Abstract
Cereal grains are major targets for genetically improving the nutritional value of food and for producing recombinant proteins. Strong and tissue-specific promoters are highly desired for effectively controlling expression in the seed or endosperm. In this study, we isolated four rice promoters from the 5′ upstream region of putative seed-specifically expressed genes: PROLAM26, RAL2, RAL4 and CAPIP. By generating transgenic rice plants carrying promoter-reporter constructs, we found these four promoters to be specifically expressed in seeds, with three having endosperm-specific or -preferential activity. The strength of each promoter in the endosperm was determined and compared to a constitutively expressed OsACTIN promoter and an endosperm-specifically expressed Glu4-B promoter in single-copy transgenic plants. The promoter of RAL2 exhibited relatively high activity, and the promoters of RAL4 and CAPIP exhibited activities comparable with those of OsACTIN and Glu4-B. In addition, monitoring activities in high-generation (T3–T4) homozygous progeny of single-copy plants revealed maintenance of expression for all four promoters, with no evidence of silencing. Taken together, our findings offer four stable rice seed-specific promoters of different strengths for endosperm expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is the most preferred staple food in both East and South-east Asia, providing nutrition to more than half of the human population (Khush 2005). Although rice grains are a main source of calories and protein for humans, the protein content is significantly lower than that of other cereals. Furthermore, rice grains contain low levels of amino acids (e.g., lysine and threonine) and essential vitamins and micronutrients (Zimmermann and Hurrell 2002). The endosperm is the main storage organ for starch and protein in cereal crops, and qualitative and quantitative enhancement of the rice endosperm is an important breeding objective. In recent years, genetic engineering has achieved some notable advances in the field of rice breeding, improving the protein and micronutrient contents of rice endosperm via expression of exogenous biosynthesis genes or transporters (Boonyaves et al. 2016; Lee et al. 2005; Ye et al. 2000). Moreover, the rice endosperm is considered to be an ideal expression platform for the production of recombinant proteins (Ou et al. 2014; Takaiwa et al. 2007). Compared to other expression systems, rice endosperm bioreactors have several advantages, such as a low cost, clear genetic background, large expression capacity, ease of scale-up production and high level of biosafety (Greenham and Altosaar 2013; Sack et al. 2015). Unlike other tissues, the endosperm naturally functions as a storage organ, providing sub-cellular storage compartments for foreign recombinant proteins. In addition, the endosperm shows clear superiority over other organs for applications that involve accumulating, preserving, purifying and delivering recombinant proteins, especially bioactive drugs and vaccines. Indeed, an increasing number of economically important recombinant proteins are being produced in rice endosperm systems via a biotechnology approach (Bundó et al. 2014; Kudo et al. 2013; Patti et al. 2012; Vamvaka et al. 2016).
Gene promoters directly determine the temporal and spatial distribution and levels of the corresponding transcripts, thus providing an efficient and economic tool for manipulating the expression pattern of target genes in plant biotechnology research. Some strong heterologous constitutive promoters, such as the cauliflower mosaic virus (CaMV) 35S promoter, nopaline synthase (NOS) promoter and maize ubiquitin promoter (P ZmUbi ), are widely used in rice genetic modification (Battraw and Hall 1990; Cornejo et al. 1993). However, heterologous promoters may have sequence structures that differ from that of the rice genome and could thus possibly be deactivated (Kilby et al. 1992; Linn et al. 1990). To avoid this risk, several highly active promoters, such as the OsACTIN1 promoter (P ACT ), OsCc1 promoter and OsDHAR1 promoter (P OsCon1 ), have been isolated and applied in rice to drive constitutively high expression of transgenes (Gao et al. 2014; Jang et al. 2002; McElroy et al. 1990). Although constitutive promoters are typically strong, their activity is often not satisfactory for seed expression requirements (Drakakaki et al. 2000). In addition, due to potential interference of plant growth and development through non-targeted expression of foreign genes and the public demand of less intrusive transgene expression, strong endosperm-specific promoters could be better candidates for biotechnology applications in rice grains (Bucchini and Goldman 2002; Desai et al. 2010).
