments were inoculated in 13 Petri dishes (repetitions) for each Coffea. All cultures were maintained in the dark at 25 ± 2 °C.
Statistical analysis
ISE responses of C. canephora, C. eugenioides, HT ‘CIFC 4106’ and C. arabica were compared with regard to two in vitro moments: (a) dedifferentiation of explant cells and FC establishment, and (b) MCSE regeneration (competence acquisition, FC cell determination and differentiation, and MCSE regeneration). For the first ISE experiment and the moment ‘a’, statistical analysis was performed using the number of responsive explants, which were defined by FC presence at 15, 30, 45, 60, 75 and 90 days. For the ISE step ‘b’, the number of regenerated MCSE was compared at 30, 60, 90, 120, 150 and 180 days, first considering all FC, then only the FC presenting MCSE.
The number of responsive explants (FC—‘a’) or MCSE (‘b’) per Petri dish were compared by analysis of variance (ANOVA), followed by Tukey’s test at 5% probability level (P ≤ 0.05), and depicted as box-plot graphics. Subsequently, regression analysis at 5% probability level (P ≤ 0.05) was performed from quantitative data: mean number of responsive leaf explants and mean number of MCSE. All analyses were accomplished using the software R 3.2.4 (R Core TEAM 2017).
For the second ISE experiment, the number of responsive explants (FC—‘a’) was compared at 30 and 60 days, and the number of regenerated MCSE (‘b’) at 150 days for the four Coffea in semisolid M2, and at 90 days in liquid M2. For the third ISE experiment, the number of responsive explants (FC—‘a’) was also compared at 30 and 60 days. The comparisons were accomplished from analysis of variance (ANOVA), followed by Tukey’s test at 5% probability level (P ≤ 0.05).
Chromosome number of the Coffea
For each Coffea, 0.5 g of a random sample of 90-day FC was collected and transferred to Erlenmeyer flasks containing FC proliferation medium (M1, Table 1) without Phytagel. At least five flasks were prepared for each Coffea, and maintained on shaker at 100 rpm and 25 °C ± 2 °C. Cell aggregate suspensions were subcultured every 15 days. After 60 days, the suspensions were treated with 4 µM of amiprophos methyl for 8 h, fixed (3:1 methanol:acetic acid) and enzymatically macerated with pectinase solution (1:20 pectinase:dH2O) for 1 h 40 min at 34 °C (Clarindo and Carvalho 2009). Cell dissociation and air-drying techniques were applied for slide preparation (Carvalho et al. 2007). After staining with 5% Giemsa solution, mitotic images were captured with a Media Cybernetics® Camera Evolution™ charge-coupled device video camera, mounted on a Nikon 80i microscope (Nikon, Japan).
Results
Nuclear DNA content and DNA ploidy level determination of the explant donors
The mean nuclear 2C DNA content value of all explant (three ISE experiments) donors was identical to previous data: 2C = 1.38 ± 0.060 pg for C. eugenioides (Fig. 1a), 2C = 1.41 ± 0.012 pg for C. canephora (Fig. 1b), 2C = 2.62 ± 0.043 pg for C. arabica (Fig. 1c), and 1C = 2.10 ± 0.003 pg for HT ‘CIFC 4106’ (Fig. 1d). Thus far, our research group had expressed the nuclear genome size of HT ‘CIFC 4106’ in 2C (Clarindo et al. 2013; Sattler et al. 2016). However, since the karyotype characterization and karyogram assembly were not performed, chromosome pairs were not identified for this allotriploid. Therefore, 1C = 2.10 pg was assumed for HT ‘CIFC 4106’ (Fig. 1d). Based on the mean nuclear 2C value, the DNA ploidy level of the explant donors was found to be diploid for C. eugenioides and C. canephora, triploid for HT ‘CIFC 4106’, and tetraploid for C. arabica.
Karyotypes, 2C DNA content values and evolutionary ages of the four Coffea. aC. eugenioides (EE)—2n = 2x = 22 chromosomes, 2C = 1.38 ± 0.060 pg. bC. canephora (CC)—2n = 2x = 22 chromosomes, 2C = 1.41 ± 0.012 pg. The two species (a, b) are diploids originated around 4.2 mya (Yu et al. 2011). cC. arabica (CaCaEaEa)—2n = 4x = 44 chromosomes, 2C = 2.62 ± 0.043 pg. This species originated ~ 0.665 mya (Yu et al. 2011). d HT ‘CIFC 4106’ (CCaEa), allotriploid karyotype with 2n = 3x = 33 chromosomes, 1C = 2.10 ± 0.003 pg. This Coffea arose from a backcrossing between C. arabica (CaCaEaEa) and its possible progenitor C. canephora (CC). HT emerged around 100 years ago (Bettencourt 1973). Due to its allotriploid origin, the nuclear genome size for HT was shown in 1C. Bar 5 µm
Dedifferentiation of explant cells and FC establishment
In the first ISE experiment, the number of responsive explants differed among the Coffea over time (Fig. 2a–f). The amount of FC (responsive leaf fragment explants) increased over time until the 60th day for all Coffea (Figs. 2a–d, 3). HT ‘CIFC 4106’ exhibited the highest mean number of FC (from 3.62 at 15 days to 4.90 at 90 days), followed by C. arabica (2.17 at 15 days to 2.37 at 90 days), and C. canephora (0.26–1.07) and C. eugenioides (0.08–1.04) which were statistically equal (Fig. 2a–f). Thus, the polyploids (HT ‘CIFC 4106’ and C. arabica) had a higher mean number of responsive explants than the diploids (C. canephora and C. eugenioides). Differently from the polyploids, the diploid Coffea species did not differed from each other over time (Fig. 2a–f), even though explants collected from ex vitro C. eugenioides plants and in vitro C. canephora plantlets.
