Abstract
CUC2 encodes a NAC domain transcription factor required for establishing boundaries around plant organ primordia. It is also associated with the initiation of the shoot apical meristem. We cloned a 1497-bp BpCUC2 promoter fragment and constructed a pBpCUC2::LUC vector for birch transgenic. A quantitative real-time polymerase chain reaction (qRT-PCR) analysis of transgenic birch buds, leaves, stems, and roots revealed that luciferase (LUC) was most highly expressed in the buds. The pBpCUC2::LUC transgenic birch plants were treated with methyl jasmonate, indoleacetic acid, abscisic acid, or gibberellic acid3 for 2 h. The subsequent qRT-PCR analysis indicated that the transgenic birch tissues responded differently to the various hormones. Our results confirmed that BpCUC2 expression is influenced by hormones. The PLACE online tool revealed that the BpCUC2 promoter sequence contains several cis-acting elements. Furthermore, an auxin response element was used to screen transcription factors in a yeast one-hybrid assay. We identified three unique cDNA sequences, with complete open reading frames containing regulatory motifs, which were related to growth and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf margin morphology was the first heritable plant trait to be studied. This plant characteristic has an important role related to the classification of plants, and influences the ornamental value of plants. Thus, botanists have been very interested in the regulatory mechanisms underlying the variability in leaf margin morphology (Bilsborough et al. 2011). Recent studies have helped to clarify the relevant mechanisms in model plants (i.e., Arabidopsis thaliana, Solanum lycopersicum, Zea mays, and Medicago truncatula), and many plant lines with mutated leaf margins have been described (Hay 2006; Nikovics et al. 2006; Ramirez et al. 2009; Koyama et al. 2010; Hasson et al. 2011). There is currently some information regarding the variability in the leaf margins of forest trees. However, because of most tree genomes are generally larger, and mostly high heterozygous, highly repetitive genomes, research into leaf margin morphologies of tree species has progressed slowly.
Betula pendula ‘Dalecarlica’ is an intraspecific variant that has leaf margins with conspicuous split lobes. Our previous study, which involved a cross-breeding test, confirmed that leaf dehiscence is a discrete characters associated with ‘Dalecarlica’ plants. To identify the key genes controlling the leaf dehiscence trait, we compared the transcriptomes of B. pendula ‘Dalecarlica’ and B. pendula plants. We observed that 17,146 unigenes were differentially expressed, with 51.13 and 48.87% of the genes being up- and down-regulated, respectively (Mu et al. 2013). Notably, of the differentially expressed genes, BpCUC2 was significantly up-regulated. This gene encodes a plant NAC transcription factor, which is important for regulating apical meristem formation, plant morphogenesis, and branch development. Additionally, CUC2 activity reorients PIN proteins and affects the polar localization of auxin. In the model plant, A. thaliana, the synergistic effects of CUC2, PINs, and auxin ensure the accumulation of different amounts of auxin in various parts of the leaf margin to generate stable serration patterns (Bilsborough et al. 2011). A previous investigation of B. pendula ‘Dalecarlica’ leaf gene expression patterns revealed that BpCUC2 is closely related to the formation of split lobes (Qu et al. 2017).
Promoters are key regulators of gene expression, and their sequences determine the specific expression levels of the associated genes (Butler and Kadonaga 2002). Analyzing the BpCUC2 promoter may help to clarify the BpCUC2 expression patterns and provide clues regarding the potential functions of the encoded protein. In this study, we constructed a pBpCUC2::LUC recombinant vector for birch transgenic. We examined the expression patterns of the BpCUC2 promoter in transgenic lines treated with hormones. Additionally, a yeast one-hybrid system involving a birch cDNA library was used to identify the regulator that interacts with the auxin response element of the promoter. The objective of this study was to characterize BpCUC2 functions and reveal the relationship between BpCUC2 and the mechanism mediating leaf margin variability.
Materials and methods
Plants and growth conditions
Betula platyphylla × Betula pendula was used as the material to be transformed, while mature birch zygotic embryos were selected as explants. Plants were grown in a tissue culture room at 22–25 °C with a 16-h light/8-h dark photoperiod at illumination intensity of 1000–1500 cd × sr/m2.