The endosperm is the main component of the seeds of monocot plants, and plant endosperm-specific promoters are typically obtained from cereal storage protein genes. Some endosperm-specific or -preferential promoters from rice have been isolated and characterized, most of which are glutelin promoters (Kawakatsu et al. 2008; Qu and Takaiwa 2004; Qu et al. 2008; Urriola and Rathore 2014). These sequences confer high expression levels in the endosperm of rice and other cereals and are therefore frequently used in molecular breeding and bioreactors. For example, the rice glutelin Glu4-B promoter (P Glu4-B ) was chosen to control specific and strong endosperm expression of foreign genes, such as phytoferritin genes, folate (Vitamin B9)-binding proteins, antihypertensive peptide and hepatitis B virus (HBV) surface antigen (Blancquaert et al. 2015; Mejima et al. 2015; Oliva et al. 2013; Qian et al. 2007; Wakasa et al. 2011). Although some endosperm promoters have been functionally identified, there is still a paucity of tissue-specific promoters for strong expression. Here, we report the isolation of four novel endosperm-specific/-preferential promoters from the rice genome. Their activities were quantitatively analyzed in transgenic rice plants using the reporter gene β-glucuronidase (GUS). Our results suggest that these promoters could be regarded as potential alternative elements for the transgenic engineering of rice and other cereals.
Materials and methods
Plant material
Rice (Oryza sativa L. ssp. japonica cv. Nipponbare) plants were used for promoter isolation and for Agrobacterium-meditated transformation. Rice seeds were germinated on solid 1/2 Murashige and Skoog (MS) medium and incubated for 10 days in a growth chamber under a light/dark cycle of 16 h/8 h at 28 °C. The seedlings were then transferred to soil and grown in a greenhouse at 25–30 °C. Mature seeds were collected after 105 days.
RNA extraction and RT-PCR
Total RNA was extracted from the leaves, roots and stems of plants at 60 days after germination (DAG), flowers, mature seed endosperm, and vegetative tissues of 10-DAG seedlings, 60-DAG plants, and 90-DAG plants using an RNAprep Pure Plant Kit (Tiangen, China). RNase-free DNase I was used to eliminate any genomic DNA, and cDNA was synthesized from approximately 2 μg RNA using Fast Quant RT Kit (Tiangen, China). Semi-quantitative PCR analysis was performed with gene-specific primers and Easy Taq PCR SuperMix (Transgen, China). A housekeeping gene, OsACTIN1, was employed as an internal control. Amplification was carried out through initial denaturation at 95 °C for 2 min, followed by 23 cycles (for the internal control) or 28 cycles (for gene detection) of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 30 s. The amplification products from each PCR reaction were separated on a 2.5 % (w/v) low-melting agarose gels.
Promoters cloning and vectors construction
According to the genomic sequence of the rice PROLAM26, RAL2, RAL4 and CAPIP genes, approximately 1.7–2.0 kb immediately upstream of the translational initiation site (ATG) was designated as the promoter (P Pro26 , P RAL2 , P RAL4 and P CAPIP ), respectively. To isolate these promoters, genomic DNA was extracted from the leaves of 10-DAG rice seedlings using a DNAsecure Plant Kit (Tiangen, China). PCR was carried out through initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 60 s using High-fidelity Fly DNA Polymerase (Transgen, China). After electrophoresis, fragments of the desired size were cloned into a pEASY-T vector (Transgen, China) and sequenced. The sequence-confirmed promoters were digested with the restriction enzymes listed in Supplemental Table 1 and fused upstream of the GUS gene in the pCAMBIA1391 vector at the corresponding sites. A 2181-bp and a 1474-bp sequence of P ACT and P Glu-4B were cloned (McElroy et al. 1990; Patti et al. 2012). All primers used for promoter cloning and plasmid construction are listed in Supplemental Table 1.
Rice transformation
Constructs were introduced into Agrobacterium-tumefaciens strain EHA105, and embryonic calli of rice were transformed following a previously reported protocol (Duan et al. 2012). Transgenic plants were regenerated using 50 mg/L hygromycin selection. T0 plants carrying a single-copy T-DNA insertion were screened using a Taqman Real-time PCR assay following a previously described method (Yang et al. 2005). For each construct, two to three independent plants with the representative expression pattern were strictly self-pollinated, and homozygous T3 and T4 plants were used for the ensuing analysis.
Histochemical GUS staining and quantitative GUS assay
For histochemical analysis, plant tissues were vacuum infiltrated for 15 min and then incubated at 37 °C in X-gluc buffer containing 50 mM sodium phosphate, pH 7.0, 0.5 mM K3[Fe(CN)6], 0.5 mM K4[Fe(CN)6]·3H2O, 10 mM Na2-EDTA, 0.1 % (v/v) Triton X-100 and 1 mM X-gluc. The incubation time varied from 30 min to overnight depending on the abundance of GUS activity. After staining, the samples were repeatedly incubated with 70 % ethanol for chlorophyll removal.