Mean number of responsive leaf explants from C. eugenioides, C. canephora, HT ‘CIFC 4106’ and C. arabica over time (15–90 days) in FC induction medium (M1, Table 1)—dedifferentiation step of the explant cells (Fig. 4a). The box plots show that the mean number of responsive explants differed among the Coffea (a–f) in the first ISE experiment, with the highest number being observed for HT ‘CIFC 4106’ (2n = 3x = 33 chromosomes, 1C = 2.10 pg), followed by C. arabica (2n = 4x = 44, 2C = 2.62 pg), C. eugenioides (2n = 2x = 22, 2C = 1.38 pg) and C. canephora (2n = 2x = 22, 2C = 1.41 pg) (Fig. 1). Notice the variation in responsive explant number in each Coffea, particularly in C. arabica and C. eugenioides. The mean number of FC followed by the same letter are not different by Tukey’s test at 5% probability level (P ≤ 0.05)
FC establishment in C. eugenioides, C. canephora, HT ‘CIFC 4106’ and C. arabica in the first ISE experiment. Graphic representing dedifferentiation of the leaf explant cells, obtained from regression analysis using the mean number of responsive leaf explants of the four Coffea. The graphic, about data of the first ISE experiment, shows that the allopolyploids (yellow—HT ‘CIFC 4106’; blue—C. arabica) had higher mean number of responsive explants than the diploids (red—C. canephora; green—C. eugenioides). Observe that, from the 60th day onwards, the mean number of responsive leaf explants was constant in each Coffea, and already from the 15th day in C. arabica. Furthermore, the response presented by each Coffea also differed in relation to the time and cellular proliferation of the FC (right). Chromosome number and 1Cx nuclear value of the four Coffea (right). Fitted quadratic models were significant (P ≤ 0.05) by regression analysis for all Coffea: C. eugenioides—Y = − 0.4236 + 0.04513X − 0.0004X2; C. canephora—Y = − 0.2367 + 0.03772X − 0.0003X2; HT ‘CIFC 4106’—Y = 2.7724 + 0.05967X − 0.0005X2; and C. arabica—Y = 2.0774 + 0.00838X − 0.0001X2. *Pinto-Maglio and Da Cruz (1998); **Hamon et al. (2009); ***Clarindo and Carvalho (2006). Bar 1 cm. (Color figure online)
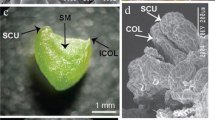
ISE achievement in C. canephora, C. eugenioides, HT ‘CIFC 4106’ and C. arabica (left to right, respectively). Above the brackets, the main chemical and physical in vitro conditions for FC induction (a, M1—Table 1), SE regeneration (b, M2—Table 1) and plantlet recovery (c, M3—Table 1) are shown. a Dedifferentiation of leaf cells in these Coffea was promoted in medium supplemented with 2,4-D and BAP, in the dark. Besides differences in mean number of responsive explants, also the cell proliferation in the FC was more pronounced in the allopolyploids (HT ‘CIFC 4106’ and C. arabica) than in the diploids (C. eugenioides and C. canephora). Bar 1 cm. b Acquisition of cell competence and determination, tissue differentiation and SE regeneration in FC occurred in medium supplemented with BAP and active charcoal, in the dark. Representative SE were obtained for all Coffea: immature cotyledonary SE of C. canephora and mature cotyledonary SE of C. eugenioides, HT ‘CIFC 4106’ and C. arabica after 180 days in medium M2 (Table 1). Note a globular SE in the apical root meristem of the C. canephora SE, evidencing the occurrence of secondary somatic embryogenesis. Bar 2 mm. c MCSE germination and seedling development was promoted in medium supplemented with GA3, under photoperiod. The ISE morphogenic pathway was established in 150 days for C. arabica, 180 days for HT ‘CIFC 4106’, 270 days for C. eugenioides, and 300 days for C. canephora. Bar 1 cm
At 15–30 days, only the polyploids showed at least one responsive explant (Fig. 2a, b), and FC were generated in larger quantities and in less time in these Coffea. The number of responsive explants (i.e. leaves exhibiting FC) was constant for all Coffea after 60 days, and after 15 days for C. arabica (Fig. 3). Furthermore, at 90 days all Coffea displayed explants which did not generated calli (Fig. 2f), except for HT ‘CIFC 4106’, which at 45 days showed all explants to be responsive (Fig. 2c–f). Considering each Coffea, variation in mean FC number was observed for C. canephora, and mainly for C. arabica and C. eugenioides (Fig. 2) that were supplied from ex vitro plants.
In addition to quantitative data, from a visual analysis, the size of the FC gradually increased until the 90th day of culture for all Coffea. However, the size of the FC, which can be influenced by cell proliferation and/or cell expansion, was visually more pronounced in the polyploids, followed by the diploids (Figs. 3, 4a).