Bioinformatics analysis and cloning of the BpCUC2 promoter
The regulatory elements in the promoter regions were analyzed using the PLACE online tool and the related database of plant cis-acting regulatory DNA elements (http://www.dna.affrc.go.jp/PLACE/) (Higo et al. 1999). Genomic DNA was isolated from birch leaves using DNAquick Plant System (TIANGEN), and a 1497-bp sequence upstream of the ATG translation start codon of BpCUC2 was analyzed. The following primers were designed according to the 5′ upstream region of BpCUC2: upstream primer F (5′-CCGGAATTCAAGGCAAGAATACAG-3′) and downstream primer R (5′-CGCGGATCCAGAAACGAAAATAAGAAAG-3′). The polymerase chain reaction (PCR) amplification was completed in a 20-μL solution consisting of 0.4 µg DNA template, 0.5 µL 10 µM forward primer, 0.5 µL 10 µM reverse primer, 1.6 µL 25 mM dNTPs, 2 µL 10 × Pfu buffer, 0.4 µL Pfu DNA Polymerase (2.5 U/µL) (TaKaRa), and double-distilled H2O up to 20 µL. The PCR cycling conditions were as follows: 94 °C for 2 min; 30 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 2.5 min; 72 °C for 15 min. The PCR product was analyzed by 1% agarose gel electrophoresis, and the expected 1497-bp amplicon was purified with the Universal DNA Purification Kit (TIANGEN).
Construction of the pBpCUC2::LUC vectors
The promoter sequence was excised by a digestion with EcoRI and BamHI, purified, and cloned into the EcoRI- and BamHI-digested pCAMBIA1300-luciferase vector at 16 °C overnight. The resulting plasmid was transformed into chemically competent E. coli cells. The cells were added to agar-solidified LB medium supplemented with hygromycin (50 mg/L) and kanamycin (50 mg/L) to screen for recombinant pBpCUC2::LUC clones. After verifying the accuracy of the construct by sequencing, the plasmid was introduced into Agrobacterium tumefaciens EHA105 cells by electroporation (Yang et al. 2015). pCAMBIA1300-luciferase vectors were stored in our laboratory, while the 35S::LUC construct was provided by Wuhan Transduction Bio Co., Ltd, Wuhan, China.
Birch transformation and analysis of transformants
The pBpCUC2::LUC construct was incorporated into mature birch zygotic embryos using a Agrobacterium-mediated transformation procedure (Zhang, unpublished). Control zygotic embryos were transformed with the 35S::LUC construct. The transformed zygotic embryos were incubated in co-cultivation medium [woody plant medium (WPM) containing 0.8 mg/L benzyladenine (BA), 0.02 mg/L naphthalene acetic acid (NAA), and 0.5 mg/L gibberellic acid (GA3)] in the dark at 25 °C for 2 days. The zygotic embryos were then incubated in selection medium (WPM containing 0.8 mg/L BA, 0.02 mg/L NAA, 0.5 mg/L GA3, 50 mg/L hygromycin, and 200 mg/L cefotaxime). After 20 days, parts of shoots and leaves were transferred to a second selection medium (WPM containing 1.0 mg/L BA, 100 mg/L hygromycin and 200 mg/L cefotaxime). The resistant shoots were transferred to differential medium (WPM containing 1.0 mg/L BA, 100 mg/L hygromycin, and 200 mg/L cefotaxime). Shoots that grew to 2 cm in height were transferred to rooting medium (WPM containing 0.2 mg/L NAA).
Total DNA was extracted from the leaves of wild-type and pBpCUC2::LUC transgenic plants using the DNAquick Plant System (TIANGEN). The extracted DNA was used as the template for the PCR amplification of LUC using the following primers: forward: 5′-ATGGAAGATGCCAAAAACATTAAG-3′ and reverse: 5′-TTACACGGCGATCTTGCCGCCC-3′. The PCR cycling conditions were as follows: 94 °C for 2 min; 30 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 2.5 min; 72 °C for 15 min. The PCR product was analyzed by 1.0% agarose gel electrophoresis.
