Abstract
The present study is the first to report somatic embryogenesis (SE) based on a plant regeneration protocol for blackberry. It uses transverse thin cell layer technique (tTCL). Two blackberry genotypes, ‘High prickle’ (Rubus sanctus) and ‘Low prickle’ (Rubus hirtus) were used as explants. The explants were soaked in ascorbic and citric acids (60 mg l−1 each) solution prior to culture on Murashige and Skoog (MS) medium containing 2.32 μM kinetin (KIN), 2.69 μM α-naphthaleneacetic acid (NAA) and 8.88 6-benzyladenine (BA). This not only reduced the phenolic compounds (in ‘High prickle’), but also produced friable and yellow-pale green calluses. The highest level of embryogenic callus initiation in both genotypes occurred in half strength MS medium containing 60 g l−1 sucrose, 9.76 μM KIN and 7.99 μM BA. The MS medium fortified with 7.57 μM abscisic acid (ABA) and malt extract (700 mg l−1) or glutamine (400 mg l−1) encouraged the formation and development of embryos on calluses originating from dermal parts of ‘High prickle’ explants. Yasuda (YA) medium enrichd with 8.88 μM BA, 10.84 μM NAA and glycerol (2%) promoted embryo development and shoot regeneration on calluses originating from dermal parts of ‘High prickle’ and ‘Low prickle’ explants respectively. Germination of embryos and growth of normal plantlets occurred on half strength MS medium containing 4.88 μM BA, 2.02 μM gibberellic acid (GA3) and 0.05 μM NAA. Histological evaluations confirmed the successful occurrence of the different stages of embryogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Rubus (Tourn) L. comes from the Rosaceae family that includes raspberries, blackberries, hybrids of the berries and others species (Jennings 1988). Blackberries (Rubus L. subgenus Eubatus Focke.) are prevalent crops in North America and Europe (Crandall 1995).
Thin cell layers (TCLs) technique is a simple and significant biotechnological technique in plant science. The tissue culture regeneration protocol is already developed concerning several dozen species of crop in which the TCLs are generated from different explant sources. TCLs are divided into two categories. The first group is the transverse TCL (tTCL) which is the most common type and which ranges from 100 µm to 1–2 mm in thickness. The second category is the longitudinal TCL (lTCL) which targets very specific layers of cells with the same thickness but with different lengths. By TCLs, it is systematically convenient to control morphogenetic characteristics and the development of specific organs, obtained from other specific or non-specific cells, tissues or organs. The TCL technique also provides a great experimental opportunity to evaluate the differentiation and totipotency in cells, tissues and organs of plants. It also makes possible the mass production of economically important species, and has great profitable advantages (Da Silva 2003).
Cotyledon explants are commonly used in many research experiments. This includes the case of somatic embryogenesis in Rubus (Fiola and Swartz 1985; Gingas and Stokes 1993). Generally, the main limitation of embryogenesis is the low rate of somatic embryo conversion. Low levels of conversion in somatic embryos can be attributed to their poor quality, and also can be considered as a result of immaturity and tolerance to dehydration (Etienne et al. 1993). Arturo et al. (2016) reported that the development of callogenesis and protoplast cell suspensions are possible in the mass propagation of pro-embryos and micro calluses developing from leaf tissue. Culturing the leaves of blackberry (R. fruticosus) has also been established successfully, and many shoots were obtained in a relevant study, using indirect organogenesis (Vujović et al. 2010). In addition to its usefulness for cloning and vegetative propagation of a given individual plant, the condition of in vitro embryogenesis can be applied for the study of molecular, cytological, physiological and developmental features underlying embryogenesis in plants (Dodeman et al. 1997). The development of a reliable method for induction of embryogenic cultures of elite Rubus and shoot regeneration would be invaluable for transformation (Súkeníková et al. 2015). One obstacle in harvesting the blackberry fruit is the prickles on the plant’s branches. Then, tTCL cultures can be useful for studying the processes of generating somatic embryos from sub-epidermal tissues. These could probably regenerate the plants without prickles if certain measures are taken.