HT ‘CIFC 4106’ and C. arabica also had the higher mean number of FC than C. eugenioides and C. canephora in the second ISE experiment. All HT ‘CIFC 4106’ leaf explants showed FC at 30 days in M1 (Table 1), statistically equal to the mean number of C. arabica (from 4.20 at 30 days to 4.50 at 60 days). C. eugenioides showed a mean number of 2.40 FC by Petri dish at 30 days and 3.00 at 60 days, and C. canephora exhibited the lowest mean number, 1.42 at 30 days and 1.45 at 60 days.
Corroborating to the previous data (first and second ISE experiments), FC were also established in higher mean number for HT ‘CIFC 4106’ (5.00 at 30 days—all responsive explants), followed by C. arabica (from 3.92 at 30 days to 4.55 at 60 days), C. eugenioides (from 2.54 at 30 days to 3.20 at 60 days) and by C. canephora (from 0.91 at 30 days to 2.10 at 60 days). Therefore, based on the results of the second and third ISE experiments, the mean number of responsive leaf explants was higher from C. arabica and C. eugenioides in vitro plantlets than ex vitro plantlets (first ISE experiment). Besides, as in the previous ISE, the more pronounced FC were observed for the polyploids.
MCSE regeneration
First signs of globular SE recovery occurred at 21, 27, 52 and 90 days for C. arabica, HT ‘CIFC 4106’, C. eugenioides and C. canephora, respectively, in the first ISE experiment. During the 180 days, SE reached different development stages (globular, heart, torpedo and cotyledonary), characterizing the Coffea ISE as asynchronous. Only the MCSE (Fig. 4b) were recorded to compare the studied Coffea (Figs. 5, 6, 7), owing to their clear identification and isolated embryo stage for plantlet recovery. MCSE emerged after 30 days in C. arabica, 51 days in HT ‘CIFC 4106’, 97 days in C. eugenioides, and 145 days in C. canephora.
Mean number of MCSE (30–180 days) obtained in the first ISE experiment for C. eugenioides, C. canephora, HT ‘CIFC 4106’ and C. arabica considering all FC in SE regeneration medium (M2, Table 1)—step of competence acquisition, FC cell determination and differentiation, and MCSE regeneration (Fig. 4b). The box plots, about data of the first ISE experiment, show that the mean number of MCSE differed among the Coffea between 60 and 180 days (a–f), with the highest number of FC exhibiting MCSE being observed for C. arabica (2n = 4x = 44 chromosomes, 2C = 2.62 pg). In addition, MCSE were recovered faster in the FC of this species (b). At 180 days, C. eugenioides, C. canephora and HT ‘CIFC 4106’ showed the same mean number of MCSE. Mean MCSE numbers followed by the same letter do not differ by Tukey’s test at 5% probability level (P ≤ 0.05)
Box plots, about data of the first ISE experiment, representing the mean number of MCSE for the responsive FC of the four studied Coffea. Because physiological and epigenetic aspects may impede competence acquisition, and consequently determination and differentiation, the mean number of MCSE in the Coffea was compared considering only the responsive FC. As shown in Fig. 5, the highest number of MCSE was observed for C. arabica between 30 and 180 days (a–f). In contrast, at 180 days (f) the FC of C. canephora presented the lowest mean number of MCSE in relation to C. eugenioides and HT ‘CIFC 4106’. Observe the variation in MCSE number mainly for C. arabica and C. eugenioides, as shown in Figs. 2 and 5. Mean MCSE numbers followed by the same letter do not differ by Tukey’s test at 5% probability level (P ≤ 0.05)
In vitro response as shown by SE recovery in C. canephora, HT ‘CIFC 4106’, C. eugenioides and C. arabica. Graphic obtained from regression analysis using the mean number of MCSE (Fig. 6) of the four Coffea. The graphic shows the highest mean number of MCSE for C. arabica (blue) during the entire period of SE conversion and maturation. C. eugenioides (green) and HT ‘CIFC 4106’ (yellow) exhibited the same mean number of MCSE at 30–180 days. After 180 days, C. canephora FC showed the lowest mean number of MCSE. These Coffea differed with regard to the chromosome number, ploidy level and 1Cx nuclear value. Fitted models were significant (P ≤ 0.05) by regression analysis: C. eugenioides—Y = − 0.8428 + 0.00522X + 0.0002X2; C. canephora—Y = 0.7–0.02512X + 0.0001X2; HT ‘CIFC 4106’—Y = − 1.6583 + 0.0441X; and C. arabica—Y = − 4.0693 + 0.1273X. *Pinto-Maglio and Da Cruz (1998); **Hamon et al. (2009); ***Clarindo and Carvalho (2006). Bar 1 cm. (Color figure online)
The mean number of MCSE per FC differed among the Coffea from 60 to 180 days (Fig. 5a–f). At 180 days, 60.1% of the FC in C. arabica exhibited MCSE, as did 30.4% in C. eugenioides, 16.2% in HT ‘CIFC 4106’, and 14.3% in C. canephora. This embryogenic response increased over time, with mean number of MCSE varying from 1.71 to 9.94 for C. arabica, 0 to 2.04 for C. eugenioides, 0.10 to 1.0 for HT ‘CIFC 4106’, and 0 to 0.29 for C. canephora at 60 and 180 days, respectively (Fig. 5a–f). C. arabica showed the highest mean number of MCSE, differing over time from the other Coffea. C. eugenioides, C. canephora and HT ‘CIFC 4106’ presented the same mean number of MCSE until 90 days (Fig. 5b, c), being that this embryo stage was firstly found in the FC of the allotriploid HT ‘CIFC 4106’. After 180 days, these three Coffea showed the same mean number of MCSE (Fig. 5f).