Detection of the BpCUC2 promoter expression pattern
The pBpCUC2::LUC transgenic lines were grown for 25 days. They were then sprayed with 0.15 mg/mL luciferin (Duchefa Biochemie, Haarlem, The Netherlands) and incubated in darkness for a few minutes. NightSHADE LB 985 (Berthold, Bad Wildbad, Germany) was used to visualize the luciferase chemiluminescence and the exposition time was 30 s.
Analysis of relative LUC expression in transgenic birch plants in response to hormone treatments
For the hormone treatments, transgenic leaves were sprayed with water (control), 100 µM abscisic acid (ABA), 100 µM methyl jasmonate (MeJA), 50 µM GA3, or 50 µM indoleacetic acid (IAA). After 2 h, the leaves were sprayed with 0.15 mg/mL luciferin, and NightSHADE LB 985 was used to visualize the luciferase chemiluminescence and the exposition time was 30 s.
Total RNA was extracted from the roots, stems, leaves, and buds of transgenic pBpCUC2::LUC plants using the Universal Plant Total RNA Extraction Kit (BioTeKe). Quantitative real-time PCR (qRT-PCR) was conducted using the SYBR Green PCR Master Mix (Toyobo Co., Ltd, Osaka, Japan) and an ABI 7500 Real-Time PCR system. BpUBC and BpSAND were used as internal controls, and the relative LUC mRNA level was then calculated by the formula: relative mRNA level = 2−∆∆CT (Schmittgen and Livak 2008).
Detection of organ-specific BpCUC2 expression patterns
Total RNA was extracted from the roots, stems, leaves, and buds of 25-day-old transgenic plants using the Universal Plant Total RNA Extraction Kit (BioTeKe). The qRT-PCR was conducted as described above. Bp18S was used as the internal control.
Screening to identify the protein that interacts with the BpCUC2 promoter
We selected an auxin response element (CATATG) based on an analysis of the BpCUC2 promoter sequence. Quadruplicate repeats of a fragment encoding this auxin response element were inserted into the pAbAi bait vector for a yeast one-hybrid screening of the library following the Yeastmaker Yeast Transformation System 2 protocol (Clontech, USA). The yeast one-hybrid assay was conducted by TaKaRa Bio Inc. In order to further confirm the results of yeast one-hybridization in plant cell environment, we inserted tripartite repeats of the putative element into the modified pCAMBIA1301 plasmid (CaMV 35S::hygromycin was removed and a minimal 35S: − 46 to + 1 bp promoter was added in front of the GUS gene) to construct a reporter vector. This report vector was named pCAM-CATATG. At the same time, the ORF (deletion terminator) sequence of 14-3-3-like protein B, 14-3-3-like protein GF14 kappa isoform X1, ribosomal protein L3 (RPL3), heat shock cognate protein 80 (HSP80), hypersensitive-induced response protein1 (HIR1) and hypersensitive-induced response protein1 (IPPase) gene was inserted into prokII vector to construct effector (Table S1), and these effect vectors were named prokII-14-3-3, prokII-14-3-3 GF14, prokII-RPL3, prokII-HSP80, prokII-HIR1 and prokII-IPPase. All reporters and effectors were transiently transformed into tobacco using the agrobacterium-mediated transformation method and all co-transformed tobacco leaves were used to measure and stain GUS activity (Jefferson 1989; Yang et al. 2016).
Results
Cloning of the BpCUC2 promoter and construction of the pBpCUC2::LUC vector
The BpCUC2 promoter fragment (approximately 1497 bp) was amplified by PCR using primers with restriction enzyme sites.
The recombinant pBpCUC2::LUC constructs (Fig. 1) were verified by DNA sequencing, and the sequences were compared using the BioEdit program.
Generation of transgenic birch plants
Birch zygotic embryos were transformed with pBpCUC2::LUC or 35S::LUC (positive control). After co-culturing, zygotic embryos were cultivated on selection medium for 20 days to generate green callus from cut sites (Fig. 2a). After 20 days, multiple transgenic shoots were generated from callus (Fig. 2b). Parts of shoots and leaves were transferred to a second selection medium (Fig. 2c). We finally obtained three independent hygromycin-resistant transgenic lines (Fig. 2d) (i.e., pBpCUC2-1, pBpCUC2-2, and pBpCUC2-3) along with two positive control lines containing the 35S::LUC construct.