The objective of this research is to study the somatic embryogenesis of shoot tTCL explants of blackberry. Histological features are incorporated into the study. This study proves to be the first report discussing the use of TCL techniques in tissue culture of the genus Rubus.
Materials and methods
The nodal segments of two Iranian native blackberry genotypes ‘High prickle’ (Rubus sanctus) and ‘Low prickle’ (Rubus hirtus) were cultured in MS medium containing 8.88 µM BA and 2.69 µM NAA. Then, derived in vitro, the sterile and actively growing shoots were used as explant sources. Transverse thin cell layers (0.5–0.8 mm) were prepared from stem of micro-shoots by help of a sharp blade and a binocular (Zeiss, semi 2000-C) under an airflow cabinet (Fig. 1a, b). Then, they were cultured on Murashige and Skoog (1962) medium (MS) containing 2.32 μM kinetin (KIN), 2.69 μM α-naphthaleneacetic acid (NAA), and 8.88 μM 6-benzylaminopurine (BA) as callus induction medium. In the first assay, in both genotypes, due to the high amount of phenolic compounds and being oxidized at the surfaces of explants tissue, the explants became brown and died (Fig. 1c). In order to prevent phenolic compound exudation, different treatments were used. Such as, control (no treatment), 0.1% turmeric powder (TP) W/V, 0.2% W/V activated charcoal (AC), 10 mg l−1 cysteine (Cys), 500 mg l−1 PVP, 100 mg l−1 citric acid (CIT) and 100 mg l−1 ascorbic acid (AS) which were included in callus induction medium. Two explant pretreatments were also applied. One included submerging tTCL sections in a solution of ascorbic and citric acid (60 mg l−1 each) for 25 min, and another treatment had the shoots (source of tTCL explants) at 4 °C under dark and sterile conditions for 24 h. Then, they were transferred to callus induction medium. After 10 and 40 days following the first culture, concentration of phenolic compounds in culture media (Sharma 1994) and the rate of callus formation on dermal and central parts of the explants were measured (using 1 mm graph paper), respectively (Fig. 1d and e).
Total phenol content in extracts was obtained based on the Folin Ciocalteu method. After removing the explants from the culture vessels, the media (20 ml) were decanted into centrifuge tubes and 10 ml of solvent (methanol, 80%) was added to each tube and were shaken for 30 min. Tubes were centrifuged (4000 rpm, 30 min), and the limpid supernatant was collected and again 10 ml methanol 80% was added to it. Then, 100 µL of each sample was poured into the test tube, and 500 µL of the Folin–Ciocalteu reagent along with 2 M and 400 µL of sodium carbonate (7.5%) were added. The tubes were mixed and allowed to stand for 2 h in the dark. Absorption at 765 nm was determined (PG instrument T60U spectrophotometer, England). The total phenolic content was shown as gallic acid equivalents (GAE) in μg gallic acid per milliliter of medium.
Embryogenic callus induction
The calli produced from the above experiment were transferred to half strength MS medium enriched with combinations of BA (μM) and KIN (μM) as fallow: IEM1- BA 7.10 + KIN 7.90; IEM2- BA 7.10 + KIN 8.83; IEM3- BA 7.10 + KIN 9.76 ; IEM4- BA 7.99 + KIN 7.90; IEM5- BA 7.99 + KIN 8.83; IEM6- BA 7.99 + KIN 9.76; IEM7- BA 8.88 + KIN 7.90; IEM8- BA 8.88 + KIN 8.83; IEM9- BA 8.88 + KIN 9.76 (µM).
Two concentrations of sucrose 30 and 60 g l−1 were used separately. After about 30 days following the first culture, the percentages of embryogenic callus (granular and friable structures) were recorded.
Embryo development medium
In order to induce the production of embryos from embryogenic callus, two different media were used. These were the MS medium containing 7.57 μM abscisic acid (ABA) or the YA (Yasuda et al. 1985) containing 8.88 μM BA and 0.11 μM NAA. In both media, the effects of different treatments and the control were studied, including treatments of 10 ml l−1 modified Staba vitamins (Skirvin and Chu 1979), 400 mg l−1 glutamine, 700 mg l−1 malt extract (ME), 15 g l−1 maltose or 2% v/v glycerol on the development of embryos. The embryogenic calluses were subcultured onto these media. After 15 to 20 days following the culturing, the presence or absence of embryos was recorded.
