Abstract
Plant cuticle plays a significant role in responses to various environmental stresses. Transcription factors were shown to regulate cuticular biosynthesis in many plants. However, no transcription factor genes involved in this biological process have been identified in wax- and cutin-rich sorghum so far. Here we clone and characterize a sorghum gene encoding an ethylene response factor (ERF) transcription factor. It consists of 204 amino acids and holds typically conserved ‘mm’, ‘cm’ and AP2 domains of WIN protein family with higher similarity to its orthologues in monocot plants. We designated this gene as sorghum WIN1-Like 1 gene (SbWINL1). Our study showed that SbWINL1 gene was highly expressed in leaf, stem, seedling and sheath, compared to root and spikelet. Notably, rosette leaves of SbWINL1-overexpressed Arabidopsis (SbWINL1-OE) were more enriched in wax crystals and shinier than those of wild-type plants. Further biochemical analysis indicated that SbWINL1-OE leaves showed 2- or 2.6-fold higher levels of total wax content or total cutin than counterparts from wild-type leaves. The drought tolerance of SbWINL1-OE plants was enhanced substantially compared with wild-type. Importantly, the expression of genes associated with wax and cutin synthesis pathways was significantly up-regulated in SbWINL1-OE plants. In summary, we demonstrate that the overexpression of sorghum WINL1 gene in Arabidopsis promotes the accumulation of wax and cutin and thus enhances drought tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorghum belongs to the family Poaceae and is a high quality food and forage crop. Sorghum has enriched wax and cutin structures, which play important roles in responses to stress and in its adaptability to the external environments, such as drought and water logging (Palmer 1992; Hwang et al. 2002). The molecular mechanism underlying the synthesis and regulation of wax and cutin in sorghum remains largely elusive, although some key genes involved in this process have been identified in model species.
The cuticular layer is composed of cutin and wax and important to the land plants (Riederer 2006; Raffaele et al. 2008; Schreiber 2010). The epidermal wax can be divided into epicuticular wax and intracuticular wax, which are deposited outside of the cuticle and in the cuticular mixtures, respectively (Pollard et al. 2008; Yeats and Rose 2013; Go et al. 2014). The compositions of cuticular cutin and wax vary among species, organs and tissues. Cuticular wax is a mixture of lipids mainly composed of very-long-chain fatty acids (VLCFAs), primary and secondary alcohols, aldehydes, ketone, alkanes and wax esters (Broun et al. 2004; Samuels et al. 2008), whereas cutin is largely composed of hydroxy, epoxy and dicarboxylic fatty acids (Pollard et al. 2008; Go et al. 2014). Cutin and wax are vital elements for the regulation of epidermal permeability and non-stomatal water loss (Schreiber 2010; Burghardt and Riederer 2003), and play crucial roles in protecting plants against insects, pathogens, UV light, and frost (Fiebig et al. 2000). In addition, it was reported that the content of wax is associated with pollen fertility and other agronomic traits (Aarts et al. 1995; Jung et al. 2006).
Genes associated with the cutin and wax pathways have been identified in the past decades (Kunst and Samuels 2009; Borisjuk et al. 2014). Several genes encoding enzymes in wax biosynthesis were also characterized, such as CER1, CER4, CER6/CUT1, KCS1, WAX2, FATB, FAE1 and FDH gene (Aarts et al. 1995; Rowland et al. 2006; Millar et al. 1999; Todd et al. 1999; Chen et al. 2003; Pruitt et al. 2000; Mao et al. 2012). For instance, the CER4 gene encoded a fatty acyl-CoA reductase, which catalyzes the two-step reduction of VLCFAs to primary alcohols (Aarts et al. 1995). CER6 gene has been shown to be essential for the formation of VLCFAs longer than 22 carbons in Arabidopsis stems (Fiebig et al. 2000), while CER1 and WAX2/CER3 genes are involved in the decarbonylation pathway responsible for the formation of aldehydes, alkanes, secondary alcohols and ketones (Chen et al. 2003; Bernard et al. 2012; Bourdenx et al. 2011; Rowland et al. 2007). Similarly, genes involved in cutin biosynthesis were reported in Arabidopsis, such as long-chain acyl-CoA synthetase 1 (LACS1), LACS2, cytochrome P450 monooxygenase (CYP86A8), CYP86A2, glycerol-3-phosphate acyltransferase 4 (GPAT4), GPAT8, BODYGUARD and HOTHEAD gene (Lu et al. 2009; Schnurr et al. 2004; Duan and Schuler 2005; Wellesen et al. 2001; Li et al. 2007; Kurdyukov et al. 2006a, b). Among them, LACS1 is a member of the long chain acyl-CoA synthetase (LACS) family, which is involved in free fatty acids to CoA process (Lu et al. 2009). In addition, ABCG11 and ABCG12(CER5) have been proposed to act in cuticular lipid export in Arabidopsis (Sieber et al. 2000; Bird et al. 2007; Panikashvili et al. 2007; Bird 2008).