The three pBpCUC2::LUC transgenic lines were analyzed by a PCR with primers LUC-F and LUC-R (Table 1). A 1653-bp band was generated for all pBpCUC2::LUC transgenic lines, but not for the no template control (double-distilled water) and wild-type plants (Fig. 3). These results provided preliminary evidence that the BpCUC2 promoter sequence had been integrated into the B. platyphylla × B. pendula genome.
Spatial BpCUC2 expression patterns
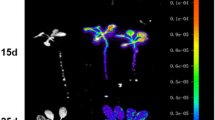
In plants sprayed with luciferin, LUC was expressed in the roots, stems, leaves, and buds (Fig. 4a), but the fluorescence intensity was different in different tissues.
Spatial BpCUC2 expression patterns. a Luciferase chemiluminescence in pBpCUC2::LUC transgenic lines. a Wild-type; b 35S::LUC transgenic lines; c pBpCUC2::LUC transgenic lines. The scale unit is mm and the pictures were merged images with luciferase signals and visible images. b Expression analysis determined by qRT-PCR analysis of LUC in transgenic lines. The experiments were replicated three times under identical conditions, error bars represent the SE. Different letters indicate a statistically significant difference when analyzed by one-way ANOVA and a multiple comparison using Duncan’s test at P < 0.05
The BpCUC2 expression levels in different tissues were also investigated by qRT-PCR. We found that LUC expression was highest in buds (i.e., sixfold higher expression level than that of the roots), followed by the leaves. The lowest expression level was observed in the roots. These findings also suggested that the BpCUC2 promoter activity varied among the buds, stems, leaves, and roots, ultimately leading to diverse BpCUC2 expression patterns (Fig. 4b).
The BpCUC2 expression levels in different tissues of 3-month-old wild-type birch plants were investigated using qRT-PCR (Fig. 5). The BpCUC2 relative expression level in wild-type birch was very similar to the LUC relative expression level in pBpCUC2::LUC transgenic birch. The highest and lowest expression levels were observed in buds and roots, respectively.
Expression analysis determined by qRT-PCR analysis of BpCUC2 in WT birch. 18S rRNA was used as an internal control. The experiments were replicated three times under identical conditions, error bars represent the SE. Different letters indicate a statistically significant difference when analyzed by one-way ANOVA and a multiple comparison using Duncan’s test at P < 0.05
Identification of the BpCUC2 promoter cis-regulatory elements
To identify the likely BpCUC2 cis-acting elements, the BpCUC2 promoter region (i.e., 1497-bp genomic region upstream of the translation start site) was used to search the PLACE database (http://www.dna.affrc.go.jp/PLACE/signalscan.html). Several cis-acting elements related to plant growth and development, phytohormone responses, and abiotic stress responses were identified. The basic transcriptional regulatory elements (i.e., TATA-box and CAAT-box) were also detected (Table 2). Additionally, the −10PEHVPSBD and CIACADIANLELHC motifs, which influence growth and development, were identified. Furthermore, many hormone-related cis-acting elements were detected, including IAA-responsive elements (i.e., ARFAT and CATATGGMSAUR), GA-responsive element (GARE-motif), MeJA-responsive element (CGTCA motif), and ABA-responsive element (ABRE). We also detected some stress-related (i.e., hydropenia and heat shock protein-related) cis-acting elements.
Response of pBpCUC2::LUC to hormone treatments
Several hormone-responsive elements were identified in the BpCUC2 promoter sequence, indicating that BpCUC2 may affect various phytohormone-related metabolic activities that regulate plant growth and development. These results prompted us to investigate the effects of hormones on pBpCUC2::LUC. The leaves of transgenic plants were sprayed with water (control), 100 µM ABA, 100 µM MeJA, 50 µM GA3, or 50 µM IAA, and then sprayed with luciferin after 2 h. The resulting fluorescence analyses revealed that the pBpCUC2 promoter was responsive to ABA, MeJA, GA, and IAA (Fig. 6a).
