Abstract
Unlike garlic and onion, the regeneration of chive (Allium schoenoprasum L.), cultivated both for culinary and ornamental purposes, has not been intensively studied. The effects of the eight cytokinins and the plant basal section thickness on regeneration efficiency and subsequent plant growth were studied. Representatives of all cytokinin structural groups: isoprenoide side chain (trans-zeatin) and aromatic side chain (benziladenine, kinetin, meta-topolin) adenine derivatives, and phenylurea derivatives (thidiazuron and N-(2-chloro-4-pyridyl)-N′-phenylurea) at 0, 1, 5 or 10 μM were used. Histological analysis revealed adventitious buds formation from the leaf sheaths’ bases and the basal plate. The highest regeneration frequency (100 %) and the mean bud number per explant (20.0) were achieved with 10 μM thidiazuron (TDZ), and 5 mm-thick basal sections were the most responsive explants. Inferior shoot and root growth characteristics of plants regenerated by this treatment was avoided by exclusion or replacement of 10 μM TDZ with 5 μM kinetin (Kin) after a 4-week bud induction period, without consequences on the regeneration efficiency. In addition, a positive correlation between peroxidase, catalase and superoxide dismutase activity and the regeneration capacity was observed. All antioxidative enzymes activity changed much faster with 10 μM TDZ than with 1 μM Kin, which provoked the weakest regeneration response. Moreover, a unique peroxidase isoform was observed only in TDZ-treated explants after 3rd day of treatment. This work is useful for genetic engineering and virus-free plant production advancement, and for the knowledge expansion regarding the role of antioxidative enzymes in plant organogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allium species are well known for their healing properties in curing of numerous medical problems (Keusgen 2002). Although less studied, Allium schoenoprasum L. (chive) has also been shown to have curative properties (Timité et al. 2013) and, as it contains sulfur compounds with prominent antibacterial activity, it was also proposed as a suitable food preservative (Rattanachaikunsopon and Phumkhachorn 2008). In addition, chive plants have widely been used for culinary purposes, as a food condiment, providing milder flavor than onion. Due to of their beautiful lilac flowers, chive plants are grown as ornamentals, as well. Chive can also be used in gardens for pests’ control because of its insect-repelling properties.
Modern biotechnology enables accelerating breeding of species and the creation of improved plant varieties, including Allium species (Umehara et al. 2006; Eady et al. 2008). However, an efficient and reliable in vitro regeneration system is a prerequisite for both gene transfer technology and interspecific hybridization. Numerous regeneration protocols are available for Allium species, especially for the most economically important, garlic and onion. Regeneration process through both caulogenesis and somatic embryogenesis was achieved from different explant types, but the basal plate and the youngest leaves’ sheath bases, just above the basal plate, has been recognized as the most responsive explants in Allium species (Kahane et al. 1992; Ayabe and Sumi 1998; Kenel et al. 2010). Unlike garlic and onion, micropropagation of chive has not been intensively studied, therefore only a few regeneration protocols are available, starting from leaf discs (Rauber and Grunewaldt 1989), root sections (Zdravković-Korać et al. 2010) and the basal plate (Tubić et al. 2011), with the efficiency that still requires further improvements.
In Allium species auxins were employed for induction of regeneration in the vast majority of studies, although they were reported to cause chromosomal aberrations (Mukhopadhyay et al. 2005), while cytokinins as a sole PGR have been used seldom (Kahane et al. 1992).
Cytokinins comprise two structurally very diverse groups of compounds: adenine derivatives with isoprenoide or aromatic side chain and phenylurea derivatives (Van Staden et al. 2008). Among the commercially available cytokinins, N6-benzyladenine (BA) has most frequently been used, due to its low cost and high effectiveness in the promotion of in vitro regeneration in numerous plant species (Van Staden et al. 2008). However, BA quite often causes morphological and physiological disorders in plants, like hyperhydricity (Bairu et al. 2007), or affects adversely rooting and subsequent acclimatization of micropropagated plants (Valero-Aracama et al. 2010). Recently, topolins, hydroxylated BA analogues, have been confirmed to be an effective and less harmful alternative to BA (Aremu et al. 2012b). Phenylurea derivatives, particularly thidiazuron (TDZ), have a potent cytokinin activity, although their structure does not resemble any naturally occurring adenine-based cytokinin (Murthy et al. 1998). TDZ is effective at lower concentrations and acts more quickly than adenine derivatives, so it is efficient in a wide range of plant species, including those recalcitrant to regeneration (reviewed by Murthy et al. 1998).