Fluorometric assays of GUS activity were performed following a standard protocol. Approximately 100 mg endosperm cells were separated from the mature seed and ground in liquid nitrogen. Total proteins were extracted in extraction buffer (100 mM phosphate buffer, pH 7.0, 10 mM EDTA, 0.2 % β-mercaptoethanol, 25 μg/mL PMSF and 25 μg/mL protease inhibitor cocktail). The homogenate was centrifuged at 12,000 rpm at 4 °C for 10 min, and the supernatant was collected. A 20-μL aliquot of the supernatant was mixed with 180 μL assay buffer containing 4-methylumbelliferyl β-d-glucuronide (MUG) substrate and incubated at 37 °C. The reactions were stopped by the addition of Na2CO3, and fluorescence signals were detected using a Fluoroskan Ascent FL (Thermo-Fisher, USA) at an emission wavelength of 455 nm and an excitation of 365 nm. GUS enzyme activity is expressed as pmol of 4-methylumbelliferone (MU) produced per minute per microgram of protein.
Results
Four candidate genes showing tissue-specific expression
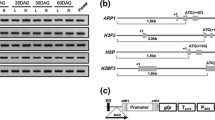
To identify strong endosperm-specific genes, we first applied the Genevestigator anatomy analysis tool to screen rice transcriptome collections (Hruz et al. 2008). Four candidates, including a prolamin precursor gene PROLAM26(LOC_Os07g10580), seed allergenic protein RA5/RA14/RA17 precursor genes RAL2 (LOC_Os07g11330) and RAL4 (LOC_Os07g11380) and a gene of unknown function, CAPIP(LOC_Os06g33640), were selected. To confirm the spatial expression pattern of these genes, transcript abundance in different tissues was examined by semi-quantitative RT-PCR. As shown in Fig. 1, mRNA for PROLAM26, RAL2 and RAL4 was only detected in the endosperm of mature seeds and not in mature leaves, roots, or stems. Furthermore, the transcripts were examined in vegetative tissues at different developmental stages, none of which could be detected in 10 DAG seedlings, 60-DAG plants or 90-DAG plants (Supplemental Fig. 1). Using primers for CAPIP, we also found strong signals in the endosperm, with weaker expression in other tissues. As the CAPIP gene exhibits extremely high similarity to other sequences (OsPYL8, LOC_Os06g33690) in the rice genome, it is technically difficult to design primers to distinguish among them. Therefore, the RT-PCR result represents the expression of the two transcripts together, suggesting that at least one gene is predominantly expressed in the endosperm.
Expression profile of the rice PROLAM26, RAL2, RAL4 and CAPIP genes by semi-quantitative RT-PCR analysis. Total RNA was isolated from the roots, leaves, stems of 60-DAG plants, flowers and endosperm and reverse transcribed into cDNA for PCR. A 23-cycle PCR amplification using specific primers for the housekeeping gene OsACTIN was used to normalize the samples. The spatial patterns of the transcripts of PROLAM26, RAL2, RAL4 and CAPIP were detected using a 28-cycle amplification
Promoter isolation and sequence analysis
To investigate whether the promoters of the selected genes could be used to control endosperm expression in rice, regulatory regions of 2062, 1768, 1942 and 1750 bp immediately upstream of the translation start site (ATG) (including the 5′ untranslated region (UTR)) of the PROLAM26, RAL2, RAL4 and CAPIP genes, respectively, were amplified from rice genomic DNA (see the Material and Methods section). The amplified promoter fragments were validated with Nipponbare genome sequences, and no variation was found within the isolated regions. The promoters were separately cloned upstream of a GUS reporter gene on the binary vector pCAMBIA 1391 to generate a promoter::GUS fusion construct. As positive controls, a widely used strong constitutive promoter from rice, P ACT (McElroy et al. 1990), and a previously identified endosperm-specific expressed promoter, P Glu-4B (Qu and Takaiwa 2004), were cloned into the binary vector.
Various cis-elements related to endosperm/seed-specific expression have been identified, and using the PLACE scan tool, we found several related cis-elements in the sequences of the isolated promoters (Higo et al. 1999). As shown in Fig. 2-box elements, which are involved in tissue specificity for a dicot seed storage protein promoter (Stålberg et al. 1996), are found in all four promoters. In addition, three, one, one and four copies of the core element for endosperm expression, the −300 core sequence (Colot et al. 1987), are present in P Pro26 , P RAL2 , P RAL4 and P CAPIP , respectively, and, three and two copies of another element related to specificity for a monocot seed storage protein, the Prolamine box (P-box) (Wu et al. 2000), are contained within P Pro26 and P CAPIP , respectively. All related tissue-specific elements are found in P Glu-4B . This abundance of seed-specific cis-elements suggests that these promoters may have tissue specificity.