Considering only the FC that generated MCSE, the mean number varied from 3.18 to 18.45 for C. arabica, 0.62 to 6.06 for HT ‘CIFC 4106’, 0–6.71 for C. eugenioides, and 0–2.0 for C. canephora at 60 and 180 days, respectively (Fig. 6a–f). From 60 days onwards, C. arabica presented the highest mean number of MCSE, while the other Coffea were similar until 150 days (Figs. 6b–e, 7). After 180 days, C. canephora presented the lowest mean number of MCSE (Figs. 6f, 7). Regarding each Coffea, variation in MCSE number was also found (Figs. 5, 6).
In the first ISE experiment, the ISE morphogenic pathway was established in relatively short time, varying from 150 days in C. arabica, 180 days in HT ‘CIFC 4106’, 270 days in C. eugenioides, to 300 days in C. canephora (Fig. 4).
After 150 days in semisolid M2 (Table 1), MCSE were recovered from C. arabica and C. canephora FC in the second ISE experiment. As previously observed, C. arabica FC provided the highest mean number of MCSE in relation to C. canephora. Only globular SE were found in the C. eugenioides FC. SE was not regenerated from HT ‘CIFC 4106’ FC until 150 days.
Corroborating, C. arabica, C. eugenioides and C. canephora cell aggregate suspensions resulted globular SE after 45 days in liquid M2 (Table 1), equivalent to three subcultures. From the 60 days (four subcultures), torpedo SE were only found in C. arabica FC, and after 90 days MCSE were only recorded for this Coffea. No SE was recovered from cell aggregate suspensions of HT ‘CIFC 4106’. From the FC of the third ISE experiment, globular to mature cotyledonary SE were only recovered for C. arabica. After 60 days in M2 (Table 1), SE were observed in ~ 54% of the C. arabica FC, corroborating to the first and second ISE experiments.
Chromosome number of the Coffea FC
The chromosome number established in the FC was identical to that reported for each Coffea: C. eugenioides and C. canephora showed 2n = 2x = 22 chromosomes (Fig. 1a, b), C. arabica had 2n = 4x = 44 (Fig. 1c), and HT ‘CIFC 4106’ presented 2n = 3x = 33 (Fig. 1d). These data confirmed the euploid series based on the basic chromosome number x = 11. All metaphases showed constant chromosome number for each Coffea, indicating that no numerical variation occurred during the in vitro tissue culture. Thus, chromosome number evidences the stability of the karyotype, indicating that no somaclonal variation related to euploidy and/or aneuploidy has occurred.
Based on chromosome number and nuclear genome size, the 2C/1C mean value was converted to 1Cx (1C DNA content for the basic chromosome set x = 11). In this way, the mean 1Cx value of C. eugenioides was equivalent to 0.680 pg, with 1Cx = 0.715 pg for C. canephora, 1Cx = 0.665 pg for C. arabica, and 1Cx = 0.700 pg for HT ‘CIFC 4106’ (Figs. 3, 7).
Discussion
Differently from other studies also involving Coffea with distinct ploidy levels (Staritsky 1970; van Boxtel and Berthouly 1996; Samson et al. 2006), the tissue culture procedure was effective for ISE establishment in all Coffea (C. canephora, C. eugenioides, HT ‘CIFC 4106’ and C. arabica) under the same in vitro conditions (Table 1). This experimental design allowed verifying the ISE responses in these Coffea with distinct ploidy level. Briefly, the karyotype of the four Coffea analyzed here differed with regard to chromosome number, evidencing distinct ploidy levels (based on x = 11 chromosomes) and nuclear 2C values (Figs. 1, 3, 7).
Based on the quantitative (mean number of responsive leaf explants, mean number of MCSE, and response time) and qualitative variables (length of the FC, which can be influenced by cell proliferation and/or cell expansion), the four Coffea differed in relation to ISE response. Due to distinct in vitro environmental conditions (M1 and M2, Table 1) and to the results, it is important to look for each ISE moment: FC induction (M1) and SE regeneration (M2). In the first ISE moment (dedifferentiation of explant cells and FC establishment), leaf explants of the allopolyploids (allotriploid HT ‘CIFC 4106’ and allotetraploid C. arabica) were the most responsive in the three ISE experiments, providing FC in relatively short time (Figs. 2, 3), and visually exhibiting more pronounced FC, mainly in HT ‘CIFC 4106’ (Figs. 4a, 6). Therefore, our data showed differences between polyploids and diploids Coffea in the first moment of the ISE—FC induction step. Other studies concomitantly analyzing Coffea with different ploidy levels also reported differences in relation to FC responses. Leaf explants of the diploid C. canephora, the allopolyploid ‘Arabusta’, and the homoploid ‘Congusta’ were more responsive than the allotetraploid C. arabica (van Boxtel and Berthouly 1996). Independently of the FC induction strategy (two steps in semisolid medium and one step in liquid medium, according to van Boxtel and Berthouly 1996, or a single step in semisolid medium, present study), differences were observed in FC responses for Coffea with different ploidy levels.