The involvement of antioxidative enzymes in the acquisition of regeneration competence was suggested (Papadakis et al. 2001; Żur et al. 2014). While the state of oxidative stress might cause a complete loss of the plant’s cells totipotency (Papadakis et al. 2001), reactive oxygen species (ROS) at low levels act as secondary messengers and are involved in the regulation of many physiological processes, including plant growth, development and differentiation (Cui et al. 1999). Furthermore, ROS are also involved in signaling pathways of plant hormones, and these in return might affect redox homeostasis by increasing antioxidative enzymes activity (Zavaleta-Mancera et al. 2007; Díaz-Vivancos et al. 2011). However, the precise mode of phytohormone action on antioxidative system is not known and not universal for all plant species (Tang and Newton 2005; Díaz-Vivancos et al. 2011). Even phytohormones sharing similar chemical structure differentially affect antioxidative system of the same plant tissue (Díaz-Vivancos et al. 2011).
In the previous study, the impact of three cytokinins (BA, kinetin or TDZ) at 5 μM on regeneration capacity from transverse stalk sections of chive plants was tested (Tubić et al. 2011). To further improve regeneration efficiency, more comprehensive research was performed in the present study, assessing the effects of representatives of all cytokinin groups, using a wider range of concentration, and plant basal section thickness on the regeneration capacity and subsequent plant growth. Furthermore, antioxidative enzymes (peroxidases, catalases and superoxid dismutases) activity was analyzed and compared in explants exposed to the treatments with the most diverse regeneration success.
Materials and methods
Basal medium
The basal medium contained MS (Murashige and Skoog 1962) macro and micro mineral salts (all purchased from Lachner, Brno, Czech Republic), 20 g l−1 sucrose, 100 mg l−1 myo-inositol, 2 mg l−1 thiamine, 2 mg l−1 pyridoxine, 5 mg l−1 nicotinic acid and 2 mg l−1 adenine (all obtained from Sigma-Aldrich, St. Louis, MO, USA). The medium pH was adjusted to 5.6 before sterilization using a pH meter. The medium was solidified with 0.7 % (w/v) agar (Torlak Institute, Belgrade, Serbia) and sterilized in an autoclave at 114 °C (80 kPa) for 25 min.
Plant material
In vitro-grown chive plants (approximately 2 mm in diameter, with 4–5 leaf sheaths), obtained in the previous study (Zdravković-Korać et al. 2010) and maintained on PGR-free basal medium, were used as a donor material. The roots were cut away up to the basal part of the stem (the basal plate) and then sequential transverse sections through the plant were made. The basal section included the basal plate, the shoot apex meristem (SAM) and the leaf sheath bases, whereas the upper sections contained only leaf blades. Each plant was sliced in three to six sections, as was specified later for each experiment. The explants with proximal side down were placed on media containing different PGRs, dispensed (25 ml) in sterile Petri-dishes (Ø 9 cm, Spektar, Čačak, Serbia).
Culture conditions
The cultures were maintained under diffuse, cool white fluorescent tubes light (Tesla, Belgrade, Serbia), with a photosynthetic photon flux density of 100 μmol m−2s−1, as measured by an LI-1400 DataLogger equipped with an LI-190SA Quantum sensor, LI-COR Biosciences, for 16 h per day at 25 ± 2 °C.
Effects of cytokinins on induction of regeneration and optimization of the regeneration procedure
For the induction of regeneration, 3 mm-thick basal transverse sections (six sections per plant) were placed on a solid medium supplemented with 0, 1, 5 or 10 µM N6-furfuryl aminopurine (kinetin, Kin, Sigma-Aldrich), N6-benzyladenine (BA, Sigma-Aldrich), trans-zeatin (ZEA, Olchemim, Olomouc, Czech Republic), meta-topolin (mT, Duchefa Biochemie, Haarlem, The Netherlands), N-phenyl-N′-1,2,3-thiadiazol-5-ylurea (thidiazuron, TDZ, Duchefa Biochemie) or N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU, Duchefa Biochemie). The experiment was performed in three replicates, each with four samples (Petri-dishes) and three subsamples (sliced plants) (n = 36) per treatment.