Transcription factors (TFs), including MYB and AP2/ERF TFs, play an important role in the regulation of wax synthesis. AtMYB41 encodes a R2R3-type MYB involved in regulating wax transport, while AtMYB96 modulates wax accumulation through regulating wax biosynthetic enzymes such as KCS1, KCS2, KCS6, KCR1, ECERIFERUM1 (CER1) and CER3 (Seo et al. 2011). WAX INDUCER1 (WIN1)/SHINE1, containing an AP2 domain, is the first transcription factor identified as a cuticle biosynthesis regulator in Arabidopsis (Broun et al. 2004). Overexpression of AtWIN1 results in significant accumulation of wax by up-regulating the expression of many genes in the wax biosynthetic pathway. Furthermore, AtWIN1 has been shown to trigger wax production, enhance drought tolerance and modulate cuticular permeability when it was overexpressed in Arabidopsis (Aharoni et al. 2004). Cutin was significantly increased in vegetative and reproductive organs in Arabidopsis plants overexpressing AtWIN1, while the opposite effects were observed when AtWIN1 expression was down-regulated. Therefore, a two-step process was proposed for the AtWIN1 regulation of cutin and wax production (Kannangara et al. 2007). An in-depth study suggested that SHN1 (AtWIN1) and its other two SHINE (SHN) clade members act redundantly to shape the surface and morphology of Arabidopsis flowers via controlling cuticular lipid metabolism and modifying the epidermis cell wall (Shi et al. 2011a, b). Recently, it has been demonstrated that WIN1 regulates epidermal cell morphology and cuticle development coordinately with MYB106 and MYB16 transcription factors in Arabidopsis and Torenia fournieri (Oshima et al. 2013). More recently, it has been shown that the WIN1 gene is closely related to drought and defense responses in plants (Sela et al. 2013; Wang et al. 2012; Al-Abdallat et al. 2014).
However, no transcription factor responsible for cuticular biosynthesis in sorghum has been identified so far and the mechanism underlying this process remains to be determined. Here, we isolate a transcription factor gene SbWINL1 from sorghum and investigate its function in Arabidopsis. Our study demonstrated that SbWINL1 gene is highly expressed in leaf, stem, seedling and sheath, particularly in leaf and stem of sorghum. Overexpressing SbWINL1 in Arabidopsis substantially enhanced the drought tolerance via the accumulation of wax and cutin. Consistently, genes related to cutin and wax biosynthesis pathway were up-regulated in the transgenic plants. Our study will further facilitate the understanding of the biological role of WIN1 orthologous genes, and shed light on the biosynthetic and regulatory pathways of cutin and wax in sorghum.
Materials and methods
Plant materials
Arabidopsis plants (Col-0 ecotype) were grown under 22 °C, 75 % relative atmospheric humidity, and 16/8 h photoperiod (day/night) with light intensity of 200 µmol m−2 s−1. Sorghum plants (line BTx622) were cultivated in a growth room with 25 °C, 50 % relative humidity, and 16/8 h photoperiod (day/night) with light intensity of 200 µmol m−2 s−1.
Construction of expression vector and screening of transgenic plants
The coding region of SbWINL1 gene was PCR-amplified from leaf cDNA of sorghum by using specific primer pair as follows: ATGGTACAGCCAAAGAAG and TCAGATGATGAAGCGACC. To study the biological functions of SbWINL1, an overexpression vector was constructed through the Gateway cloning technology. Firstly, the target gene containing the AttB sites was obtained by PCR amplification with AttB sites added at the 5′ end of the primers. The PCR product was recombined into the entry vector pDONR221 (Amp) through BP reaction. Secondly, the target gene was replaced into overexpression vector pGWB14 (Kan) by LR reaction, and under the control of 35S promoter. The resulting plasmid was introduced into Agrobacterium strain GV3101, and transformed into Arabidopsis plants by the floral dip method. Positive transgenic plants were then selected on 1/2 MS medium containing 50 mg L−1 = kanamycin.
Environment scanning electron microscopy (ESEM) analysis
For cuticular wax structure analysis, fresh leaves from four-week-old wild type and transgenic Arabidopsis plants were placed in a clean petri dish and dried at room temperature for 2–3 days. Leaf samples were then treated with gold sputtering and examined by a Quanta250FEG (ESEM mode) scanning electron microscope (FEI).