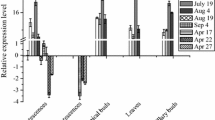
Response of pBpCUC2::LUC to hormone treatments. a Fluorescence analysis of rooted seedlings 2 h after being sprayed with water (control), 100 µM ABA, 100 µM MeJA, 50 µM GA3, or 50 µM IAA. b The changes of pBpCUC2:LUC transgenic birch buds, stems, leaves and roots in response to 100 µM MeJA, 100 µM ABA, 50 µM IAA, 50 µM GA after 2 h. Two stars indicate a statistically significant difference between hormone treatments and water treatment at P < 0.05 when analyzed by one-way ANOVA
To further determine the hormone-induced expression levels of genes under the control of the BpCUC2 promoter, qRT-PCR was also used to analyze LUC transcript abundance in response to ABA, GA3, MeJA, and IAA in different tissues. Transgenic lines that were not sprayed with plant hormones were used as controls (Fig. 6b). In the buds of MeJA- and IAA-treated plants, the LUC expression levels were 6 and 10% lower than the control levels, respectively. In contrast, the LUC expression levels were 30 and 20% higher in the buds of the ABA- and GA3-treated plants than in the controls. In stems, LUC expression was down-regulated by 15, 41, 62, and 32% in response to four plant hormones. In leaves, the MeJA treatment increased the LUC relative expression level by only 3%, while the ABA, IAA, and GA3 treatments down-regulated the LUC relative expression level by 42, 34, and 74%, respectively. In roots, the MeJA, ABA, and GA3 treatments up-regulated LUC expression by 94, 168, and 45%, respectively. However, LUC expression increased by only 17% in IAA-treated roots. These results confirmed that the birch tissues responded differently to various hormones. In MeJA-treated plants, the LUC transcript levels increased in the roots, but decreased in the stems. Meanwhile, ABA significantly promoted LUC relative expression in the buds and roots, but inhibited LUC relative expression in the leaves and stems. We observed that IAA significantly inhibited LUC relative expression in the buds, leaves, and stems, but not in the roots. Additionally, GA3 significantly induced LUC expression in the buds and roots, but had the opposite effect in the stems and leaves.
Identification of the protein that interacts with the BpCUC2 promoter using a yeast one-hybrid assay
CUC2 affects the polar localization of auxin (Bilsborough et al. 2011), and our analyses revealed that the BpCUC2 promoter activity was responsive to IAA in birch buds, stems, and leaves. We constructed a bait vector carrying an IAA-responsive element (i.e., CATATG) to screen for the protein that interacts with the BpCUC2 promoter in a yeast one-hybrid assay.
Ninety-six positive clones for the candidate binding protein cDNA sequences were obtained using the yeast one-hybrid library. After a second selection step, six positive yeast colonies were analyzed by DNA sequencing with the pGADT7-Rec primers (Table 3). The subsequent sequence alignment indicated that the six sequences were unique, while a BLAST search detected homologs in Gossypium raimondii, Betula luminifera, Jatropha curcas L., Camelina sativa, Vitis vinifera, and Sesamum indicum. Furthermore, the potential functional domains were analyzed using the Simple Modular Architecture Research Tool. All of the sequences contained regulatory motifs, indicating that these transcription factors might influence the responses of auxin signaling pathways. In order to further validate the binding of 14-3-3-like protein B (14-3-3), 14-3-3-like protein GF14 kappa isoform X1 (14-3-3 GF14), ribosomal protein L3 (RPL3), heat shock cognate protein 80 (HSP80), hypersensitive-induced response protein1 (HIR1) and soluble inorganic pyrophosphatase-like isoform X1 (IPPase) proteins to auxin response element (CATATG) in a plant cell environment, we constructed a related reporter and effector and transform them into tobacco cells by transient transformation experiments (Fig. 7a). The results showed that the GUS activity of the co-transformed pCAM-CATATG with pROKII-14-3-3-like protein B, pCAM-CATATG with pROKII-RPL3 and pCAM-CATATG with pROKII-IPPase were similar to that of the positive control group, whereas other effectors did not activate GUS expression (Fig. 7b, c). Indicating that 14-3-3-like protein B, RPL3 and IPPase and auxin response element (CATATG) in the plant cell environment can still be combined, that is, 14-3-3-like protein B, RPL3 and IPPase is a regulator of BpCUC2 gene upstream.