In the second ISE moment (competence acquisition, FC cell determination and differentiation, and MCSE regeneration), a higher mean number of MCSE was observed for the true allotetraploid C. arabica, which were also recovered faster in the FC of this Coffea (Figs. 6, 7). Since the first ISE study (Staritsky 1970), differences in relation to SE recovering have been noted in Coffea with different ploidy levels (van Boxtel and Berthouly 1996; Samson et al. 2006). Hence, van Boxtel and Berthouly (1996) highlighted the importance of considering the genetic background of selected Coffea. In that sense, since differences in relation to SE formation have been pointed out but not debated, the present study showed and compared the ISE responses for each Coffea with marked karyotype features (chromosome number, ploidy level and nuclear DNA content), considering also each karyotype origin (diploid, allotriploid–anorthoploid, and allotetraploid). Therefore, the experimental design of the present study constitutes a further step towards understanding the ISE response in Coffea.
Nuclear genome size and karyotype divergences have been pointed out as factors that directly influence the gene regulation involved in morphogenesis, interfering with the in vitro response (Chandler et al. 2008; Irikova et al. 2012; Xu and Huang 2014). In an euploid series (monoploid, diploid, triploid and tetraploid) of Zea mays L., the most analyzed genes exhibited a relative dosage effect in multiples of the basic chromosome complement, x = 10. The expression level rose proportionally to the ploidy level, increasing from monoploid up to tetraploid (Guo et al. 1996).
The high mean number of responsive leaf explants in relatively short time (Figs. 2, 3) and the FC were more pronounced in HT ‘CIFC 4106’ (2n = 3x = 33 chromosomes) compared with the other three Coffea species (Figs. 3, 4a). However, the mean number of MCSE in HT ‘CIFC 4106’ was the same as in C. eugenioides (2n = 2x = 22) and lower than in C. arabica (2n = 4x = 44) in the first ISE experiment (Fig. 6f), and no HT ‘CIFC 4106’ SE was recovered in the second and third experiments. Possibly, HT ‘CIFC 4106’ FC cells exhibited a high level of endogenous auxins, such as indole-3-acetic acid, which maintain the cells undifferentiated (Fehér et al. 2003). This physiological aspect, which may be related to the allotriploidy of HT ‘CIFC 4106’, prevents the regeneration, conversion and maturation of SE under the tissue culture conditions established here (M2, Table 1). Therefore, for future ISE in the allotriploid HT ‘CIFC 4106’, the tissue culture medium should be adjusted to consider the ploidy level, so as to increase the mean number of MCSE. Further suggestions are to decrease the 2,4-D level in the FC induction medium (M1, Table 1), and/or increase the concentration of active charcoal or sucrose, and/or add abscisic acid to the SE regeneration medium (M2, Table 1).
Epigenetic features may also have influenced the MSCE regeneration in HT ‘CIFC 4106’. Epigenetics involves chromatin remodeling, mainly by methylation, which alters the gene expression (Reyes 2006; Jarillo et al. 2009). Chromatin methylation may involve histones (H3 and H4) and/or nitrogen bases, such as cytosine (Karim et al. 2016). DNA hypomethylation has been associated with the suppression of somatic embryos conversion and maturation. Contrarily, hypermethylation improved the rates of somatic embryogenesis (Karim et al. 2016). For C. canephora, somatic embryos recovering from direct embryogenesis as well as their conversion and maturation were accompanied by DNA hypermethylation (Nic-Can et al. 2013). Hence, the measurement of the DNA methylation level should be performed to understand the changes during the ISE in Coffea displaying distinct ploidy levels.
As well as the MSCE regeneration in HT ‘CIFC 4106’, intraspecific variation related to the mean number of responsive leaf explants (Fig. 2) and MCSE (Figs. 5, 6) was observed in each Coffea, particularly in C. arabica and C. eugenioides (Figs. 2f, 5f, 6f). In addition, distinct mean numbers of responsive leaf explants were accounted for C. arabica and C. eugenioides in the first and in second/third experiments. Therefore, besides ploidy level (chromosome number and nuclear DNA content), the ISE responses were also influenced by ex vitro and in vitro conditions of the Coffea explant donors, and, so, by physiological and epigenetic features. These aspects can also be related to the results observed in van Boxtel and Berthouly (1996) and in the present study for each moment of the ISE in C. canephora and C. arabica.
The ISE establishment for C. canephora, C. eugenioides, HT ‘CIFC 4106’ and C. arabica under the same in vitro conditions was fundamental, since in vitro response is influenced by the in vitro environment and the species’ genotype (Fehér et al. 2003). Some authors have assumed a species-specific response for Coffea (Molina et al. 2002; Samson et al. 2006) based on the ISE response rates among hybrids, species and lines of the same species. Figure 4 is a guideline that summarizes the ISE procedure, showing the chemical and physical conditions of each step (FC induction, SE regeneration and plantlet recovery). This guideline represents a substantial advancement for the conduction of future ISE studies in Coffea.
For the first time, SE and plantlets were regenerated for C. eugenioides via ISE. All steps of the ISE procedure (dedifferentiation, Figs. 2, 3, 4a; SE conversion and maturation, Figs. 4b, 5, 6, 7; and seedling development, Fig. 4c), in particular the chemical and physical in vitro conditions, were adapted from van Boxtel and Berthouly (1996). The procedure proposed by these authors has represented the basis for the in vitro propagation of C. canephora and C. arabica lines, and has been improved in our ISE routine (Sattler et al. 2016; Sanglard et al. 2017).