Analysis of wax and cutin composition
For wax and cutin analysis, each sample with about ten mature rosette leaves of four-week-old Arabidopsis plants was used for extraction of wax and cutin. The analysis protocol of wax and cutin contents was described in detail in previous studies (Kurdyukov et al. 2006a; Shi et al. 2011; Franke et al. 2005). The main instruments for above analysis are GC-MS (Agilent Technologies) and GC-FID (Agilent Technologies).
Drought treatment
Seven-day-old seedlings of wild type and SbWINL1-OE, grown on 1/2 MS medium, were transplanted into nutrient soil for another 7 days, then drought treatment was carried out for 20 days (Zou et al. 2015; Liu et al. 2016; Wang et al. 2016). The survival rates of plant were measured 3 days after rewatering.
Gene expression analysis
For expression pattern analysis of SbWINL1 gene, quantitative real time PCR (q RT-PCR) was performed as follows. Total RNA was extracted from root, 10-day-old seedling, stem, leaves, sheath and spikelet of sorghum, respectively. 2 μg of total RNA was used for first-strand cDNA synthesis by SuperScript III transcriptase (Invitrogen). Sorghum β-ACTIN gene acted as the internal control. For the expression analysis of wax and cutin related synthetic genes and SbWINL1 gene in SbWINL1-OE plants, 2 μg of total RNA was extracted from 4-week-old rosette leaves of SbWINL1-OE and wild type Arabidopsis plants. The first-strand cDNA was then synthesized using SuperScript III transcriptase (Invitrogen). ACTIN2 gene was quantified as an internal control.
The resultant cDNA solution was used for semi-quantitative PCR and qRT-PCR. The qRT-PCR analysis was performed using an Applied Biosystems 7500 real-time PCR system. The SYBR Premix Ex Taq Kit (TakaRa) was used for reaction according to the manufacturer’s instruction.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: X79378 for Sorghum β-ACTIN, At1g01600 for CYP86A4, At1g63710 for CYP86A7, At1g12570 for HTH-like, At1g49430 for LACS2, At1g01610 for GPAT4, At1g02205 for CER1, At4g24510 for CER2, At5g57800 for WAX2, At1g01120 for KCS1 and At2g04570.
Results
Cloning and structural analysis of SbWINL1 from sorghum
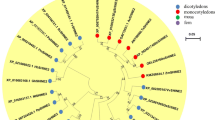
The coding region of SbWINL1 was isolated from the sorghum stem. It contains 615 bp and encodes 204 amino acids. Sequence analysis showed a highly conserved AP2 domain and two WIN protein characteristic ‘mm’ and ‘cm’ domains (Aharoni et al. 2004; Shi et al. 2013), suggesting SbWINL1 from sorghum is a typical WIN protein, (Fig. 1a). Further sequence comparison indicated an 85 % sequence identity to OsWR1 of rice, 81 % to BdWIN1 of Brachypodium distachyon and 75 % to HvNud of Hordeum vulgare, though a much lower sequence identity to VvWIN1of Vitis vinifera (64 %). Overall, SbWINL1 presented higher homology with WIN1 proteins from the monocots than dicots. The phylogenetic tree analysis showed that SbWINL1 shared high similarity to orthologous proteins from rice and Brachypodium distachyon (Fig. 1b).
Comparative analysis of WIN1 proteins in various plant species. a Multiple alignment analysis of WIN1 protein sequences. All nine proteins contain a single AP2 domain at their N termini, a conserved middl1e domain (termed mm), and a conserved C-terminal domain (termed cm). White letters shaded indicate amino acids that are either 100 % identical (black) or identical in at least 75 % (dark gray) or identical in at least 50 % (light gray) of all proteins. Vv Vitis vinifera; Mt Medicago truncatula; At Arabidopsis thaliana; Bd Brachypodium distachyon; Os Oryza sativa Japonica Group; Sb Sorghum bicolor; Gm Glycine max; Pt Populus trichocarpa; Hv Hordeum vulgare. b Phylogenetic tree analysis of WIN1 proteins
Expression profile analysis of SbWINL1 gene and functional evaluation in Arabidopsis
To investigate the spatial expression pattern of SbWINL1, we performed quantitative real-time PCR (qRT-PCR) analysis in different tissues from sorghum. Our data showed that SbWINL1 was expressed in root, seedling, leaf, stem, sheath and spikelet (Fig. 2a), with higher expression in leaf, stem, seedling and sheath than root and spikelet. To elucidate the physiological role of the SbWINL1 gene, we generated transgenic Arabidopsis plants (SbWINL1-OE) with the SbWINL1 gene controlled by the CAMV 35S promoter (Fig. 2b). After validation of the transgenic plants, we chose T2 transgenic Arabidopsis plants for subsequent studies. The expression levels of SbWINL1-OE-5# and SbWINL1-OE-9# were analyzed using semi-quantitative RT-PCR methods and the results showed that SbWINL1 gene overexpressed in transgenic Arabidopsis plants while there were no transcripts of foreign gene detected in wild type plants (Fig. 2c). Strikingly, SbWINL1-OE plants exhibited much glossier rosette leaves compared with control plants (Fig. 3), suggesting that the overexpression of SbWINL1 might influence the synthesis pathway of cuticular wax. Similar phenotypes were found in AtWIN1-OE Arabidopsis plants (Broun et al. 2004; Kannangara et al. 2007), implying a functional similarity between SbWINL1 and AtWIN1.