Analysis of the promoter activity in a transgenic rice T0 generation
To assess the activity of the selected promoters, promoter::GUS constructs were separately introduced into japonica rice (Nipponbare) via Agrobacterium-mediated transformation. More than 20 independent plants were generated for each construct, and transformation was confirmed using PCR. To determine the tissue specificity of the promoters, GUS activity in the leaves, stems, roots, flowers and mature seeds of in all transgenic plants was detected by histochemical staining (Table 1). GUS staining was robustly restricted to seed tissue (proper expression) in 48.0, 76.9, 81.8 and 62.5 % of the P Pro26 , P RAL2 , P RAL4 and P CAPIP T0 populations, respectively. In the remaining plants, GUS activity was either not detected or only detected in tissues other than seeds (improper or null expression). Moreover, the spatial pattern of seed expression for the four promoters differed, though they were all detected in the endosperm. In P RAL4 transgenic plants, staining was restricted to endosperm cells. In contrast, blue color was more intense in the embryo and aleurone compared to the endosperm in P Pro26 transgenic plants, and pale blue staining was observed in the margin of the embryo of P RAL2 and P CAPIP plants (Fig. 3a). To further evaluate the activities of the promoters, single-copy (Supplemental Table 2) transgenic plants with proper expression patterns were screened, and GUS activity was quantitatively determined in the endosperm of the seeds from these plants (Fig. 3b). Based on the expression strength, the promoters could be divided into two groups. P RAL4 showed high activity, with a mean GUS activity of 59.8 ± 35.2 pmol 4-µ min−1 μg−1 protein in the nine individual plants of the P RAL4 transformants. The other promoters, namely, P Pro26 , P RAL2 and P CAPIP , showed relatively lower expression: the average GUS activities were 12.9 ± 5.7, 29.6 ± 22.4 and 22.7 ± 4.5 pmol 4-MU min−1 μg−1 protein in six, five and six independent plants carrying the corresponding construct. Expression levels of the control promoters P ACT and P Glu-4B were also determined in five and eight plants, respectively, showing average activities of 23.4 ± 11.3 and 26.3 ± 4.1pmol 4-MU min−1 μg−1 protein, slightly lower than that of the P RAL4 plants but comparable to the expression levels of P RAL2 and P CAPIP transgenic plants.
Expression patterns of PPRO26, PRAL2, PRAL4 and PCAPIP in transgenic plants. a Histochemical staining of GUS activity driven by P PRO26 , P RAL2 , P RAL4 and P CAPIP in different tissues. In transgenic plants with proper expression, no obvious GUS staining was detected in the roots, stems and leaves (from left to right) of 60-DAG plants after 24 h of X-gluc incubation, but strong blue staining could be observed in longitudinal sections of mature seeds only after 30 min to 1 h of incubation. Scale bars represent 2 mm. b GUS activity for various promoters in the endosperm of mature seeds of single-copy transgenic plants. GUS activity is expressed as pmol 4-MU per min per microgram protein. The dot indicates the GUS activity of promoters in each plant. The whisker cap represents 10th/90th percentiles. The median is depicted by the line, and the box represents outliers of 10th/90th percentiles
Analysis of the promoter activity in high-generation transgenic rice
To analyze promoter expression patterns in later generations, two to three single-copy plants of the transgenic plants of each construct with proper and representative expression patterns were strictly self-pollinated to generated homozygous plants. Promoter expression was monitored in late generations (T3 and T4). According to histochemical staining, GUS activity remained specific to seeds and was not detected in vegetative organs (data not shown). To further evaluate the strength of the promoters, GUS activity in the endosperm of the high-generation seeds was quantitatively determined. As indicated in Fig. 4, the promoter activity of the same construct in the T3 and T4 generations was not significantly different (P < 0.05, t test), suggesting stable expression in these later generations of the transgenic plants. P RAL4 showed strong activity, exhibiting 4.1- and 3.7-fold greater GUS expression than in P ACT in the T3 and T4 generations, respectively. Similar to the early generation, the expression levels of P RAL2 and P CAPIP were comparable to those of P ACT and P GluB-4 in the T3 and T4 plants. However, the strength of P Pro26 was much lower, with 58.7 and 44.7 % of the GUS activity of P ACT in the T3 and T4 generations, respectively.