Considering the ISE responses and the more adequate in vitro procedures summarized in Table 1, certain in vitro conditions should be compiled for Coffea. FC formation and cell proliferation in Coffea leaf explants (Figs. 2, 3, 4a) confirmed the role of the growth regulators 2,4-D and BAP in cell dedifferentiation. Thus, the use and balance of these compounds was crucial for callus induction, a precondition for ISE in Coffea. The essential role of 2,4-D in dedifferentiation has been demonstrated via a stress-related mechanism (Fehér 2015). Such stress is promoted by an increased amount of oxidative compounds, such as hydrogen peroxide, produced in plant cells exposed to this growth regulator (Pfeiffer and Hoftberger 2001). Another advantage of the synthetic 2,4-D is associated to its chemical structure (Gaspar et al. 1996), which is stable under in vitro conditions and not recognized by intracellular enzymes (Gaspar et al. 1996; Karami and Saidi 2010). Similarly to 2,4-D, the cytokinin BAP, a derivative of a purine base not cleaved by cytokinin oxidase, affects the plant growth and development, such as cell division, shoot initiation and growth, and apical dominance (Kieber and Schaller 2014). Despite being fundamental for the Coffea ISE (Table 1), the role of BAP, as of other cytokinins, should be further investigated in tissue culture studies.
SE formation in Coffea is promoted after removal of the exogenous auxin from the culture medium, eliminating the main chemical component that maintains plant cells in a totipotent condition (Rose et al. 2010; Nic-Can and Loyola-Vargas 2016). Accordingly, activated charcoal has been added to plant tissue culture due to its capacity of adsorbing residues of exogenous 2,4-D (Pan and van Staden 1998), which has been demonstrated by its inhibitory effect on direct somatic embryogenesis in C. canephora (Hatanaka et al. 1991). In the present study, for the FC providing MCSE in all Coffea, the 2,4-D excess was adsorbed by active charcoal, enabling to speed up the determination and differentiation process.
Coffea MCSE inoculation in medium supplemented with GA3 provided seedlings at 30 days. This compound is metabolized by gibberellin 3-oxidase, resulting in GA3 conversion to its bioactive forms. Hence, GA3 plays a direct role in bioactive gibberellin levels (Mitchum et al. 2006), promoting SE germination.
Differently from the previous reports for Coffea ISE, which generally required at least four steps, we have adapted an ISE strategy involving three steps in semisolid media. Regardless remarkable karyotype differences, the ISE strategy was reproducible in four Coffea, including the two relevant crops, C. arabica and C. canephora, as well as C. eugenioides and HT ‘CIFC 4106’.
Conclusions
The tissue culture procedure was effective for ISE establishment in the four Coffea under the same in vitro conditions, independently of the differences regarding (a) ploidy level, (b) nuclear DNA content, and (c) physiological conditions of the explant donors (greenhouse—C. arabica and C. eugenioides, or in vitro—C. canephora and HT ‘CIFC 4106’). So, this study revised and updated the data about ISE responses in Coffea with different ploidy levels. Therefore, for future ISE studies in Coffea, it is imperative to establish the ploidy level, as well as the epigenetic and physiological backgrounds.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BAP:
-
6-Benzylaminopurine
- FC:
-
Friable calli
- GA3 :
-
Gibberellic acid
- HT:
-
“Híbrido de Timor”
- ISE:
-
Indirect somatic embryogenesis
- MCSE:
-
Mature cotyledonary somatic embryo
- SC:
-
Secondary constriction
- SE:
-
Somatic embryos
References
Almeida JAS, Silvarolla MB, Fazuoli LC, Stancato GC (2008) Embriogênese somática em genótipos de Coffea arabica L. Coffee Sci 3:143–151. https://doi.org/10.25186/cs.v3i2.85
Bettencourt AJ (1973) Considerações gerais sobre o “Híbrido de Timor”. Instituto Agronômico de Campinas, Campinas
Carvalho CR, Clarindo WR, Almeida PM (2007) Plant cytogenetics: still looking for the perfect mitotic chromosomes. Nucleus 50:453–462
Chandler J, Nardmann J, Werr W (2008) Plant development revolves around axes. Trends Plant Sci 13:78–84. https://doi.org/10.1016/j.tplants.2008.09.008
Clarindo WR, Carvalho CR (2006) A high quality chromosome preparation from cell suspension aggregates culture of Coffea canephora. Cytologia 71:243–249. https://doi.org/10.1508/cytologia.71.243
Clarindo WR, Carvalho CR (2009) Comparison of the Coffea canephora and C. arabica karyotype based on chromosomal DNA content. Plant Cell Rep 28:73–81. https://doi.org/10.1007/s00299-008-0621-y
Clarindo WR, Carvalho CR, Mendonça MAC (2012) Cytogenetic and flow cytometry data expand knowledge of genome evolution in three Coffea species. Plant Syst Evol 298:835–844. https://doi.org/10.1007/s00606-012-0595-7
