Abstract
Black ash (Fraxinus nigra) is an endangered hardwood tree species under threat of extirpation by the emerald ash borer (EAB), an aggressive exotic phloem-feeding beetle. We have developed an efficient regeneration system through adventitious shoot organogenesis in F. nigra using in vitro-derived leaf explants. Two types of leaf explants were cultured on Murashige and Skoog (MS) medium supplemented with different concentrations of plant growth regulators to induce callus and adventitious shoot bud formation. Significant effects of explant, and plant growth regulator interactions were found. The frequency of callus formation ranged from 77.8 to 94.4% and 88.9–100% from single leaflets and intact compound leaves, respectively, with no significant difference between treatments. For adventitious shoot bud induction, however, 22.2 µM 6-benzylaminopurine (BA) combined with 31.8 µM thidiazuron (TDZ) was the best treatment regardless of the initial leaf explant type, showing 21.1 and 28.8% shoot bud induction, with 1.5 and 1.9 adventitious shoots per explant, from single leaflets and intact compound leaves, respectively. The regenerated shoot buds were elongated on MS medium supplemented with Gamborg B5 vitamins plus 2 mg L−1 glycine (MSB5G), 13.3 µM BA, 1 µM indole-3-butyric acid (IBA), and 0.29 µM gibberellic acid. The elongated shoots were continuously micropropagated through nodal stem sectioning until used for rooting. An average of 85.2% of the microshoots were successfully rooted in woody plant medium containing 5.7 µM indole-3-acetic acid plus 4.9 µM IBA with a 10-day initial dark culture, followed by culture under a 16-h photoperiod. Rooted plantlets were acclimatized to the greenhouse and showed normal plant growth and development with 100% survival. This regeneration protocol would be useful for mass propagation for conservation of F. nigra and for use in genetic transformation for EAB resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black ash (Fraxinus nigra Marsh.) is a hardwood tree species in North America with a native range in wetland forests from Newfoundland west to Manitoba, south to Indiana and West Virginia (Wright and Rauscher 1990). The strongly ring-porous wood is preferred by Native Americans for making splints for basketry, and also used commercially for furniture, veneer, pulpwood, and non-timber forest products (Benedict and Frelich 2008). Black ash is ecologically valuable as the seeds are consumed by a number of birds and mammals, while twigs and foliage are eaten by white-tailed deer and moose (Anderson and Nesom 2003). While most of the urban and residential ash trees are predominantly white and green ash (F. americana L. and F. pennsylvanica Marsh.) (Kovacs et al. 2010), black ash inhabits wetland forests and is integral to riparian ecosystems (Nisbet et al. 2015). However, the emerald ash borer (EAB; Agrilus planipennis Fairmaire), an aggressive exotic phloem-feeding beetle from Asia, has destroyed tens of millions of ash trees in the United States since the first detection in 2002 in Michigan. EAB is fatal to all native North American ash trees, showing 99% mortality of black, green, and white ash trees with stems greater than 2.5 cm in diameter in forests of southeastern Michigan (Klooster et al. 2014). To date, there is no means to completely eradicate the beetle, and it appears that EAB could functionally extirpate ash in North America with a huge economic and ecological loss (Poland and McCullough 2006; Herms and McCullough 2014). According to a modeling study conducted by Iverson et al. (2016), climate change along with the devastating short-term effects of EAB offered a bleak prospect for the continued existence of black ash in Minnesota.