Expression level of SbWINL1 in Sorghum and transgenic Arabidopsis. a Detection of expression levels of SbWINL1 gene in various tissues of sorghum using qRT-PCR. b Schematic representation of the SbWINL1 over-expression construction. 35S, 35S promoter; TNOS, NOS terminator. c Detection of expression levels of SbWINL1 gene in WT, SbWINL1-OE-5# and SbWINL1-OE-9#. Semi-quantitative RT-PCR was also performed with AtACTIN2 primers as control. SbWINL1 semi-quantitative RT-PCR products are shown after 35 PCR cycles, and actin products are shown after 25 PCR cycles. N negative control
Overexpression of SbWINL1 significantly contributes to the accumulation of wax and cutin in Arabidopsis
To understand the structural alteration accounting for the observed phenotypes, we examined the leaf surface of SbWINL1-OE plants with ESEM. The results showed that there were more wax crystals and films distributed on the surface of transgenic plant leaves than on wild-type leaves (Fig. 4), supporting the idea that overexpressing SbWINL1 enhances the synthesis of wax. The chemical analysis of wax and cutin in 4-week-old rosette leaves by GC-FID and GC-MS revealed a significant difference in the amount of wax and cutin between SbWINL1-OE and wild type plants. The total content of wax in leaves from SbWINL1-OE plants was significantly increased up to twofold higher than control plants (Fig. 5a). The main components of wax such as fatty acids, alcohols, alkanes and aldehydes were increased significantly in SbWINL1-OE plants, whereas 16:0 fatty acid, 18:0 fatty acid and 32:0 alcohol were decreased, compared with wild-type controls (Fig. 5c).
Changes of wax and cutin in WT and SbWINL1-OE rosette leaves. Rosette leaves of 4-week-old wild-type (Col-0) and SbWINL1-OE plants grown in soil were used for analysis of wax and cutin composition. Each value represents the mean of five replicates. Statistically analyzed using a Student’s t test (*0.05 < p < 0.01, **p < 0.01). Bars indicate SD of the mean. FA fatty acid, ALK alkane, OL alcohols, ALD aldehydes, 2HFA 2-OH fatty acid, DFA dioic acid, ω-HFA ω-OH fatty acid, C16:0 9/10 16-DHFA, 9/10 16 di-OH C16 FA. a Total leaf wax of SbWINL1-OE and wild type plants. b Total leaf cutin of SbWINL1-OE and wild type plants. c Wax profiles of SbWINL1-OE and wild type plants. d Cutin profiles of SbWINL1-OE and wild type plants
It was reported that overexpressing AtWIN1 can dramatically affect cutin biosynthesis in Arabidopsis leaves, so we next assessed whether SbWINL1 could exert the same effect on cutin biosynthesis. We analyzed the levels of cutin in SbWINL1-OE rosette leaves. The results showed that the total cutin content in transgenic plant leaves was about 2.6-fold higher than control plants (Fig. 5b). Notably, most of the cutin monomers were dramatically increased in SbWINL1-OE plants compared with wild-type controls (Fig. 5d). Also the overexpression of SbWINL1 significantly promoted the accumulation of C24:0 2-OH fatty acids, C16:0 and C18:2 dioic acid. The contents of ω-hydroxy fatty acids, such as C16:0, C18:0, C18:1 and C18:2, were also substantially increased (Fig. 5d). These results indicated that overexpression of SbWINL1 contributed to the accumulation of wax and cutin.