Embryo germination medium
The embryos were subcultured on half basal salt MS media enriched with 0, 3.99, 4.88, or 5.77 μM of BA in combination with 0, 2.02, or 4.04 μM of gibberellic acid (GA3). These combinations were experimented to find the most suitable medium for embryo germination. Finally, plantlets derived in vitro were subcultured on PGRs-free MS medium.
Culture conditions
The incubation of cultures was done at 25 ± 2 °C under a 16/8 h (light/darkness) photoperiod and light intensity of approximately 45 µmol m−2 s−1 photosynthetic photon flux density (PPFD) emitted by cool-white fluorescent tubes in 35% relative humidity.
Histological analysis
Histological examination of embryogenic calluses and the different stages of embryo formation were performed. After the samples were situated in a fixative solution (formaldehyde:pure acetic acid:alcohol 8:1:1), they were treated with graded ethanol so as to be dehydrated, and were then infiltrated and embedded in paraffin wax. Thin Sections (12 μm) of the samples were cut by the help of a microtome device (model Spenser 820, AMERICAN OPTICAL COMPANY) according to Johansen’s method (1940). Subsequently, they were stained with haematoxylin and eosin and observed with Letiz (wetzlab, SM-LUX) optical microscope and were photographed by Canon EOS 600D camera.
Acclimatization
After 6 weeks, all of the plantlets were moved into paper teacups containing autoclaved peat and perlite by a ratio of 1:1 v/v. The cultures were placed in a growth chamber at 25 ± 2 °C under a 16/8 h (light/dark) photoperiod, light intensity of approximately 45 µmol m−2 s−1 PPFD emitted by cool-white fluorescent tubes and 80% relative humidity. Subsequently they were transferred in a greenhouse at 30 ± 4 °C, natural light and 50% relative humidity. Initially, they were maintained under plastic film and then gradually exposed to greenhouse conditions. After a few weeks (about 45 days), all surviving plants were relocated to pots ex vitro.
Statistical analysis
The study was on a factorial layout based on a completely randomized design with five replications. Each step of the experiments was repeated twice and data pertaining to the average of five explants were used as the value of each replication. Data were analyzed using SAS statistical software version 9.0, and the mean values were compared using the least significant difference (LSD) test at a 5% level of significance (P ≤ 0.05).
Results and discussion
Control of phenolic compounds and callus induction
The excessive exudation of phenolic compounds, as an oxidized form, under conditions of in vitro culture for some plant species causes the browning and death of explants, and therefore preventing this process could improve the percentage of explant survival. The analysis of variance showed a high interaction between different treatments and genotypes in reducing phenolic secretion into the media. In ‘High prickle’, all treatments significantly reduced phenolic exudation in media (Table 1). However, in ‘Low prickle’, AC and CIT significantly increased phenolic secretion. In all treatments (except in the AC), callus was initiated from both dermal and central parts of tTCL explants with reduction of phenolic compounds. Soaking the explants in a solution of ascorbic and citric acids (60 mg l−1 each) prior to culture produced friable and fresh callus. As a result, this treatment was applied to prevent phenolic exudation in culture media in other experiments.
Efforts have been done for minimizing the “phenolization” of cultures in order to reduce in vitro explant browning, mostly by adding antioxidants such as ascorbic acid, citric acid or by absorbing polymers like polyvinylpyrrolidone (PVP) (López Arnaldos et al. 2001). In this experiment, pre-culturing treatments were performed for 24 h at 4 °C which reduced phenolic compounds in culture medium. This finding is in agreement with the results obtained by Poudyal et al. (2008) which claimed that treating explants for 12 h with a temperature of 4 °C prior to explants’ sterilization seems to be the best approach for controlling browning. This was claimed to increase the survival of explants of the Yali pear. It has already been mentioned that soaking the explants in a solution of citric and ascorbic acids not only diminishes phenolic exudation but also produces friable and good-looking callus. Citric acid is a weak organic acid (Anthony et al. 2004) and it acts as an excellent cleaning and chelating agent of phenolic components. Both ascorbic and citric acids are involved in cell division and elongation (Smirnoff 1996). Activated charcoal serves to be an efficient adsorbent that can adsorb toxic substances (Zhou et al. 2010). In this study, it seems that the AC could adsorb many components (polyphenols and PGRs) and therefore the explants remained fresh for a longer duration and survived.