In vitro plant regeneration is a powerful tool for germplasm conservation of endangered plant species (Jin et al. 2014; Slazak et al. 2015; Wang et al. 2014). Several features of black ash, such as irregular seed production intervals, embryo immaturity at seed set, and complex stratification and germination requirements, make the use of in vitro regeneration technology more feasible (Benedict and David 2003; Gucker 2005; Vanstone and LaCroix 1975). This technology is also useful for production of important secondary metabolites, and a pre-requisite for genetic transformation to confer a new trait such as EAB-resistance. Adventitious shoot regeneration has been established in a number of ash species including white ash (Bates et al. 1992; Palla and Pijut 2011), green ash (Du and Pijut 2008), common ash (F. excelsior) (Mockeliunaite and Kuusiene 2004), narrowleaf ash (F. angustifolia) (Tonon et al. 2001), pumpkin ash (F. profunda) (Stevens and Pijut 2012), and black ash (Beasley and Pijut 2013), using various seed-derived organs such as hypocotyls and cotyledons. But there are no reports on adventitious shoot regeneration from leaf explants and regeneration of whole plants in Fraxinus. The ash seed bank was rapidly depleted and no viable ash seeds were found in several Michigan sites following invasion by EAB (Klooster el al. 2014), indicating limited availability of the use of seed-derived materials. Thus, there is a great need to develop an efficient protocol for shoot regeneration from leaf explants. Black ash leaves are deciduous, opposite, pinnately compound with 7–11 sessile leaflets (Anderson and Nesom 2003). Leaves are more readily available and usually do not produce inhibitory compounds when cultured in vitro, making this type of explant ideal for use in regeneration systems. Furthermore, development of an in vitro regeneration protocol using leaf explants would be useful to establish a genetic transformation system for multiple gene manipulation via gene stacking. The present study was designed to establish an efficient protocol for adventitious shoot regeneration from in vitro leaf explants of black ash.
Materials and methods
Plant material and culture medium
In vitro shoot cultures of black ash (established from open-pollinated seed, National Tree Seed Centre, Fredericton, New Brunswick, Canada) were maintained in Magenta™ GA-7 vessels (Magenta Corp., Chicago, IL) containing a modified Murashige and Skoog (1962) (MS) basal medium (M499; PhytoTechnology Laboratories, Shawnee Mission, KS) with Gamborg B5 vitamins (Gamborg et al. 1968) plus 2 mg L−1 glycine (MSB5G), supplemented with 13.3 µM 6-benzylaminopurine (BA), 1 µM indole-3-butyric acid (IBA), 0.2 g L−1 casein hydrolysate, and 0.29 µM gibberellic acid (GA3) (Beasley and Pijut 2013). Unless noted otherwise, all media contained 3% (w/v) sucrose and 0.7% (w/v) Bacto agar (No. 214030; Becton Dickinson and Co., Sparks, MD) with the pH adjusted to 5.7 before autoclaving for 20 min at 121 °C. Cultures were maintained in a growth room at 24 ± 2 °C under a 16-h photoperiod at approximately 80 µmol m−2 s−1 provided by cool-white fluorescent lamps. The in vitro shoots were regularly subcultured to fresh medium every 4 weeks, and micropropagated by nodal stem sectioning.
Effect of explant type and plant growth regulator on callus formation and shoot bud induction
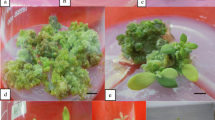
The whole compound leaf (five leaflets attached; Fig. 1a) and single leaflets (Fig. 1b) were used as explants. Leaf explants obtained from 4-week old in vitro cultures (after micropropagation) were transversely cut two or three times across the midrib and cultured with the abaxial surface in contact with the shoot bud induction medium [MSB5G medium supplemented with 0.5 µM IBA, 10% (v/v) coconut water (C195; PhytoTechnology Laboratories), plus BA and thidiazuron (TDZ)]. To study the effect of different concentrations of plant growth regulators (PGRs) on callus formation and adventitious shoot bud induction, we tested 0, 22.2, 26.2, 31.1, or 35.5 µM BA in combination with 27.2 or 31.8 µM TDZ (selected by preliminary factorial experiments with different concentrations of BA (0–35.5 µM) and TDZ (0–36.3 µM); data not shown). Three replicates of 12–15 leaflets or compound leaves each were cultured for each treatment. Cultured leaf explants were incubated in the dark at 26 ± 2 °C for 3 weeks, and then transferred to 80 µmol m−2 s−1 light intensity for culture one additional week before evaluating the frequency of callus formation and adventitious shoot bud induction. Explants forming callus were then transferred to MS medium containing 13.3 µM BA, 4.5 µM TDZ, 0.05 g L−1 adenine hemisulfate, and 10% coconut water. After an additional 3 weeks the number of shoots per explant was recorded.