Overexpression of SbWINL1 enhances drought tolerance in Arabidopsis
It was reported that WIN1 gene is involved in drought and defense responses (Sela et al. 2013; Wang et al. 2012; Al-Abdallat et al. 2014). OsWR1 and SlSHN1 transgenic plants were insensitive to drought stress. To examine the sensitivity of SbWINL1-OE plants to drought stress, we performed drought treatment on seedlings of transgenic and wild-type plants. The results showed that SbWINL1-OE Arabidopsis was insensitive to drought stress compared with wild- type plants (Fig. 6a). Importantly, the survival rates of SbWINL1-OE Arabidopsis plants were significantly higher than those of control plants (Fig. 6b), indicating that overexpression of SbWINL1 enhances drought tolerance in Arabidopsis.
Drought stress tolerance was enhanced in SbWINL1-OE plants. a Reponses of SbWINL1-OE and wild type plants to drought stress. b Survival rates of SbWINL1-OE and wild type plants under drought stress. The experiments were repeated three times with similar results. Data shown are mean values ± SD (n = 36). Date are statistically analyzed using a Student’s t test (**p < 0.01)
Overexpression of SbWINL1 up-regulates the expression of wax and cutin related genes in SbWINL1-OE plants
As reported previously, the transcription factor AtWIN1 triggered the expression of genes involved in the biosynthesis of wax in 35S:WIN1 Arabidopsis (Broun et al. 2004; Kannangara et al. 2007). To examine the effect of SbWINL1 on wax biosynthesis-related genes, we measured the expression of CER1, CER2, WAX2 and KCS1, which are important genes involved in wax biosynthesis, in rosette leaves from 4-week-old Arabidopsis by quantitative RT-PCR. The results demonstrated that the expression of four genes related to wax biosynthesis was significantly increased, particularly for CER1 gene expression (Fig. 7a).
Changes in the expression levels of genes related to wax (a) and cutin (b) biosynthesis in SbWINL1-OE plants. Quantitative RT-PCR was carried out to analyze the patterns of gene expression. The experiments were repeated two times with similar results. Data shown are mean values ± SD (n = 3). Difference levels among transgenic and wild type plants were statistically analyzed using a Student’s t test (**p < 0.01)
To further validate the notion that the overexpression of SbWINL1 gene might modulate the expression of genes associated with cutin synthesis in SbWINL1-OE plants, we examined the expression of six cutin synthesis relevant genes with quantitative RT-PCR. As expected, the tested genes were significantly up-regulated in SbWINL1 overexpressing plants (Fig. 7b). These results indicated that SbWINL1 plays an important role in regulating the expression of genes involved in wax and cutin biosynthesis and thus enhancing the accumulation of wax and cutin, resulting in glossy and shiny leaves.
Discussion
Wax and cutin play important roles in plant growth, development and in response to environmental stresses (Kunst and Samuels 2009, 2003; Borisjuk et al. 2014; Sieber et al. 2000; Woloshuk and Kolattukudy 1986; Fauth et al. 1996; Preuss et al. 1993; Scott et al. 2004). WIN1 transcription factor has been shown to be a positive regulator of wax and cutin synthesis in some plant species (Broun et al. 2004; Kannangara et al. 2007; Shi et al. 2011; Wang et al. 2012; Al-Abdallat et al. 2014), however there is scarce report on the molecular mechanism of wax and cutin synthesis in sorghum, a crop with enriched wax and cutin in the cuticular layer (Hwang et al. 2002; Lochte-Watson and Weller 1999; Burow et al. 2008; Peters et al. 2009; Lee et al. 2014). In this study, we cloned and characterized the SbWINL1gene from sorghum, which encodes an AP2 type transcription factor with high similarity to WIN1 proteins from other plant species (Shi et al. 2011, 2013; Wang et al. 2012; Taketa et al. 2008). It was reported that AtWIN1 transcription factor activates wax and cutin synthesis in Arabidopsis and thus contributes to shiny plants (Broun et al. 2004; Kannangara et al. 2007). Here we demonstrated that the overexpression of SbWINL1 in Arabidopsis also increased the wax and cutin synthesis and resulted in glossy and shiny plants, consistent with previous reports on its orthologues OsWR1 and AtWIN1 (Broun et al. 2004; Aharoni et al. 2004; Kannangara et al. 2007; Wang et al. 2012).
Chemical analysis revealed that waxes such as C26:0, C30:0, C32:0 and C34:0 fatty acids, campesterol, B-sitosterol and C31:0 alkanes were dramatically increased in SbWINL1-OE plants. Interestingly, although cutin monomers such as C16:0 and C18:2 dioic acids, and all of the ω-hydroxy fatty acids were increased dramatically in SbWINL1-OE plants, unlike AtWIN1, there was no significant change in C16:0 and C18:0 fatty acids in cutin monomers, indicating that functional differences might exist between SbWINl and AtWIN1 and this merits further investigation.