Yeast one-hybrid analyses of the upstream regulators of BpCUC2. a Schematic diagram of the reporter and effector vectors that constructed for yeast one-hybrid verification. b The co-expression of the reporter and effector vectors in tobacco leaves. c GUS activity according to (b). 14-3-3, RPL3, IPPase indicate 14-3-3-like protein B, ribosomal protein L3, soluble inorganic pyrophosphatase-like isoform X1 gene, respectively; pCAMBIA1301 indicates the positive control. Data indicate means ± SE of three biological replicates (P < 0.05)
Discussion
The CUC2 transcription factor is important for the initiation of the shoot apical meristem via the regulation of STM (SHOOT MERISTEMLESS) expression. It is also involved in establishing the cotyledon and floral organ boundaries (Aida et al. 1999). However, the promoter and function of the birch BpCUC2 have not been fully characterized. In this study, we generated pBpCUC2::LUC transgenic birch plants, and analyzed LUC expression levels in the buds, leaves, stems, and roots using qRT-PCR. We observed that LUC was most highly expressed in the buds, which is consistent with the fact that CUC2 is a boundary-specific gene (Takada et al. 2001). This result is also in agreement with the spatial expression patterns of BpCUC2. Using AtGenExpress Visualization Tool (http://jsp.weigelworld.org/expviz/expviz.jsp), we found that our results was similar in Arabidopsis, as AtCUC2 gene is also highly expressed in apex, suggesting that BpCUC2 may have the same function in apical meristem as AtCUC2. However, it is unclear where in the apical meristem BpCUC2 is primarily expressed, which need further investigation.
Several cis-acting elements involved in plant growth and development, phytohormone responses, and abiotic stress responses were identified in the BpCUC2 promoter, including many hormone-responsive elements. This implies that BpCUC2 may be associated with the metabolism of various phytohormones. Furthermore, the hormones differentially affected the various birch tissues, suggesting that the regulation of birch growth and development by BpCUC2 might be related to the biosynthesis and signal transduction of four hormones. The IAA treatment down-regulated LUC expression in the shoots, stems, and leaves. This is consistent with the model for the regulation of A. thaliana leaf margin development in which auxin inhibits CUC2 expression. This implies that BpCUC2 may mediate the mechanism underlying the formation of birch leaf margin lobes. Additional research will be needed to verify this possibility.
The regulation of the transcription factors is mainly divided into transcriptional regulation, post-transcriptional regulation, and post-translational regulation. Transcription factors bind to cis-acting elements in the promoter of specific genes. Using a yeast one-hybrid assay, we screened for proteins that interact with the BpCUC2 promoter. We identified three development-related proteins including 14-3-3 protein, ribosomal protein L3 (RPL3) and soluble inorganic pyrophosphatase-like isoform X1. Among them, 14-3-3 proteins, which are present in almost all eukaryotes can bind to target proteins to enhance or inhibit catalytic activities (Camoni et al. 1998; van Hemert et al. 2001). Additionally, 14-3-3 proteins are also involved in transducing signals in diverse plant hormone signaling pathways (Ishida et al. 2004; Del et al. 2007), suggesting that they may also affect the auxin-induced response of BpCUC2. Up-regulated RPL3 expression stimulates plant organ growth during the vegetative stage (Popescu and Tumer 2004). The production of a bacterial soluble inorganic pyrophosphatase (IPPase) in the cytosol of transgenic tobacco and potato plants considerably alters metabolic activities, growth, and development (Sonnewald 1992). These results indicated that BpCUC2 may also associated with growth and development in Betula platyphylla × Betula pendula. However, it is unclear if the relationship between these proteins and the formation of birch leaf lobes involves hormone signaling pathways. It is important to note that in this study, only one of the auxin response elements was analyzed. Thus, relatively few upstream regulatory genes were screened, and other auxin response elements should be investigated in future experiment.
Identifying important cis-acting elements in the promoter of specific target genes is important for characterizing the regulation of gene expression levels. Many studies have focused on gene functions, but there is considerably less information available about gene promoters. In this study, we confirmed that the 1497-bp BpCUC2 promoter region upstream of the translation initiation codon can induce LUC expression in the buds, leaves, roots, and stem of transgenic birch plants, especially in buds. The regulatory genes upstream of auxin response elements in promoter sequences were analyzed based on the results of a yeast one-hybrid assay. We will further analyze the relationship between these genes and the regulation of BpCUC2 expression. The results of this study provide novel insights into birch BpCUC2 expression characteristics and the upstream regulatory genes, and represent a solid foundation for future investigations of BpCUC2 functions.