Discussion
Seed-specific promoters have broad applications, such as tissue-specific targeting of industrial and pharmaceutical compounds, better functional quality of milled grain, and development of transgenic seeds with improved nutritional quality. Several endosperm-specific or -preferential promoters have been isolated from rice, maize, barley and other crops (Chen et al. 2007; Gunadi et al. 2016; Kawakatsu et al. 2008). The promoters of Glutelin genes from rice, Glutenin genes from wheat and Hordein genes from barley have been exploited for producing recombinant proteins in grains (Furtado et al. 2008; Kawakatsu and Takaiwa 2010; Lamacchia et al. 2001; Stöger et al. 2001). Nonetheless, additional promoters are still needed because the various purposes of transgene expression require a range of activities and temporal patterns. In addition, a battery of different promoters are required to stack multiple transgenes to avoid homology-dependent gene silencing caused by the repetitive use of the same promoter (Potenza et al. 2004). In this study, we isolated four novel rice promoters from putative seed-specific genes. By examining promoter-reporter transgenic plants, activity of all four promoters was found exclusively in seeds and not in other vegetative tissues, such as roots, leaves, sheaths and stems. Although these promoters are specifically or primarily expressed in the endosperm, the details of their spatial patterns differ. Prolamin is a major component of the total seed storage proteins of monocots (Shewry and Halford 2002), and many studies have employed the prolamin promoter to efficiently produce recombinant proteins in cereal grains (Iizuka et al. 2014; Ogo et al. 2014; Wakasa et al. 2015; Wakasa and Takaiwa 2013). In rice, 10, 13 and 16 kDa prolamin gene promoters exhibit endosperm-preferential expression, with strong activities in the aleurone and outer portion of the endosperm but weaker activities in the inner portion of the endosperm (Qu and Takaiwa 2004). P Pro26 is also a seed-specific promoter, though it does show high expression in the embryo, with weaker, evenly distributed activity in the endosperm. P RAL4 is specifically expressed in the endosperm, with the strongest activity among the four promoters studied here. Indeed, the activity of P RAL4 was even several fold higher than that of the strong promoters P Glu-4B and P ACT , suggesting that it may serve as an ideal alternative in genetic engineering. P RAL2 and P CAPIP expression was also high in the endosperm, though slight activity could be detected along the edge of the embryo, suggesting that these are endosperm-preferential but not -specific promoters. Although P RAL2 and P CAPIP exhibited relatively lower activity than P RAL4 , their activity was nonetheless still comparable to that of P ACT . Most monocot seed-specific promoters have been isolated from genes encoding seed storage proteins. A previous genome-wide analysis indicated that some rice RALs should be restrictively expressed in the endosperm (Nie et al. 2013). Consistently, our results showed two rice RAL promoters are specifically or preferentially expressed in the endosperm with strong activity, thus providing a potential direction for screening new endosperm-specific promoters. Moreover, it has been reported that OsPYL8 and CAPIP (also known as OsPYL7) transcripts mainly accumulate in the embryo, partly in the endosperm, and slightly in vegetative tissues (Tian et al. 2015). As the activity of P CAPIP suggested that the CAPIP gene may be preferentially expressed in the endosperm, OsPYL8 transcripts would accumulate in the embryo and also in vegetative tissues. The endosperm is not a tissue generally targeted by ABA; thus, we speculate that OsPYL8 may be involved in ABA sensing and signaling, more so than OsPYL7, which is consistent with the fact that OsPYL8 exhibits stronger interactions with rice PP2Cs (Tian et al. 2015).
It is a common strategy to elucidate the activity of a promoter by generating stably transformed plants. However, insertions often are multiple copies that are randomly dispersed throughout the genome by Agrobacterium- or biolistic-mediated transformation. Higher copy numbers may lead to transgene instability and gene silencing (Hernandez-Garcia and Finer 2014; Tang et al. 2007), whereas random insertions may result in great variability in expression patterns due to positional effects and potential loss of chromatin-mediated regulation. In addition, promoter expression may vary between different generations of the same transformation event (Chen et al. 2013). Therefore, to evaluate potential utility in plant biotechnical applications, promoter activities need to be progressively examined in several generations of single-copy events. Although many engineering-purpose promoters have been reported, very few, including seven rice constitutive promoters and the stress-inducible OsNCED3 promoter, have been examined with regard to long-term expression stability in single-copy populations (Bang et al. 2012, 2015; Park et al. 2012). In this study, we observed variation in promoter expression in the T0 population and further monitored the activity of single-copy events of early (T0) and late (T3 and T4) generations. Our results indicated that all four promoters are seed specific and have strong activity in the endosperm in single-copy populations, with stable expression between generations. Therefore, these newly isolated promoters have potential value in the future for stable grain-specific gene expression in efforts to enhance nutritional value and/or for bioreactor production.