It was reported that WIN1 overexpression enhanced drought tolerance and defense responses by increasing wax and cutin accumulation in plants (Sela et al. 2013; Wang et al. 2012; Al-Abdallat et al. 2014). Similarly, overexpression of SbWINL1 in Arabidopsis enhanced drought tolerance compared with wild-type. Statistical analysis showed that the survival rate of SbWINL1-OE was obviously higher than control plants in drought treatment experiments. These results demonstrated that SbWINL1 enhanced drought tolerance by accumulation of wax and cutin.
Transcription factors can promote or block the transcription of target genes and thus control various biological processes. Several transcription factors have been identified to regulate wax and cutin synthesis by directly or indirectly interacting with relevant target genes (Go et al. 2014; Seo et al. 2011; Kannangara et al. 2007; Oshima et al. 2013). Our study showed that genes known to be involved in the biosynthesis pathway of cutin and wax were up-regulated. Among them, the expression of four wax biosynthetic genes and six cutin biosynthetic genes were dramatically increased in SbWINL1-OE plants. This indicated that overexpression of SbWINL1 had the same effect on wax and cutin biosynthesis as its Arabidopsis orthologues AtWIN1 (Broun et al. 2004).
Taken together, our study showed that SbWINL1 functions in a similar manner to its orthologous genes in other species (Broun et al. 2004; Oshima et al. 2013; Sela et al. 2013; Wang et al. 2012; Al-Abdallat et al. 2014) and enhanced the drought tolerance.
Abbreviations
- WIN1:
-
WAX INDUCER1
- ERF:
-
Ethylene response factor
- SbWINL1 :
-
Sorghum WIN1-Like 1 gene
- VLCFAs:
-
Very-long-chain fatty acids
- TFs:
-
Transcription factors
- FA:
-
Fatty acid
- ALK:
-
Alkane
- OL:
-
Alcohols
- ALD:
-
Aldehydes
- 2HFA:
-
2-OH fatty acid
- DFA:
-
Dioic acid
- ω-HFA:
-
ω-OH fatty acid
- LACS:
-
Long-chain acyl-CoA synthetase
- CER :
-
ECERIFERUM
References
Aarts MG, Keijzer CJ, Stiekema WJ, Pereira A (1995) Molecular characterization of the CER1 gene of Arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 7:2115–2127
Aharoni A, Dixit S, Jetter R, Thoenes E, Arkel G, Pereira A (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16:2463–2480
Al-Abdallat AM, Al-Debei HS, Ayad JY, Hasan S (2014) Over-expression of SlSHN1 gene improves drought tolerance by increasing cuticular wax accumulation in tomato. Int J Mol Sci 15:19499–19515
Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J (2012) Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 24:3106–3118
Bird DA (2008) The role of ABC transporters in cuticular lipid secretion. Plant Sci 174:563–569
Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L (2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52:485–498
Borisjuk N, Hrmova M, Lopato S (2014) Transcriptional regulation of cuticle biosynthesis. Biotechnol Adv 32:526–540
Bourdenx B, Bernard A, Domergue F, Pascal S, Léger A, Roby D, Pervent M, Vile D, Haslam RP, Napier JA, Lessire R, Joubès J (2011) Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol 156:29–45
Broun P, Poindexter P, Osborne E, Jiang CZ, Riechmann JL (2004) WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc Natl Acad Sci USA 101:4706–4711
Burghardt M, Riederer M (2003) Ecophysiological relevance of cuticular transpiration of deciduous and evergreen plants in relation to stomatal closure and leaf water potential. J Exp Bot 54:1941–1949
Burow GB, Franks CD, Xin Z (2008) Genetic and physiological analysis of an irradiated bloomless mutant (epicuticular wax mutant) of sorghum. Crop Sci 48:41–48
Chen XB, Goodwin SM, Boroff VL, Liu XL, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15:1170–1185
Duan H, Schuler MA (2005) Differential expression and evolution of the Arabidopsis CYP86A subfamily. Plant Physiol 137:1067–1081
Fauth M, Merten A, Hahn MG, Jeblick W, Kauss H (1996) Competence for elicitation of H2O2 in hypocotyls of cucumber is induced by breaching the cuticle and is enhanced by salicylic acid. Plant Physiol 110:347–354
Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12:2001–2008
Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues-a typical suberin and a particular cutin. Phytochemistry 66:2643–2658
Go YS, Kim H, Kim HJ, Suh MC (2014) Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-Type transcription factor. Plant Cell 26:1666–1680