Abbreviations
- CUC2 :
-
CUP-SHAPED COTYLEDON2
- SAM:
-
Shoot apical meristem
- BpCUC2 :
-
Betula platyphylla × Betula pendula CUC2
- pBpCUC2:
-
BpCUC2 promoter
- LUC :
-
Luciferase
- PINs:
-
PIN-formed proteins
- SD:
-
Synthetic dropout medium
- Leu:
-
Leudne
References
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570
Bilsborough GD et al (2011) Model for the regulation of Arabidopsis thaliana leaf margin development. PNAS 108:3424–3429
Butler JE, Kadonaga JT (2002) The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev 16:2583–2592
Camoni L, Harper JF, Palmgren MG (1998) 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett 430:381–384
Del VF, Casaretto JA, Quatrano RS (2007) 14-3-3 Proteins are components of the transcription complex of the ATEM1 promoter in Arabidopsis. Planta 227:167–175
Hasson A, Plessis A, Blein T, Adroher B, Grigg S, Tsiantis M, Boudaoud A, Damerval C, Laufs P (2011) Evolution and diverse roles of the CUP-SHAPED COTYLEDON genes in Arabidopsis leaf development. Plant Cell 23:54–68
Hay A, Barkoulas M, Tsiantis M (2006) ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133:3955–3961
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27:297–300
Ishida S, Fukazawa J, Yuasa T, Takahashi Y (2004) Involvement of 14-3-3 signaling protein binding in the functional regulation of the transcriptional activator REPRESSION OF SHOOT GROWTH by Gibberellins. Plant Cell 16:2641–2651
Jefferson RA (1989) The GUS reporter gene system. Nature 342:837
Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2010) TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 22:3574–3588
Mu H, Lin L, Liu G, Jiang J (2013) Transcriptomic analysis of incised leaf-shape determination in birch. Gene 531:263–269
Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, Aida M, Laufs P (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18:2929–2945
Popescu SC, Tumer NE (2004) Silencing of ribosomal protein L3 genes in N. tabacum reveals coordinate expression and significant alterations in plant growth, development and ribosome biogenesis. Plant J 39:29–44
Qu C, Bian X, Jiang J, Chen S, Liu G (2017) Leaf morphological characteristics and related gene expression characteristic analysis in Betula pendula ‘Dalecarlica’ and Betula pendula. J Beijing For Univ 39(8):9–16
Ramirez J, Bolduc N, Lisch D, Hake S (2009) Distal Expression of knotted1 in maize leaves leads to reestablishment of proximal/distal patterning and leaf dissection. Plant Physiol 151:1878–1888
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Sonnewald U (1992) Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J 2:571–581
Takada S, Hibara K, Ishida T, Tasaka M (2001) The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128:1127–1135
van Hemert MJ, Steensma HY, van Heusden GP (2001) 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays 23:936–946
Yang G, Chen S, Wang S, Liu G, Li H, Huang H, Jiang J (2015) BpGH3.5, an early auxin-response gene, regulates root elongation in Betula platyphylla × Betula pendula. Plant Cell Tissue Org 120(1):239–250
Yang G, Wang C, Wang Y, Guo Y, Zhao Y, Yang C, Gao C (2016) Overexpression of ThVHAc1 and its potential upstream regulator, ThWRKY7, improved plant tolerance of cadmium stress. Sci Rep 6:18752
Acknowledgements
This work was supported by the National Natural Science Foundation of China “Cloning and Functional Study on the Leaf Margin-related Genes of Betula platyphylla” Grant (Grant No. 31370660) and 111 Project (B16010). The funding source had no other involvement.
Author information
Authors and Affiliations
Contributions
CL, HX: performed all the experiments. SW assisted with DNA sequence analysis. GL, JJ together with CL, HX and SW designed the experiments and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by John J Finer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, C., Xu, H., Jiang, J. et al. Analysis of the promoter features of BpCUC2 in Betula platyphylla × Betula pendula . Plant Cell Tiss Organ Cult 132, 191–199 (2018). https://doi.org/10.1007/s11240-017-1324-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1324-2