Callus formation from leaf explants of Fraxinus nigra (black ash). Compound leaves with five leaflets attached (a) or single leaflets (b) were placed on induction medium. Each leaf was transversally cut two or three times across the midrib and cultured with the abaxial surface in contact with the medium. c, d Callus was induced from the cuts on the abaxial side and petiole ends after 4 weeks (3 weeks in the dark followed by 1 week in the light) bar 1 cm
Adventitious shoot elongation, rooting, and acclimatization
Once adventitious shoot buds were initiated, shoot elongation, rooting, and acclimatization followed our previous protocol (Beasley and Pijut 2013). Briefly, all explants initiating shoot buds were transferred to MSB5G medium supplemented with 6.7 µM BA, 1 µM IBA, and 0.29 µM GA3 in Magenta™ GA-7 vessels for 3 weeks. Cultures were then transferred to MSB5G medium with 13.3 µM BA, 1 µM IBA, 0.2 g L−1 casein hydrolysate, and 0.29 µM GA3. Elongated shoots were excised from leaf explants, subcultured every 4 weeks to fresh medium, and micropropagated through nodal stem sectioning. Elongated microshoots (3–4 cm) were induced to form roots on woody plant medium (WPM; Lloyd and McCown 1980) supplemented with 5.7 µM indole-3-acetic acid (IAA) and 4.9 µM IBA in Magenta™ GA-7 vessels (Beasley and Pijut 2013). Three replications with nine microshoots each were conducted to verify our previous protocol. Microshoots on root induction medium were incubated in the dark at 26 ± 2 °C for 10 days and then transferred to a 16-h photoperiod (80 µmol m−2 s−1). After 6 weeks on root induction medium, the frequency of root formation, number of roots and lateral roots per microshoot, and length of roots were evaluated. Rooted plantlets were acclimatized to the greenhouse as described by Beasley and Pijut (2013).
Statistical analysis
Data were analyzed using SPSS 23.0 statistical software (IBM-SPSS 2015). The mean with standard error (±SE) was presented. The percent callus formation, shoot bud induction, and number of shoots were subjected to analysis of variance (ANOVA). Significant difference between treatments was tested by a Duncan’s multiple comparison test (p = 0.05). The effects of explant type, BA, and TDZ and their interactions were examined using a three-way ANOVA.
Results and discussion
Effect of explant type and plant growth regulator on callus formation and shoot bud induction
In the present study, we developed the first protocol on plant regeneration from leaf explants of F. nigra. Although several studies have reported regeneration protocols for Fraxinus spp. using seed-derived explants such as hypocotyls or cotyledons, there has been no study using leaf explants. In vitro leaf explants were used in this study because leaves often show a better regeneration potential than explants derived from mature tissue (Harding et al. 1996). Furthermore, in vitro leaf explants are more feasible for use in genetic transformation studies because they are aseptic, and gene stacking techniques would be feasible.
We initially tested leaf explants on the best adventitious shoot induction medium (MS medium supplemented with 13.3 µM BA and 4.5 µM TDZ) previously developed in our laboratory with black ash hypocotyl explants, but no response for callus formation and shoot bud induction was obtained (data not shown). We then optimized adventitious shoot regeneration for leaf explants using a combination of BA and TDZ at various concentrations. After the first 4 weeks on shoot bud induction medium, the first visible change was the enlargement in size of leaf explants with callus formation on the cuts in midrib and the petiole base (Fig. 1c, d). Through these cut edges more nutrients and PGRs could be absorbed efficiently from the induction medium, as proposed by Sarwar and Skirvin (1997). Most explants produced callus, with the frequency of callus formation ranging from 77.8–94.4% to 88.9–100% from single leaflet and compound leaf, respectively (Table 1). Average percent callus formation was 87.5 ± 1.9 and 94.5 ± 1.4 from single leaflet and compound leaf, respectively, with a significant effect of explant type on callus formation (F = 8.74, p < 0.01; Tables 1, 2). A three-way ANOVA revealed a significant interaction between BA and TDZ on percent callus formation (Table 2).