Embryogenic callus induction
The interaction between sucrose, BA and KIN significantly affected the embryogenic callus induction (Fig. 2). The highest percentage of embryogenic callus (80%) was obtained on IEM6 (MS/2 medium, 7.99 μM BA, and 9.76 μM KIN) containing 60 g l−1 sucrose, while the minimum percentage of embryogenic callus induction (0%) was observed in the IEM2 with 60 g l−1 sucrose, IEM9 with 30 g l−1, IEM5 and IEM8 enriched with 30 and 60 g l−1.
The effects of PGRs and different sucrose concentrations on percentage of embryogenetic callus from dermal part of ‘High prickle’ IEM1-BA 7.10 + KIN 7.90; IEM2-BA 7.10 + KIN 8.83; IEM3-BA 7.10 + KIN 9.76 ; IEM4-BA 7.99 + KIN 7.90; IEM5-BA 7.99 + KIN 8.83; IEM6-BA 7.99 + KIN 9.76; IEM7-BA 8.88 + KIN 7.90; IEM8-BA 8.88 + KIN 8.83; IEM9-BA 8.88 + KIN 9.76 (µM)
It has been reported that sucrose at 20–30 g l−1 is used as the only carbon source feeding most cases of Rosa spp. tissue culturing (Da Silva et al. 2007). However, in this study, it was observed that a higher concentration of sucrose (60 mg l−1) is more effective than having a lower concentration (30 mg l−1) aimed at embryo induction.
Da Silva et al. (2007) reported that 20–30 g l−1 sucrose is the most optimum concentration for the majority of Rosa spp. tissue culturing. However, based on our research, a high concentration of sucrose (60 mg l−1) was more effective than a low concentration (30 mg l−1) for embryo induction.
High concentrations of sucrose result in osmotic stress, however. The resulting stress could affect in vitro induction of somatic embryos which might cause a general stress response that would trigger the reorganization of chromatin. Extended reorganizations of chromatin appear to result in random outcomes of embryogenic inductions (Fehér 2005).
Cytokinins can act to promote embryogenesis (Kavathekar et al. 1978), and Jha et al. (1982) indicated that embryogenesis in celery can be facilitated under conditions where high levels of kinetin are present. Arturo et al. (2016) reported that 2.28 μM zeatin induced embryogenic callus in blackberry as they posited that cytokinins are important for in vitro somatic embryogenesis. Our results are in agreement with these findings.
Embryo initiation and development media
Results show that the MS medium containing 7.57 μM ABA alone or in combination with malt extract (ME) or glutamine is effective in forming embryos (Table 2, ‘+’ sign) from embryogenic callus of dermal parts of explants. In these media, many embryos initiated and developed gradually, but not all of them were normal embryos. There were instances where it seemed that the germination of embryos occurred before they completed their maturities (globular, heart and torpedo shapes). In this medium, maltose, glycerol or the modified Staba vitamins were not effective in embryo induction on the callus initiated from dermal or central parts of both genotypes.
Akula et al. (2000) studied the role of diverse osmotic elements and abscisic acid in the induction of somatic embryos in tea (Camellia sinensis). Abscisic acid is often reported to act as a “stress hormone” in plants and also would assist in the induction of somatic embryos in experimental systems (Charrière et al. 1999; Nishiwaki et al. 2000; Senger et al. 2001). The maturity of somatic embryos may also be achieved by the application of exogenous ABA (Klimaszewska and Smith 1997). In the mentioned research, it was reported that ABA is a suitable agent in assisting embryo formation from stem dermal parts of ‘Low prickle’. However, complementary effects of glutamine or ME are necessary for provoking somatic embryogenesis in ‘High prickle’.