References
Bang SW, Park S-H, Jeong JS, Kim YS, Jung H, Ha S-H, Kim J-K (2012) Characterization of the stress-inducible OsNCED3 promoter in different transgenic rice organs and over three homozygous generations. Planta 237:211–224. doi:10.1007/s00425-012-1764-1
Bang SW, Park S-H, Kim YS, Choi Y, Kim J-K (2015) The activities of four constitutively expressed promoters in single-copy transgenic rice plants for two homozygous generations. Planta 241:1529–1541. doi:10.1007/s00425-015-2278-4
Battraw MJ, Hall TC (1990) Histochemical analysis of CaMV 35 S promoter-β-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol 15:527–538. doi:10.1007/bf00017828
Blancquaert D, Van Daele J, Strobbe S, Kiekens F, Storozhenko S, De Steur H, Gellynck X, Lambert W, Stove C, Van Der Straeten D (2015) Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat Biotech 33:1076–1078. doi:10.1038/nbt.3358
Boonyaves K, Gruissem W, Bhullar NK (2016) NOD promoter-controlled AtIRT1 expression functions synergistically with NAS and FERRITIN genes to increase iron in rice grains. Plant Mol Biol 90:207–215
Bucchini L, Goldman LR (2002) Starlink corn: a risk analysis. Environ Health Perspect 110:5–13
Bundó M, Montesinos L, Izquierdo E, Campo S, Mieulet D, Guiderdoni E, Rossignol M, Badosa E, Montesinos E, San Segundo B (2014) Production of cecropin A antimicrobial peptide in rice seed endosperm. BMC Plant Biol 14:1
Chen X, Wang Z, Wang J et al (2007) Isolation and characterization of Brittle2 promoter from Zea mays and its comparison with Ze19 promoter in transgenic tobacco plants. Plant Cell Tissue Organ Cult 88:11. doi:10.1007/s11240-006-9165-4
Chen Z, Wang J, Ye M-X, Li H, Ji L-X, Li Y, Cui D-Q, Liu J-M, An X-M (2013) A novel moderate constitutive promoter derived from poplar (Populus tomentosa Carrière). Int J Mol Sci 14:6187–6204
Colot V, Robert L, Kavanagh T, Bevan M, Thompson R (1987) Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J 6:3559
Cornejo M-J, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:567–581. doi:10.1007/bf00019304
Desai PN, Shrivastava N, Padh H (2010) Production of heterologous proteins in plants: strategies for optimal expression. Biotechnol Adv 28:427–435
Drakakaki G, Christou P, Stöger E (2000) Constitutive expression of soybean ferritin cDNA intransgenic wheat and rice results in increased iron levels in vegetative tissues but not in seeds. Transgenic Res 9:445–452
Duan Y, Zhai C, Li H, Li J, Mei W, Gui H, Ni D, Song F, Li L, Zhang W (2012) An efficient and high-throughput protocol for Agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in Japonica rice (Oryza sativa L.) Plant Cell Rep 31:1611–1624
Furtado A, Henry RJ, Takaiwa F (2008) Comparison of promoters in transgenic rice. Plant Biotechnol J 6:679–693
Gao S, Zhu Z, Liu S, Jin R, Yang G, Tan L (2014) Estimating the spatial distribution of soil moisture based on Bayesian maximum entropy method with auxiliary data from remote sensing. Int J Appl Earth Obs Geoinf 32:54–66
Greenham T, Altosaar I (2013) Molecular strategies to engineer transgenic rice seed compartments for large-scale production of plant-made pharmaceuticals. Rice Protocols 956:311–326
Gunadi A, Rushton PJ, McHale LK et al (2016) Characterization of 40 soybean (Glycine max) promoters, isolated from across 5 thematic gene groups. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-016-1038-x
Hernandez-Garcia CM, Finer JJ (2014) Identification and validation of promoters and cis-acting regulatory elements. Plant Sci 217:109–119
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27:297–300
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform. doi:10.1155/2008/420747
Iizuka M, Wakasa Y, Tsuboi H, Asashima H, Hirota T, Kondo Y, Matsumoto I, Takaiwa F, Sumida T (2014) Suppression of collagen-induced arthritis by oral administration of transgenic rice seeds expressing altered peptide ligands of type II collagen. Plant Biotechnol J 12:1143–1152. doi:10.1111/pbi.12223