Visible protuberances and multiple outgrowths which subsequently developed into adventitious shoot buds were observed (Fig. 2a–c). Most of the shoot buds developed from callus formed on the abaxial side of the leaf in contact with the medium (Fig. 2d), but some shoot buds developed on the adaxial side (Fig. 2e) or from callus formed on the petiole base (Fig. 2f). This result was similar with those of Pérez-Tornero et al. (2000), who reported most adventitious buds originated from the leaf tissue of apricot (Prunus armeniaca L.) in contact with the medium. Different regeneration responses also have been observed in European beech (Fagus sylvatica L.) leaf explants, with better shoot formation from proximal half-leaves than distal leaf explants that might be a result of differentials of endogenous hormone transport and maturity between the distal and proximal leaf tissues (Vieitez and San-José 1996). Whereas, petioles were reported to be an excellent explant for adventitious shoot regeneration in several woody plants (Bergmann and Moon 1997; Mohammed et al. 2015). The regeneration response of princess tree (Paulownia tomentosa (Thunb.) Siebold and Zucc. ex Steud.) and dragon tree (P. fortune (Seem.) Hemsl.) were stimulated by the presence of the leaf lamina along with the attached petiole as explants, compared to using intact petioles only, suggesting the promotive effect of leaf lamina through the establishment of a gradient of diffusible factors (Corredoira et al. 2008; Kumar et al. 1998). Similarly, a possible explanation for our observation (significantly higher frequency of callus formation and shoot bud induction from compound leaf compared to single leaflet; Table 2) might be because of a promotive effect of attached leaflets through enhanced transportation of endogenous phytohormones or uptake of PGRs from the medium. We also found that compound leaves were more feasible initial starting materials than single leaflets in terms of being simple and easy to handle.
Adventitious shoot bud initiation. a Protuberance (arrow) development and (b, c) adventitious shoot bud initiation (bar 1 mm). Adventitious shoot buds arising from callus formed on the abaxial side (d), adaxial side (e), and on the petiole (f) after 4 weeks on MSB5G medium with 22.2 µM BA, 31.8 µM TDZ, and 0.5 µM IBA (bar 2 mm)
Although adventitious shoot buds were observed from all BA and TDZ concentrations tested in our study, the response of leaf explants was variable based on the relative concentrations of the two PGRs. The percent of explants with shoot bud induction ranged from 7.9 to 21.1% in single leaflet, while it varied from 11.7 to 28.8% in the compound leaf (Table 1). Average percent shoot bud induction was 15.9 ± 1.7 and 20.8 ± 1.3 from single leaflets and compound leaves, respectively, showing a significant effect of explant type on shoot bud induction (F = 5.49, p < 0.05; Tables 1, 2). The combination of 22.2 µM BA and 31.8 µM TDZ gave the best results on shoot bud induction from both single leaflet and compound leaf. There was no significant difference between treatments for mean number of adventitious shoots per explant using single leaflet; ranged from 1 ± 0.6 to 1.8 ± 1.4 (Table 1). However, the combination of 22.2 µM BA and 31.8 µM TDZ proved to produce a significantly higher number of adventitious shoots (1.9 ± 0.5 shoots per explant) when using the compound leaf (Table 1). There was no significant effect of explant on mean number of adventitious shoots (Table 2).