Two conditions were used in this study to induce embryo formation and development that allow different cells to make proper dedifferentiated cells. Plant growth regulators and stress factors (such as osmotic shock, culture medium dehydration, water stress, heavy metal ions, heat or cool treatments, antibiotics can also be involved in the processes along with PGRs and chemical treatments.
According to our results, glutamine was an effective treatment in causing the formation of embryo in ‘High prickle’ which is in agreement with the result obtained by Rijven (1952) on Capsella bursa. Furthermore, glutamine is an efficient compound for embryo development (Thorpe 1995).
The reasons for the usefulness of ME in causing embryo formation could be explained by that the embryos are very sensitive to the action of plant extracts, and they can be used as a tool for determining the effective substances in a plant. Another reason is the enhancement of growth resulting from plant extracts, indicating that synthetic media lack certain substances that can enhance growth triggered by plant extracts. This is specifically an indication that synthetic media probably lack certain biological compounds (Thorpe 1995). Furthermore, it can be assumed that the better development of embryos obtained by a medium supplemented with plant extracts is probably due to the fact that the composition of the medium is closer to that of the ovular sap (Thorpe 1995).
The YA medium containing 8.88 μM BA and 0.11 μM NAA was not effective in embryo formation. Nonetheless, enriching the medium with glycerol and maltose encourage the formation of embryos on callus, from dermal parts of ‘High prickle’. The modified Staba vitamins facilitate the formation of embryos from central parts of the same genotype. Moreover, glycerol not only promoted embryo induction on YA medium (on callus of ‘High prickle’), but also provoked organogenesis on the callus initiated from dermal parts of ‘Low prickle’ (Table 2, ++ sign) (Fig. 3).
In most studied plants, it has been reported that the inclusion of PGRs is necessary for somatic embryogenesis. Auxins and cytokinins are key factors, probably because they strongly participate in cyclic regulations in plant physiology and cell division (Francis and Sorrell 2001; Gaj 2004). However, in this research, (Table 2) BA and NAA alone were not beneficial for embryo development, and it was needed to add glycerol, maltose or the modified Staba vitamin into the media. Swartz et al. (1990) also reported organogenesis from apple and blackberry leaves by applying modified Staba vitamins in the MS medium. However, we showed that the modified Staba vitamins were useful for somatic embryogenesis in stem tTCL of blackberry. It has been shown that vitamins, especially of the B group, are important for cell division, elongation and the process of embryogenesis (George et al. 2008), while the modified Staba vitamins are a complex of B vitamins.
Exogenous sugar was prepared to be added to the culture medium since it is known to affect somatic embryogenesis in the genus. Maltose was found to be the best carbon source for the development and maturation of somatic embryos in Prunus incisa x P. serrula (Druart 1989). Secondary somatic embryogenesis was also promoted in Malus domestica B. by 30 and 60 g l−1 maltose (Daigny et al. 1996).
Strickland et al. (1987) compared maltose and sucrose with each other and showed that maltose is stronger in improving the yield and development of somatic embryos at equal osmolarity. They also indicated that the effect of maltose was mainly nutritional and not osmotically mediated. In this study, 15 g l−1 from each of the two carbohydrate sources—sucrose and maltose—were used for the improvement in embryogenesis. Moreover, Nørgaard et al. (1997) noted that maltose can be effective in the process of causing maturation in somatic embryos of Abies nordmanniana. It is commonly known that maltose is directly obtained from the medium, while sucrose is hydrolyzed into monosacharides. Reidiboym-Talleux and March (1999) demonstrated that sucrose is more quickly metabolized than maltose which is slower in molecular conversion and therefore could lead to hypoxia and accumulation of ethanol in cells. Thus, the slow metabolism of maltose would lead to sufficient oxygen being present in the cells to allow survival and subsequent somatic embryo development. In this study, glycerol was used as a source of carbohydrates and promoted embryogenesis on stem dermal parts of ‘High prickle’ but also caused organogenesis on explants of the ‘Low prickle’ genotype. In many of Citrus genotypes and Cichorium (Bellettre et al. 1999), it was seen that glycerol is a useful carbohydrate for morphogenesis and embryogenesis.