Jang I-C, Choi W-B, Lee K-H, Song SI, Nahm BH, Kim J-K (2002) High-level and ubiquitous expression of the rice cytochromec gene OsCc1 and its promoter activity in transgenic plants provides a useful promoter for transgenesis of monocots. Plant Physiol 129:1473–1481
Kawakatsu T, Takaiwa F (2010) Cereal seed storage protein synthesis: fundamental processes for recombinant protein production in cereal grains. Plant Biotechnol J 8:939–953
Kawakatsu T, Yamamoto MP, Hirose S, Yano M, Takaiwa F (2008) Characterization of a new rice glutelin gene GluD-1 expressed in the starchy endosperm. J Exp Bot 59:4233–4245. doi:10.1093/jxb/ern265
Khush GS (2005) What it will take to Feed 5.0 billion rice consumers in 2030. Plant Mol Biol 59:1–6. doi:10.1007/s11103-005-2159-5
Kilby NJ, Leyser HO, Furner IJ (1992) Promoter methylation and progressive transgene inactivation in Arabidopsis. Plant Mol Biol 20:103–112
Kudo K, Ohta M, Yang L, Wakasa Y, Takahashi S, Takaiwa F (2013) ER stress response induced by the production of human IL-7 in rice endosperm cells. Plant Mol Biol 81:461–475. doi:10.1007/s11103-013-0016-5
Lamacchia C, Shewry PR, Di Fonzo N, Forsyth JL, Harris N, Lazzeri PA, Napier JA, Halford NG, Barcelo P (2001) Endosperm-specific activity of a storage protein gene promoter in transgenic wheat seed. J Exp Bot 52:243–250
Lee TTT, Chung M-C, Kao Y-W, Wang C-S, Chen L-J, Tzen JTC (2005) Specific expression of a sesame storage protein in transgenic rice bran. J Cereal Sci 41:23–29. doi:10.1016/j.jcs.2004.08.014
Linn F, Heidmann I, Saedler H, Meyer P (1990) Epigenetic changes in the expression of the maize A1 gene in Petunia hybrida: role of numbers of integrated gene copies and state of methylation. Mol Gen Genet 222:329–336
McElroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
Mejima M, Kashima K, Kuroda M et al (2015) Determination of genomic location and structure of the transgenes in marker-free rice-based cholera vaccine by using whole genome resequencing approach. Plant Cell Tissue Organ Cult 120:35–48. doi:10.1007/s11240-014-0575-4
Nie D-M, Ouyang Y-D, Wang X, Zhou W, Hu C-G, Yao J (2013) Genome-wide analysis of endosperm-specific genes in rice. Gene 530:236–247. doi:10.1016/j.gene.2013.07.088
Ogo Y, Wakasa Y, Hirano K, Urisu A, Matsuda T, Takaiwa F (2014) Generation of transgenic rice with reduced content of major and novel high molecular weight allergens. Rice 7:1–9. doi:10.1186/s12284-014-0019-0
Oliva N, Chadha-Mohanty P, Poletti S, Abrigo E, Atienza G, Torrizo L, Garcia R, Dueñas C, Poncio MA, Balindong J, Manzanilla M, Montecillo F, Zaidem M, Barry G, Hervé P, Shou H, Slamet-Loedin IH (2013) Large-scale production and evaluation of marker-free indica rice IR64 expressing phytoferritin genes. Mol Breed 33:23–37. doi:10.1007/s11032-013-9931-z
Ou J, Guo Z, Shi J, Wang X, Liu J, Shi B, Guo F, Zhang C, Yang D (2014) Transgenic rice endosperm as a bioreactor for molecular pharming. Plant Cell Rep 33:585–594
Park S-H, Bang SW, Jeong JS, Jung H, Redillas MCFR, Kim HI, Lee KH, Kim YS, Kim J-K (2012) Analysis of the APX, PGD1 and R1G1B constitutive gene promoters in various organs over three homozygous generations of transgenic rice plants. Planta 235:1397–1408. doi:10.1007/s00425-011-1582-x
Patti T, Bembi B, Cristin P, Mazzarol F, Secco E, Pappalardo C, Musetti R, Martinuzzi M, Versolatto S, Cariati R, Dardis A, Marchetti S (2012) Endosperm-specific expression of human acid beta-glucosidase in a waxy rice. Rice 5:1–15. doi:10.1186/1939-8433-5-34
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol Plant 40:1–22
Qian B, Shen H, Liang W, Guo X, Zhang C, Wang Y, Li G, Wu A, Cao K, Zhang D (2007) Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 17:621–631. doi:10.1007/s11248-007-9135-6
Qu LQ, Takaiwa F (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J 2:113–125