The concentration of BA played a key role in determining shoot bud induction, showing a significantly lower frequency of shoot bud induction on medium containing only 27.2 µM TDZ (Table 1). This result was consistent with the observation that shoot bud induction capacity of physic nut (Jatropha curcas) leaf-discs was reduced in the absence of BA (Deore and Johnson 2008). The same BA concentration (22.2 µM) was also found to be successful for shoot formation from hypocotyl explants of black ash with the highest frequency (62.5%) of shoot formation on medium with BA plus 2.3 µM TDZ (Beasley and Pijut 2013). Efficiency of BA over other cytokinins was found in tamarillo (Cyphomandra betacea) shoot regeneration from leaf explants, showing more microshoot regeneration with BA treatment compared to TDZ treatment (Kahia et al. 2015). Similarly, high BA concentration efficiently induced multiple bud formation from explants of Cavendish banana (Musa spp.) (Subramaniam et al. 2008). However, some contrary results were reported that TDZ was more effective than BA in inducing shoot buds on leaf explants of European beech (Vieitez and San-José 1996), apricot (Pérez-Tornero et al. 2000), and blackberry (Rubus hybrid) (Gupta and Mahalaxmi 2009). Rathore et al. (2016) suggested that differential responses of explants caused by different cytokinins may be a result of their varied translocation rates, differential uptake, various effects on metabolic processes, and ability to change the level of endogenous cytokinins.
In this study, a significant interaction was found between BA and TDZ on shoot bud induction (F = 3.51, p < 0.05; Table 2). The higher concentration of TDZ (31.8 µM) in combination with 22.2 µM BA produced more shoot buds, while there was negative correlation between TDZ concentration and shoot bud induction in combination with BA concentration higher than 22.2 µM (Table 1). Negative effects of over-abundance of TDZ were reported in pumpkin ash adventitious shoot formation, showing a decreased percent shoot formation with TDZ concentrations higher than 4.5 µM in combination with BA (Stevens and Pijut 2012). Lower concentrations of TDZ were reported to produce a better response in callus formation from leaf explants of Indian sandalwood (Santalum album L.) as higher concentrations were toxic to the explants and caused browning (Singh et al. 2013).
TDZ is well known as a multidimensional PGR which may have both auxin- and cytokinin-like effects, inducing diverse morphogenic responses (Guo et al. 2011). Although cytokinin-like activity of TDZ is well documented, a role of TDZ as a modulator of auxin metabolism has been suggested in several reports of TDZ-induced somatic embryogenesis which is a response commonly associated with auxins (Murthy et al. 1998). Increases in the level of IAA and its precursor, tryptophan, were observed in response to TDZ treatment that caused stimulation of de novo synthesis of auxins in peanut (Arachis hypogaea L.) (Murthy et al. 1995). In this study, we obtained good callus formation and shoot regeneration without exogenous auxin application, suggesting black ash leaf explants may contain sufficient levels of endogenous auxin or TDZ may be involved in auxin metabolism to stimulate auxin synthesis. In addition, the dark treatment may influence the levels of endogenous auxin contributing to the induction process (Miguel et al. 1996). Shoot bud browning followed by deterioration was observed when the explants were continuously cultured on the induction medium for more than the first 4 weeks (data not shown). This may be a result of adverse effects of continuous high concentration of cytokinins.