Embryo germination medium
Among the combinations of BA and GA3, somatic embryos merely germinated on MS/2 medium containing 4.88 μM BA and 2.02 μM GA3 after 7 days of culture, and the growth of radicles were stopped. However, with the exogenous application of NAA (0.05 μM) in the same medium, the result culminated in normal root and shoot development (Fig. 4i). Thereafter the plantlets were relocated to a sterile soil mixture.
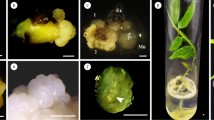
Different stages of somatic embryogenesis in tTCL explants of Rubus and histological sections of these stages; a embryogenic callus with globular structure(Gl); b sections of globular embryos on the surface of a tTCL embryogenic callus; c heart-stage (Hs) of somatic embryo; d section of heart-shaped somatic embryo; e torpedo-shaped embryo with root primordium; f section of torpedo-shaped embryo with root primordia (Rp) and cotyledons (Ct); g germinated and growth of embryos; h section of shoot vascular tissue (Vs); i normal plantlet growth from embryo on half strength MS medium containing 4.88 μM BA, 2.02 μM GA and 0.05 μM NAA; leaf (L), shoot (S), Root (R)
By adding growth substances to the medium at usual concentrations, modifications in the ontogenic pattern of embryos were observed, including repression of root growth and premature leaf expansion with kinetin, while longer and thinner embryos were achieved as a result of applying gibberellins (Raghavan 1964; Thorpe 1995). Our findings confirmed that BA and GA are not suitable for normal embryo production. The process of embryo germination would perform normally if NAA is added to the growth substances.
Histological analysis
After the formation of proper meristematic cells, they continue to proliferate and form pro-embryonic clusters. In this research, the mentioned structures were almost located at the surface of the tissue forming pro-embryonic nodules. However, not all meristematic cells turn into embryogenic cells. Both embryogenic and non-embryogenic regions, which can be easily distinguished by morphological structure, color and cellular characteristics, are present in the calluses during the indirect somatic embryogenesis process (Carvalho et al. 2013). Embryogenic zones have yellow or dark-yellow color, nodular features, and smooth surfaces. After 3 weeks of culture on induction medium, the pro-embryogenic nodules developed into globular embryos (Fig. 4a) and gradually converted to heart and torpedo shape embryos (Fig. 4c, e and related sections d, f). These structures are easily separated from the surrounding cells of calluses. After four more weeks, transition to the cotyledonary stage occurred concurrently with the inception of root primordium. After three more weeks, the cotyledonary embryos were observed to germinate which was succeeded by elongation of the radicle and shoot development (Fig. 4g and its section h; normal plantlet, Fig. 4i).
Conclusion
In this research, we demonstrated that embryogenesis typically occurs on cells from dermal parts, in addition to the occurrence on cells from central parts. However, results pertaining to embryos derived from the pith cells are not conclusive, and therefore definite deductions cannot be made regarding that specific area. Embryogenesis on the dermal parts of cells can be evidently attributed to the ability of cells to initiate different developmental processes, such as callus production and somatic embryogenesis. In the end, it can be claimed that somatic embryogenesis and plant regeneration are generally observed to be more efficient in ‘High prickle’ than in ‘Low prickle’ types of blackberry.