Hwang KT, Cuppett SL, Weller CL, Hanna MA (2002) Properties, composition and analysis of grain sorghum wax. J Am Oil Chem Soc 79:521–527
Jung KH, Han MJ, Lee DY, Lee YS, Schreiber L, Franke R, Faust A, Yephremov A, Saedler H, Kim YW, Hwang I, An G (2006) Wax-deficient anther1is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 18:3015–3032
Kannangara R, Branigan C, Liu Y, Penfield T, Rao V, Mouille G, Höfte H, Pauly M, Riechmann JL, Broun P (2007) The transcription factor WIN1/SHN1 regulates cutin biosynthesis in Arabidopsis thaliana. Plant Cell 19:1278–1294
Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42:51–80
Kunst L, Samuels L (2009) Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol 12:721–727
Kurdyukov S, Faust A, Nawrath C, Bär S, Voisin D, Efremova N, Franke R, Schreiber L, Saedler H, Métraux JP, Yephremov A (2006a) The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 18:321–339
Kurdyukov S, Faust A, Trenkamp S, Bar S, Franke R, Efremova N, Tietjen K, Schreiber L, Saedler H, Yephremov A (2006b) Genetic and biochemical evidence for involvement of HOTHEAD in the biosynthesis of long-chain alpha-omega- dicarboxylic fatty acids and formation of extracellular matrix. Planta 224:315–329
Lee BH, Carr TP, Weller CL, Cuppett S, Dweikat IM, Schlegel V (2014) Grain sorghum whole kernel oil lowers plasma and liver cholesterol in male hamsters with minimal wax involvement. J Funct Foods 7:709–718
Li Y, Beisson F, Koo AJ, Molina I, Pollard M, Ohlrogge J (2007) Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc Natl Acad Sci USA 104:18339–18344
Liu S, Wang C, Jia F, An Y, Liu C, Xia X, Yin W (2016) Secretory peptide PdEPF2 enhances drought tolerance by modulating stomatal density and regulates ABA response in transgenic Arabidopsis thaliana. Plant Cell Tiss Org 125:419–431
Lochte-Watson KR, Weller CL (1999) Wax yield of grain sorghum (Sorghum bicolor) as affected by mechanical harvesting, threshing, and handling methods. Appl Eng Agric 15:69–72
Lu SY, Song T, Kosma DK, Parsons EP, Rowland O, Jenks MA (2009) Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J 59:553–564
Mao B, Cheng Z, Lei C, Xu F, Gao S, Ren Y, Wang J, Zhang X, Wang J, Wu F, Guo X, Liu X, Wu C, Wang H, Wan J (2012) Wax crystal-sparse leaf 2, a rice homologue of WAX2/GL1, is involved in synthesis of leaf cuticular wax. Planta 235:39–52
Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L (1999) CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 11:825–838
Oshima Y, Shikata M, Koyama T, Ohtsubo N, Mitsuda N, Ohme-Takagi M (2013) MIXTA-Like transcription factors and WAX INDUCER1/SHINE1 coordinately regulate cuticle development in Arabidopsis and Torenia fournieri. Plant Cell 25:1609–1624
Palmer GH (1992) Sorghum-food, beverage and brewing potentials. Process Biochem 27:145–153
Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Hofer R, Schreiber L, Chory J, Aharoni A (2007) The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 145:1345–1360
Peters PJ, Jenks MA, Rich PJ, Axtell JD, Ejeta G (2009) Mutagenesis, selection, and allelic analysis of epicuticular wax mutants in Sorghum. Crop Sci 49:1250–1258
Pollard M, Beisson F, Li YH, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246
Preuss D, Lemieux B, Yen G, Davis RW (1993) A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev 7:974–985
Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ (2000) FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc Natl Acad Sci USA 97:1311–1316
Raffaele S, Vailleau F, Leger A, Joubes J, Miersch O, Huard C, Blée E, Mongrand S, Domergue F, Roby D (2008) A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis. Plant Cell 20:752–767
Riederer M (2006) Thermo dynamics of the water permeability of plant cuticles: characterization of the polar pathway. J Exp Bot 57:2937–2942
Rowland O, Zheng HQ, Hepworth SR, Lam P, Jetter R, Kunst L (2006) CER4 encodes an alcohol-forming fatty acyl-coenzyme a reductase involved in cuticular wax production in Arabidopsis. Plant Physiol 142:866–877
Rowland O, Lee R, Franke R, Schreiber L, Kunst L (2007) The CER3 wax biosynthetic gene from Arabidopsis thaliana is allelic to WAX2/YRE/FLP1. FEBS Lett 581:3538–3544
Samuels L, Kunst L, Jetter R (2008) Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol 59:683–707
Schnurr J, Shockey J, Browse J (2004) The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16:629–642