Adventitious shoot elongation, rooting, and acclimatization
The regenerated shoot buds were cultured on MS medium with a lower concentration of BA (6.7 µM) plus 1 µM IBA and 0.29 µM GA3, but without TDZ to continue adventitious shoot bud enhancement (Fig. 3a). While TDZ is a powerful inducer of shoot organogenesis in woody plants, various effects of TDZ on explants and shoots in tissue culture have been reported, including excessive callus formation, bushy shoots, and inhibiting shoot elongation (Beasley and Pijut 2013; Chalupa 1988; Huetteman and Preece 1993). A two-stage culture procedure consisting of a TDZ-treatment of explants followed by TDZ-free cultivation proved efficient in regeneration of Rhododendron sichotense with the highest frequency of shoot regeneration along with maximum number of shoots per explant (Zaytseva et al. 2016). In addition to removing TDZ, lowered BA was necessary in the medium for black ash regeneration from hypocotyl explants to continuously enhance shoot buds (Beasley and Pijut 2013). Rathore et al. (2016) also found that continuous high level of BA produced hyper-hydration in subculture of regenerated Paneer dodi (Withania coagulans Dunal) shoot buds, causing adverse effects on the growth and regeneration potential of cultures. A significant elongation of microshoots was obtained on MS medium with lowered BA (from 4.44 to 1.11 µM) (Rathore et al. 2016). However, for routine elongation of shoots regenerated from black ash hypocotyl explants, the concentration of BA needed to be increased after a lower exposure (from 6.7 to 13.3 µM) (Beasley and Pijut 2013). After 3 weeks on the shoot bud enhancement medium (Fig. 3b), regenerated shoots were cultured on shoot elongation medium with increased BA (13.3 µM) along with 0.2 g L−1 casein hydrolysate. When shoots had reached 3–4 cm in height with several nodes (Fig. 3c), micropropagation was routinely achieved through nodal stem sectioning until we obtained an adequate number of microshoots for rooting.
Leaf-explant derived shoot regeneration of Fraxinus nigra (black ash). a Adventitious shoot on shoot induction medium (bar 0.5 mm); b shoots on shoot bud enhancement medium (bar 1 cm); c microshoot elongating on shoot elongation medium (bar 1 cm); d in vitro root production (bar 1 cm); e acclimatization of a rooted plantlet in the culture room; and f acclimatized black ash plant in the greenhouse
Elongated shoots with two or three nodes were rooted on WPM supplemented with 5.7 µM IAA and 4.9 µM IBA (Fig. 3d). Callus formation was first observed at the basal end of the shoot, and roots developed from the callus 2 weeks after culture on root induction medium. We achieved 85.2% rooting with a mean of 5.6 ± 0.4 roots per shoot, with a mean root length of 2.6 ± 0.2 cm, and a mean of 2 ± 0.6 lateral roots per shoot (Table 3). Twenty-five rooted plantlets with well-developed roots were transferred to pots and acclimatized in the culture room. Normal growth was observed 2–3 weeks after acclimatization (Fig. 3e), and plants were then moved to the greenhouse. After an additional 4 weeks, plants were transplanted to larger pots for further growth. One-hundred-percent of the regenerated black ash plants survived in the greenhouse with no morphological abnormalities (Fig. 3f). Our laboratory also reported 93% rooting with 4.1 roots per shoot using this rooting procedure for black ash shoots regenerated from hypocotyls (Beasley and Pijut 2013).
Conclusions
We developed a useful protocol for complete plant regeneration of F. nigra via adventitious shoot formation from callus using leaf explants. This protocol will provide the basis for the further applications such as black ash conservation, mass propagation, as well as experimental studies to produce transgenic F. nigra, especially to introduce multiple traits of interest by gene stacking.