Abbreviations
- ABA:
-
Abscisic acid
- AC:
-
Activated charcoal
- AS:
-
Ascorbic acid
- BA:
-
6-Benzyladenine
- CIT:
-
Citric acid
- Cys:
-
Cysteine
- GA3 :
-
Gibberellic acid
- KIN:
-
Kinetin
- ME:
-
Malt extract
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthaleneacetic acid
- PVP:
-
Polyvinyl pyrolidine
- tTCL:
-
Transverse thin cell layers
- TP:
-
Turmeric powder
- YA:
-
Yasuda
References
Akula A, Akula C, Bateson M (2000) Betaine a novel candidate for rapid induction of somatic embryogenesis in tea (Camellia sinensis (L.) O. Kuntze). Plant Growth Regul 30:241–246. doi:10.1007/978-94-011-4774-3_15
Anthony J, Senaratna T, Dixon K, Sivasithamparam K (2004) The role of antioxidants for initiation of somatic embryos with Conostephium pendulum (Ericaceae). Plant Cell Tissue Organ Cult 78:247–252. doi:10.1023/B:TICU.0000025661.56250.b4
Arturo RD, Andrea A, Pedro R, Bryan R, Gooty JM (2016) Obtaining protoplasts from leaf tissue plantlets of Rubus glaucus Benth (Blackberry) to develop proembryos. Indian J Sci Technol 9:1–8. doi:10.17485/ijst/2016/v9i10/81983
Bellettre A, Couillerot J-P, Vasseur J (1999) Effects of glycerol on somatic embryogenesis in Cichorium leaves. Plant Cell Rep 19:26–31. doi:10.1007/s002990050705
Carvalho MAF et al. (2013) Morphogenetic potential of native passion fruit (Passiflora gibertii NE Brown.) calli. Brazilian J Bot 36:141–151. doi:10.1007/s40415-013-0015-4
Charrière F, Sotta B, Miginiac É, Hahne G (1999) Induction of adventitious shoots or somatic embryos on in vitro cultured zygotic embryos of Helianthus annuus: variation of endogenous hormone levels. Plant Physiol Biochem 37:751–757
Crandall PC (1995) Bramble production. The management and marketing of raspberries and blackberries. Food Products Press, New York
Da Silva JAT (2003) Thin cell layer technology in ornamental plant micropropagation and biotechnology Afr J Biotechnol 2:683–691. http://www.academicjournals.org/AJB
Da Silva J, Van K, Biondi S, Nhut DT, Altamura MM (2007) Thin cell layers: developmental building blocks in ornamental biotechnology. Floric Ornam Biotechnol 1:1–13. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.587.2503&rep=rep1&type=pdf
Daigny G, Paul H, Sangwan R, Sangwan-Norreel B (1996) Factors influencing secondary somatic embryogenesis in Malus x domestica Borkh.(cv ‘Gloster 69’). Plant Cell Rep 16:153–157
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509. https://pdfs.semanticscholar.org/e1cb/06bc8b9c56f7ab60761cf77f49362c55f130.pdf
Druart P (1989) Improvement of somatic embryogenesis of the cherry dwarf rootstock INMIL/GM9 by the use of different carbon sources. In: proceedings of the international symposium on in vitro culture and horticultural breeding 280, pp 125–130. doi:10.17660/ActaHortic.1990.280.19
Etienne H, Montoro P, Michaux-Ferriere N, Carron M (1993) Effects of desiccation, medium osmolarity and abscisic acid on the maturation of Hevea brasiliensis somatic embryos. J Exp Bot 44:1613–1619
Fehér A (2005) Why somatic plant cells start to form embryos? Somatic embryogenesis. Springer, Berlin, pp 85–101. http://springerlink.bibliotecabuap.elogim.com/chapter/10.1007%2F7089_019#page-2
Fiola JA, Swartz H (1985) Somatic embryogenesis, organogenesis and proliferation in vitro from Rubus embryos. In: Proceedings of the IV international rubus and ribes symposium 183, pp 91–98. doi:10.17660/ActaHortic.1986.183.11
Francis D, Sorrell DA (2001) The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regul 33:1–12
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47
George EF, Hall MA, De Klerk G-J (2008) Plant tissue culture procedure-background. In: Plant propagation by tissue culture. Springer, Berlin, pp 1–28. https://download.e-bookshelf.de/download/0000/0038/77/L-G-0000003877-0002333095.pdf
Gingas V, Stokes B (1993) Rubus plant regeneration via asexual embryogenesis. HortScience 28:58. http://hortsci.ashspublications.org/content/28/1/58.full.pdf+html
Jennings D (1988) Raspberries and blackberries: their breeding, diseases and growth. Academic Press, Cambridge. https://www.cabdirect.org/cabdirect/abstract/19901610038