Clarindo WR, Carvalho CR, Caixeta ET, Koehler AD (2013) Following the track of “Híbrido de Timor” origin by cytogenetic and flow cytometry approaches. Genet Resour Crop Evol 60:2253–2259. https://doi.org/10.1007/s10722-013-9990-3
Etienne H, Anthony F, Dussert S, Fernandez D, Lashermes P, Bertrand B (2002) Biotechnological applications for the improvement of coffee (Coffea arabica L.). In Vitro Cell Dev Plant 38:129–138. https://doi.org/10.1079/IVP2001273
Fehér A (2015) Somatic embryogenesis-stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849:385–402. https://doi.org/10.1016/j.bbagrm.2014.07.005
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Org Cult 74:201–228. https://doi.org/10.1023/A:1024033216561
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirement of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Plant 32:272–289. https://doi.org/10.1007/BF02822700
Gatica-Arias AM, Arrieta-Espinoza G, Espinoza Esquivel AM (2008) Plant regeneration via indirect somatic embryogenesis and optimisation of genetic transformation in Coffea arabica L. cvs. Caturra and Catuaí. Electron J Biotechno 11:101–112. https://doi.org/10.2225/vol11-issue1-fulltext-9
Guo M, Davis D, Birchler JA (1996) Dosage effects on gene expression in a maize ploidy series. Genetics 142:1349–1355
Hamon P, Siljak-Yakovlev S, Srisuwan S, Robin O, Poncet V, Hamon S, De Kochko A (2009) Physical mapping of rDNA and heterochromatin in chromosomes of 16 Coffea species: a revised view of species differentiation. Chromosome Res 17:291–304. https://doi.org/10.1007/s10577-009-9033-2
Hamon P, Hamon S, Razafinarivo NJ, Guyot R, Siljak-Yakovlev S, Couturon E, Crouzillat D, Rigoreau M, Akaffou S, Rakotomalala JJ, de Kochko A (2015) Coffea genome organization and evolution. In: Coffee in health and disease prevention. Academic, San Diego, pp 29–37. https://doi.org/10.1016/B978-0-12-409517-5.00004-8
Hatanaka T, Arakawa O, Yasuda T, Uchida N, Yamaguchi T (1991) Effect of plant growth regulators on somatic embryogenesis in leaf cultures of Coffea canephora. Plant Cell Rep 10:179–182. https://doi.org/10.1007/BF00234290
Herrera JC, Moreno LG, Acuna JR, De Pena M, Osorio D (2002) Colchicine-induced microspore embryogenesis in coffee. Plant Cell Tissue Org Cult 71:89–92. https://doi.org/10.1023/A:101656481
Ibrahim MSD, Hartati RRS, Rubiyo R, Purwito A, Sudarsono S (2015) The induction of primary and secondary somatic embryo to support Arabica coffee propagation. J Trop Crop Sci 2:3
Irikova T, Grozeva S, Denev I (2012) Identification of BABY BOOM and LEAFY COTYLEDON genes in sweet pepper (Capsicum annuum L.) genome by their partial gene sequences. Plant Growth Regul 67:191–198. https://doi.org/10.1007/s10725-012-9676-4
Jarillo JA, Piñeiro M, Cubas P, Martínez-Zapater JM (2009) Chromatin remodeling in plant development. Int J Dev Biol 53:1581–1596. https://doi.org/10.1387/ijdb.072460jj
Karami O, Saidi A (2010) The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol Biol Rep 37:2493–2507. https://doi.org/10.1007/s11033-009-9764-3
Karim R, Nuruzzaman M, Khalid N, Harikrishna JA (2016) Importance of DNA and histone methylation in in vitro plant propagation for crop improvement: a review. Ann Appl Biol 169:1–16. https://doi.org/10.1111/aab.12280
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239
Kieber J, Schaller G (2014) Cytokinins. Arabidopsis Book. https://doi.org/10.1199/tab.0168
Lashermes P, Combes MC, Robert J, Trouslot P, D’Hont A, Anthony F, Charrier A (1999) Molecular characterization and origin of the Coffea arabica L. genome. Mol Gen Genet 261:259–266. https://doi.org/10.1007/s004380050965
Mitchum MG, Yamaguchi S, Hanada A, Kuwahara A, Yoshioka Y, Kato T, Tabata S, Kamiya Y, Sun TP (2006) Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 45:804–818. https://doi.org/10.1111/j.1365-313X.2005.02642.x
Molina DM, Aponte ME, Cortina H, Moreno G (2002) The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell Tissue Org Cult 71:117–123. https://doi.org/10.1023/A:1019965621041
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Murashige T, Nakano R (1966) Tissue culture as a potential tool in obtaining polyploid plants. J Hered 57:114–118. https://doi.org/10.1093/oxfordjournals.jhered.a107486
Nic-Can GI, Loyola-Vargas VM (2016) The role of the auxins during somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Somatic embryogenesis: fundamental aspects and applications. Springer, New York, pp 171–182. https://doi.org/10.1007/978-3-319-33705-0_10
Nic-Can GI, Lopez-Torres A, Barredo-Pool F, Wrobel K, Loyola-Vargas VM, Rojas-Herrera R, De-la-Pena C (2013) New insights into somatic embryogenesis: LEAFY COTYLEDON1, BABY BOOM1 and WUSCHEL-RELATED HOMEOBOX4 are epigenetically regulated in Coffea canephora. PLoS ONE 8:e72160. https://doi.org/10.1371/journal.pone.0072160