Qu LQ, Xing YP, Liu WX, Xu XP, Song YR (2008) Expression pattern and activity of six glutelin gene promoters in transgenic rice. J Exp Bot 59:2417–2424. doi:10.1093/jxb/ern110
Sack M, Hofbauer A, Fischer R, Stoger E (2015) The increasing value of plant-made proteins. Curr Opin Biotechnol 32:163–170
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958. doi:10.1093/jexbot/53.370.947
Stålberg K, Ellerstöm M, Ezcurra I, Ablov S, Rask L (1996) Disruption of an overlapping E-box/ABRE motif abolished high transcription of the napA storage-protein promoter in transgenic Brassica napus seeds. Planta 199:515–519
Stöger E, Parker M, Christou P, Casey R (2001) Pea legumin overexpressed in wheat endosperm assembles into an ordered paracrystalline matrix. Plant Physiol 125:1732–1742
Takaiwa F, Takagi H, Hirose S, Wakasa Y (2007) Endosperm tissue is good production platform for artificial recombinant proteins in transgenic rice. Plant Biotechnol J 5:84–92
Tang W, Newton RJ, Weidner DA (2007) Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J Exp Bot 58:545–554
Tian X, Wang Z, Li X, Lv T, Liu H, Wang L, Niu H, Bu Q (2015) Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice. Rice 8:1–13
Urriola J, Rathore KS (2014) Temporal and spatial activities of a rice glutelin promoter in transgenic sorghum. Plant Cell Tissue Organ Cult 116:227–234. doi:10.1007/s11240-013-0398-8
Vamvaka E, Twyman RM, Murad AM, Melnik S, Teh AY-H, Arcalis E, Altmann F, Stoger E, Rech E, Ma JKC, Christou P, Capell T (2016) Rice endosperm produces an underglycosylated and potent form of the HIV-neutralizing monoclonal antibody 2G12. Plant Biotechnol J 14:97–108. doi:10.1111/pbi.12360
Wakasa Y, Takaiwa F (2013) The use of rice seeds to produce human pharmaceuticals for oral therapy. Biotechnol J 8:1133–1143. doi:10.1002/biot.201300065
Wakasa Y, Zhao H, Hirose S, Yamauchi D, Yamada Y, Yang L, Ohinata K, Yoshikawa M, Takaiwa F (2011) Antihypertensive activity of transgenic rice seed containing an 18-repeat novokinin peptide localized in the nucleolus of endosperm cells. Plant Biotechnol J 9:729–735. doi:10.1111/j.1467-7652.2010.00576.x
Wakasa Y, Takagi H, Watanabe N, Kitamura N, Fujiwara Y, Ogo Y, Hayashi S, Yang L, Ohta M, Thet Tin WW, Sekikawa K, Takano M, Ozawa K, Hiroi T, Takaiwa F (2015) Concentrated protein body product derived from rice endosperm as an oral tolerogen for allergen-specific immunotherapy? A new mucosal vaccine formulation against Japanese cedar pollen allergy. PLoS One 10:e0120209. doi:10.1371/journal.pone.0120209
Wu CY, Washida H, Onodera Y, Harada K, Takaiwa F (2000) Quantitative nature of the prolamin-box, ACGT and AACA motifs in a rice glutelin gene promoter: minimal cis-element requirements for endosperm-specific gene expression. Plant J 23:415–421
Yang L, Ding J, Zhang C, Jia J, Weng H, Liu W, Zhang D (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23:759–763
Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (ß-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305
Zimmermann MB, Hurrell RF (2002) Improving iron, zinc and vitamin A nutrition through plant biotechnology. Curr Opin Biotechnol 13:142–145. doi:10.1016/S0958-1669(02)00304-X
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31401454 and No. 31501239) and the Creative Foundation of Anhui Agricultural Academy of Sciences (Nos. 13C0101, 15B0101, 15B0128 and 15A0115).
Author contributions
R.X., D.L. and J.L. cloned promoters and constructed vectors. H.L., Y.Y., R.Q. and L.L. performed rice transformations. R.X., D.L. and R.Q. determined the gene expression level and GUS biochemical activities. R.X. and P.W. analyzed the data and drew illustrations. P.W. and J.Y. designed experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, R., Li, D., Li, H. et al. Isolation of four rice seed-specific promoters and evaluation of endosperm activity. Plant Cell Tiss Organ Cult 128, 125–132 (2017). https://doi.org/10.1007/s11240-016-1091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1091-5