Jha T, Roy S, Mitra G (1982) Brief review on in vitro studies on umbelliferous spice plants. In: tissue culture of economically important plants: proceedings of the international symposium held at the Botany Department, National University of Singapore, Singapore, 28–30 April 1981/edited by AN Rao, 1982. http://agris.fao.org/agris-search/search.do?recordID=US201301415723
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Kavathekar A, Ganapathy P, Johri B (1978) In vitro responses of embryoids of Eschscholzia californica. Biol Plant 20:98–106
Klimaszewska K, Smith DR (1997) Maturation of somatic embryos of Pinus strobus is promoted by a high concentration of gellan gum. Physiol Plant 100:949–957
López Arnaldos T, Muñoz R, Ferrer MA, Calderón AA (2001) Changes in phenol content during strawberry (Fragaria × ananassa, cv. Chandler) callus culture. Physiol Plant 113:315–322
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759
Nørgaard JV (1997) Somatic embryo maturation and plant regeneration in Abies nordmanniana Lk. Plant Sci 124:211–221
Poudyal BK, Du G, Zhang Y, Liu J, Shi Q (2008) Studies on browning problem and phenols content on shoots of Yali, Aikansui and Abbe Fetel pears for in vitro culture. Front Agric China 2:321–330
Raghavan V (1964) Interaction of growth substances in growth and organ initiation in the embryos of Capsella. Plant Physiol 39:816
Reidiboym-Talleux L, March GD (1999) Lipid and fatty acid composition in non-embryogenic calli and embryogenic tissues in wild cherry (Prunus avium). Physiol Plant 105:513–520
Rijven AHGC (1952) In vitro studies on the embryo of Capsella bursa-pastoris. Acta Botanica Neerlandica 1:157–200. doi:10.1111/j.1438-8677.1952.tb00007.x
Senger S, Mock H-P, Conrad U, Manteuffel R (2001) Immunomodulation of ABA function affects early events in somatic embryo development. Plant Cell Rep 20:112–120
Sharma RR (1994) In vivo and in vitro polyphenol oxidase activity in grape (Vitis vinifera L.). Thesis, Post Graduate School, IARI, New Delhi
Skirvin RM, Chu MC (1979) In vitro propagation of ‘forever yours’ rose. HortScience 14:608–610
Smirnoff N (1996) Botanical briefing: the function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Strickland SG, Nichol JW, McCall CM, Stuart DA (1987) Effect of carbohydrate source on alfalfa somatic embryogenesis. Plant Sci 48:113–121
Súkeníková M, Libiaková G, Moravčíková J, Hricová A, Gajdošová A (2015) Agrobacterium tumefaciens-mediated transformation of blackberry (Rubus fruticosus L.). Plant Cell Tiss Organ Cult 120:351–354. doi:10.1007/s11240-014-0569-2
Swartz HJ, Bors R, Mohamed F, Naess SK (1990) The effect of in vitro pretreatments on subsequent shoot organogenesis from excised Rubus and Malus leaves. Plant Cell Tissue Org Cult 21:179–184
Thorpe TA (1995) In vitro embryogenesis in plants. Kluwer Academic, Dordrecht
Vujović T, Ružić Đ, Cerović R, Momirović GÅ (2010) Adventitious regeneration in blackberry (Rubus fruticosus L.) and assessment of genetic stability in regenerants. Plant Growth Regul 61:265–275. doi:10.1007/s10725-010-9474-9
Yasuda T, Fujii Y, Yamaguchi T (1985) Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol 26:595–597
Zhou B, Wei X, Wang R, Jia J (2010) Quantification of the enzymatic browning and secondary metabolites in the callus culture system of Nigella glandulifera Freyn et Sint. Asian J Tradit Med 5:109–116 http://218.25.35.236:8080/Jwk_yzctyy/EN/Y2010/V5/I3/109
Acknowledgements
We would like to thank Ali Pourkhaloee for his scientific advices. We also like to thank Mohsen Hamedpour-Darabi for assisting in editing the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Barbara M. Reed.
Rights and permissions
About this article
Cite this article
Sabooni, N., Shekafandeh, A. Somatic embryogenesis and plant regeneration of blackberry using the thin cell layer technique. Plant Cell Tiss Organ Cult 130, 313–321 (2017). https://doi.org/10.1007/s11240-017-1225-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1225-4