Noirot M, Poncet V, Barre P, Hamon P, Hamon S, Kochko A (2003) Genome size variations in diploid African Coffea species. Ann Bot 92:709–714. https://doi.org/10.1093/aob/mcg183
Otto FJ (1990) DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Darzynkiewiez Z, Crissman HA, Robinson JP (eds) Methods in cell biology, vol. 33. Academic Press, San Diego, pp 105–110
Pan MJ, van Staden J (1998) The use of charcoal in vitro culture: a review. Plant Growth Regul 26:155–163
Pfeiffer W, Höftberger M (2001) Oxidative burst in Chenopodium rubrum suspension cells: induction by auxin and osmotic changes. Physiol Plant 111:144–150. https://doi.org/10.1034/j.1399-3054.2001.1110203.x
Pinto-Maglio CAF, Da Cruz ND (1998) Pachytene chromosome morphology in Coffea L. II. C. arabica L. complement. Caryologia 51:19–35. https://doi.org/10.1080/00087114.1998.10589117
Praça-Fontes MM, Carvalho CR, Clarindo WR (2011) C-value reassessment of plant standards: an image cytometry approach. Plant Cell Rep 30:2303–2312. https://doi.org/10.1007/s00299-011-1135-6
R Core TEAM (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available on the Internet via http://www.Rproject.org/. Accessed 19 Jan 2017
Reyes JC (2006) Chromatin modifiers that control plant development. Curr Opin Plant Biol 9:21–27. https://doi.org/10.1016/j.pbi.2005.11.010
Rose RJ, Mantiri FR, Kurdyukov S, Chen SK, Wang XD, Nolan KE, Sheahan MB (2010) Developmental biology of somatic embryogenesis. In: Pua EC, Davey MR (eds) Plant developmental biology-biotechnological perspectives. Springer, Berlin, pp 3–26. https://doi.org/10.1007/978-3-642-04670-4_1
Samson NP, Campa C, Gal LL, Noirot M, Thomas G, Lokeswari TS, De Kochko A (2006) Effect of primary culture medium composition on high frequency somatic embryogenesis in different Coffea species. Plant Cell Tissue Org Cult 86:37–45. https://doi.org/10.1007/s11240-006-9094-2
Sanglard NA, Amaral-Silva PM, Sattler MC, Oliveira SC, Nunes ACP, Soares TCB, Carvalho CR, Clarindo WR (2017) From chromosome doubling to DNA sequence changes: outcomes of an improved in vitro procedure developed for allotriploid Híbrido de Timor (Coffea arabica L. × Coffea canephora Pierre ex A. Froehner). Plant Cell Tissue Org Cult 131:223–231. https://doi.org/10.1007/s11240-017-1278-4
Sattler MC, Carvalho CR, Clarindo WR (2016) Regeneration of allotriploid Coffea plants from tissue culture: resolving the propagation problems promoted by irregular meiosis. Cytologia 81:125–132. https://doi.org/10.1508/cytologia.81.125
Staritsky G (1970) Embryoid formation in callus tissues of coffee. Plant Biol 19:509–514. https://doi.org/10.1111/j.1438-8677.1970.tb00677.x
van Boxtel J, Berthouly M (1996) High frequency somatic embryogenesis from coffee leaves. Plant Cell Tissue Org Cult 44:7–17. https://doi.org/10.1007/BF00045907
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462. https://doi.org/10.1093/oxfordjournals.aob.a087127
Xu L, Huang H (2014) Genetic and epigenetic controls of plant regeneration. Curr Top Dev Biol 108:1–33. https://doi.org/10.1016/B978-0-12-391498-9.00009-7
Yu Q, Guyot R, Kochko A, Byers A, Navajas-Pérez R, Langston BJ, Dubreuil-Tranchant C, Paterson AH, Poncet V, Nagai C, Ming R (2011) Micro-collinearity and genome evolution in the vicinity of an ethylene receptor gene of cultivated diploid and allotetraploid coffee species (Coffea). Plant J 67:305–317. https://doi.org/10.1111/j.1365-313X.2011.04590.x
Acknowledgements
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília—DF, Brazil, Grant: 443801/2014-2), Fundação de Amparo à Pesquisa do Espírito Santo (FAPES, Vitória—ES, Brazil, Grant: 65942604/2014 and 82/2017), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília—DF, Brazil) for financial support. We also thank Dr. Eveline Teixeira Caixeta (Embrapa Café, Empresa Brasileira de Pesquisa Agropecuária, BIOAGRO, Laboratório BioCafé, Universidade Federal de Viçosa, MG, Brazil) for providing leaves in 2015 of the C. eugenioides and C. arabica ‘Catuaí Vermelho’ plants.
Author information
Authors and Affiliations
Contributions
The authors NAS, PMA, LMC and WRC conceived, designed and conducted the tissue culture experiments. MCS, SCDO and WRC carried out the cytogenetic analyses. WRC and CRC contributed with flow cytometry analysis. The authors NAS and AF performed the statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Jochen Kumlehn.
Rights and permissions
About this article
Cite this article
Sanglard, N.A., Amaral-Silva, P.M., Sattler, M.C. et al. Indirect somatic embryogenesis in Coffea with different ploidy levels: a revisiting and updating study. Plant Cell Tiss Organ Cult 136, 255–267 (2019). https://doi.org/10.1007/s11240-018-1511-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1511-9