Schreiber L (2010) Transport barriers made of cutin, suberin and associated wax. Trends Plant Sci 15:546–553
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16:S46–S60
Sela D, Buxdorf K, Shi JX, Feldmesser E, Schreiber L, Aharoni A, Levy M (2013) Overexpression of AtSHN1/WIN1 provokes unique defense responses. PLoS One 8:e70146
Seo PJ, Lee SB, Suh MC, Park MJ, Go YS, Park CM (2011) The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23:1138–1152
Shi J, Tan H, Yu XH, Liu Y, Liang W, Ranathunge K, Franke RB, Schreiber L, Wang Y, Kai G, Shanklin J, Ma H, Zhang D (2011a) Defective pollen wall is required for anther and microspore development in rice and encodes a fatty acyl carrier protein reductase. Plant Cell 23:2225–2246
Shi JX, Malitsky S, de Oliveira S, Branigan C, Franke RB, Schreiber L, Aharoni A (2011b) SHINE transcription factors act redundantly to pattern the archetypal surface of Arabidopsis flower organs. PLoS Genet 7:e1001388
Shi JX, Adato A, Alkan N, He Y, Lashbrooke J, Matas AJ, Meir S, Malitsky S, Isaacson T, Prusky D, Leshkowitz D, Schreiber L, Granell AR, Widemann E, Grausem B, Pinot F, Rose JK, Rogachev I, Rothan C, Aharoni A (2013) The tomato SlSHINE3 transcription factor regulates fruit cuticle formation and epidermal patterning. New Phytol 197:468–480
Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Metraux J, Nawrath C (2000) Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12:721–738
Taketa S, Amano S, Tsujino Y, Sato T, Saisho D, Kakeda K, Nomura M, Suzuki T, Matsumoto T, Sato K, Kanamori H, Kawasaki S, Takeda K (2008) Barley grain with adhering hulls is controlled by an ERF family transcription factor gene regulating a lipid biosynthesis pathway. Proc Natl Acad Sci USA 105:4062–4067
Todd J, Post-Beittenmiller D, Jaworski JG (1999) KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17:119–130
Wang Y, Wan L, Zhang L, Zhang Z, Zhang H, Quan R, Zhou S, Huang R (2012) An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol Biol 78:275–288
Wang F, Zhu H, Chen D, Li Z, Peng R, Yao Q (2016) A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss Org 125:387–398
Wellesen K, Durst F, Pinot F, Benveniste I, Nettesheim K, Wisman E, Steiner-Lange S, Saedler H, Yephremov A (2001) Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different robes of fatty acid omegahydroxylation in development. Proc Natl Acad Sci USA 98:9694–9699
Woloshuk CP, Kolattukudy PE (1986) Mechanism by which contact with plant cuticle triggers cutinase gene expression in the spores of Fusariumsolanif. Proc Natl Acad Sci USA 83:1704–1708
Yeats TH, Rose JKC (2013) The formation and function of plant cuticles. Plant Physiol 163:5–20
Zou JJ, Li XD, Ratnasekera D, Wang C, Liu WX, Song LF, Zhang WZ, Wu WH (2015) Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE 8 and CATALASE3 function in abscisic acid-mediated signaling and H2 O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 27:1445–14460
Acknowledgments
We are grateful to Professor Dabing Zhang and Dr. Dierdre McLachlan for the critical reading of the manuscript and stimulating advice and to Ms. Qian Luo and Dr. Guorun Qu from Shanghai Jiao Tong University for their assistance in the GC-FID and GC-MS analysis. This work was supported by Thousand Talents Program (Physiological and Genetic Mechanism of Sorghum Stress Resistance), Innovative Group Scheme of Tianjin (TD12-5017), Science and Technology Support Program (16YFZCNC00630) and Special Funds of Agricultural Ministry for Public Welfare Professions (201503134).
Author contributions
The experiments were designed and supervised by Prof. Shoujun Sun and Prof. Xiaodong Xie. Mr. Shuguang Bao carried out the main experiments, statistical analysis, figure preparation and manuscript editing; Associate Prof. Jianxin Shi performed data acquisition and statistical analysis of wax and cutin measuring experiments. Mr. Feng Luo and Mrs. Bo Ding performed the gene expression analysis. Mr. Feng Luo, Mrs. Bo Ding and Mrs. Jinyu Hao performed the drought stress tolerance experiment. Prof. Xie and Associate Prof. Shi revised the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bao, SG., Shi, JX., Luo, F. et al. Overexpression of Sorghum WINL1 gene confers drought tolerance in Arabidopsis thaliana through the regulation of cuticular biosynthesis. Plant Cell Tiss Organ Cult 128, 347–356 (2017). https://doi.org/10.1007/s11240-016-1114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1114-2