References
Anderson MK, Nesom G (2003) Black ash, Fraxinus nigra Marsh. NRCS Plant Guide. Natl Res Conserv Serv https://plants.usda.gov/plantguide/pdf/cs_frni.pdf
Bates S, Preece JE, Navarrete NE, Van Sambeek JW, Gaffney GR (1992) Thidiazuron stimulates shoot organogenesis and somatic embryogenesis in white ash (Fraxinus americana L.). Plant Cell Tiss Organ Cult 31:21–29
Beasley RR, Pijut PM (2013) Regeneration of plants from Fraxinus nigra Marsh. hypocotyls. HortScience 48:887–890
Benedict L, David R (2003) Propagation protocol for black ash (Fraxinus nigra Marsh.). Native Plants J 4:100–103
Benedict MA, Frelich LE (2008) Site factors affecting black ash ring growth in northern Minnesota. Forest Ecol Manag 255:3489–3493
Bergmann BA, Moon HK (1997) In vitro adventitious shoot production in Paulownia. Plant Cell Rep 16:315–319
Chalupa V (1988) Large scale micropropagation of Quercus robur L. using adenine-type cytokinins and thidiazuron to stimulate shoot proliferation. Biol Plantarum 30:414–421
Corredoira E, Ballester A, Vieitez AM (2008) Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tiss Organ Cult 95:197–208
Deore A, Johnson T (2008) High-frequency plant regeneration from leaf-disc cultures of Jatropha curcas L.: an important biodiesel plant. Plant Biotechnol Rep 2:7–11
Du N, Pijut PM (2008) Regeneration of plants from Fraxinus pennsylvanica hypocotyls and cotyledons. Sci Hortic 118:74–79
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gucker CL (2005) Fraxinus nigra. In: Fire Effects Information System. USDA, Forest Service, Washington DC http://www.fs.fed.us/database/feis/plants/tree/franig/all.html.
Guo B, Abbasi B, Zeb A, Xu L, Wei Y (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Gupta S, Mahalaxmi V (2009) In vitro high frequency direct plant regeneration from whole leaves of blackberry. Sci Hortic 120:22–26
Harding K, Benson EE, Roubelakis-Angelakis KA (1996) Methylated DNA changes associated with the initiation and maintenance of Vitis vinifera in vitro shoot and callus cultures: a possible mechanism for age-related changes. Vitis 35:79–85
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impacts, and management. Annu Rev Entomol 59:13–30
Huetteman C, Preece J (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Organ Cult 33:105–119
IBM-SPSS (2015) SPSS version 23.0, IBM-SPSS, Armonk, NY
Iverson L, Knight KS, Prasad A, Herms DA, Matthews S, Peters M, Smith A, Hartzler DM, Long R, Almendinger J (2016) Potential species replacements for black ash (Fraxinus nigra) at the confluence of two threats: emerald ash borer and a changing climate. Ecosystems 19:248–270
Jin S, Wang J, Wang X, Sun D, Li G, Genovesi AD, Liu S (2014) Direct and indirect shoot and bulblet regeneration from cultured leaf explants of Lilium pumilum, an endangered species. In Vitro Cell Dev Biol Plant 50:69–75
Kahia J, Sallah PK, Diby L, Kouame C, Kirika M, Niyitegeka S, Asiimwe T (2015) A novel regeneration system for tamarillo (Cyphomandra betacea) via organogenesis from hypocotyl, leaf, and root explants. HortScience 50:1375–1378
Klooster WS, Herms DA, Knight KS, Herms C, McCullough DG, Smith A, Gandhi, KJK, Cardina J (2014) Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol Invasions 16:859–873
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578
Kumar PP, Dimps C, Goh CJ (1998) Influence of petiole and lamina on adventitious shoot initiation from leaf explants of Paulownia fortunei. Plant Cell Rep 17:886–890
Lloyd GB, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Int Plant Prop Soc 30:421–427
Miguel CM, Druart P, Oliveira MM (1996) Shoot regeneration from adventitious buds induced on juvenile and adult almond (Prunus dulcis Mill.) explants. In Vitro Cell Dev Biol Plant 32:148–153
Mockeliunaite R, Kuusiene S (2004) Organogenesis of Fraxinus excelsior L. by isolated excelsior mature embryo culture. Acta Univ Latviensis Biol 676:197–676
Mohammed A, Chiruvella KK, Namsa ND, Ghanta RG (2015) An efficient in vitro shoot regeneration from leaf petiolar explants and ex vitro rooting of Bixa orellana L.—a dye yielding plant. Physiol Mol Biol Plants 21:417–424
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1995) Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): Endogenous growth regulator levels and significance of cotyledons. Physiol Plantarum 94:268–276
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 34:267
Nisbet D, Kreutzweiser D, Sibley P, Scarr T (2015) Ecological risks posed by emerald ash borer to riparian forest habitats: a review and problem formulation with management implications. Forest Ecol Manag 358:165–173
Palla KJ, Pijut PM (2011) Regeneration of plants from Fraxinus americana hypocotyls and cotyledons. In Vitro Cell Dev Biol Plant 47:250–256
Pérez-Tornero O, Egea J, Vanoostende A, Burgos L (2000) Assessment of factors affecting adventitious shoot regeneration from in vitro cultured leaves of apricot. Plant Sci 158:61–70
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J Forestry 104:118–124
Rathore MS, Mastan SG, Yadav P, Bhatt VD, Shekhawat NS, Chikara J (2016) Shoot regeneration from leaf explants of Withania coagulans (Stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. S Afr J Bot 102:12–17
Sarwar M, Skirvin RM (1997) Effect of thidiazuron and 6-benzylaminopurine on adventitious shoot regeneration from leaves of three strains of McIntosh apple (Malus x domestica Borkh.) in vitro. Sci Hortic 68:95–100
Singh C, Raj S, Patil V, Jaiswal P, Subhash N (2013) Plant regeneration from leaf explants of mature sandalwood (Santalum album L.) trees under in vitro conditions. In Vitro Cell Dev Biol Plant 49:216–222
Slazak B, Sliwinska E, Saługa M, Ronikier M, Bujak J, Słomka A, Göransson U, Kuta E (2015) Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tiss Organ Cult 120:179–190
Stevens M, Pijut P (2012) Hypocotyl derived in vitro regeneration of pumpkin ash (Fraxinus profunda). Plant Cell Tiss Organ Cult 108:129–135
Subramaniam S, Rathinam X, Poobathy R, Sinniah U (2008) In vitro production of multiple bud clumps (Mbcs) from Cavendish banana cultivar, Brasilian (AAA). Am Eurasian J Sustain Agr 2:300–307
Tonon G, Capuana M, Di Marco A (2001) Plant regeneration of Fraxinus angustifolia by in vitro shoot organogenesis. Sci Hortic 87:291–301
Vanstone DE, LaCroix LJ (1975) Embryo immaturity and dormancy of black ash [Fraxinus nigra]. J Amer Soc Hort Sci 100:630–632
Vieitez AM, San-José MC (1996) Adventitious shoot regeneration from Fagus sylvatica leaf explants in vitro. In Vitro Cell Dev Biol Plant 32:140–147
Wang Y, Chen F, Wang Y, Li X, Liang H (2014) Efficient somatic embryogenesis and plant regeneration from immature embryos of Tapiscia sinensis Oliv., an endemic and endangered species in China. HortScience 49:1558–1562
Wright JK, Rauscher HM (1990) Fraxinus nigra Marsh., black ash. In: Burns RM, Honkala BH (eds) Silvics of North America: 2. Hardwoods, Agric Handbook. USDA Forest Services, Washington DC, p 688–693
Zaytseva YG, Poluboyarova TV, Novikova TI (2016) Effects of thidiazuron on in vitro morphogenic response of Rhododendron sichotense Pojark. and Rhododendron catawbiense cv. Grandiflorum leaf explants. In Vitro Cell Dev Biol Plant 52:56–63
Acknowledgements
This research was supported by partial funding from the USDA-APHIS-PPQ Center for Plant Health Science and Technology, the U.S. Endowment for Forestry and Communities, and members of the Indiana Hardwood Lumbermen’s Association. The authors gratefully acknowledge Drs. Armand Séguin and Jayasankar Subramanian for their constructive review and suggestions for the improvement of this manuscript, and Dale Simpson for the black ash seed. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that also may be suitable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Angeles Revilla.
Rights and permissions
About this article
Cite this article
Lee, J.H., Pijut, P.M. Adventitious shoot regeneration from in vitro leaf explants of Fraxinus nigra . Plant Cell Tiss Organ Cult 130, 335–343 (2017). https://doi.org/10.1007/s11240-017-1228-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1228-1