To optimize the regeneration procedure further, the effect of section thickness was tested. Each plant was sliced in six 0.5 mm-thick or three 2 mm- or 5 mm-thick sequential basal transverse sections by hand, using a very sharp razor blade, and placed on medium supplemented with 10 μM TDZ. The experiment was performed in three replicates, each with 4 samples (Petri-dishes) and 3 subsamples (sliced plants) (n = 36) per treatment.
To test carry-over effect of TDZ on further growth and development of shoots, 5 mm-thick basal transverse sections (three sections per plant) were cultivated on medium supplemented with 10 µM TDZ for 4 weeks and then subcultivated on media supplemented with 10 µM TDZ, 5 µM Kin or 1 µM mT, or on PGR-free medium for an additional 4 weeks. Three replicates, each with 4 samples (Petri-dishes) and 4 subsamples (sliced plants) (n = 48) were prepared per treatment.
In all experiments, the number of shoots was recorded with the aid of a stereomicroscope (Carl Zeiss, Jena, Germany) following an 8-week period. The results are expressed as the regeneration frequency, defined as the frequency of SAM-containing cross sections regenerating buds, the mean bud number per SAM-section, and an index of bud-forming capacity (BFC), which was used to evaluate the cumulative effect of the two aforementioned variables, calculated as follows: BFC = (the mean bud number per SAM-section) × (% of regenerating SAM-sections). The term regeneration capacity was used as a descriptive term referring to overall ability of the explants to regenerate buds.
Growth parameters of plants (the longest shoot and root length, and the mean root number) were also determined following an 8-week culture period. The rooting frequency was expressed as the portion of explants formed roots.
Scanning electron microscopy
Scanning electron microscopy was employed to analyze initiation of bud regeneration. The explants were directly inserted inside the scanning electron microscope (JOEL-JSM-6390LV, Tokyo, Japan) without prior fixation. Scanning electron images were taken at 15 kV.
Histological analysis
The basal transverse sections of chive plants grown on medium supplemented with 10 μM TDZ for 22 days, were processed for histological analysis. The basal transverse sections cut from plants maintained on PGR-free medium were used as control. Samples were fixed in FAA (formalin:acetic acid:ethanol, 10:5:85), dehydrated in graded ethanol series and embedded in paraffin wax at 58 °C. Sections (8 μm thick) were stained with haematoxylin and photographed under a Zeiss Axiovert microscope (Carl Zeiss GmbH, Göttingen, Germany).
Total proteins isolation
Total proteins were extracted from the same explant type used for the induction of regeneration. Briefly, 5 mm-thick basal transverse sections (one section per plant), cultivated on the media supplemented with cytokinins with the most diverse regeneration success, 1 µM Kin or 10 µM TDZ, were collected after 1, 3, 5, 7, 10 and 14 days of cultivation. Foliage leaves of the emerging shoots, that were developed during the period of cultivation, were cut away up to the initial explant size prior to total proteins isolation. Freshly prepared explants taken from the 4-week old plantlets maintained on the PGR-free medium were used as controls. For each treatment, ten explants were pooled in a sample and ground to fine powder by liquid nitrogen with addition of ice-cold extraction buffer in 2:1 (v:w) proportion. The extraction buffer consisted of 50 mM Tris–HCl pH 7.6, 10 mM ethylenediaminetetraacetic acid (EDTA) pH 8.0, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 % (v/v) glycerol and 5 % (w/v) insoluble polyvinylpolypyrrolidone (PVP). The homogenates were cleared by centrifugation at 12,000×g for 10 min at 4 °C and the obtained crude extracts were used for further analyses. Total soluble protein contents were determined by the method of Bradford (1976) with bovine serum albumin (BSA) as a standard. Experiments were done in two biological replicates for each treatment.
Native-PAGE separation of POX, CAT and SOD enzymes
Samples containing 15 µg of total proteins were subjected to non-denaturing polyacrylamide gel electrophoresis (native-PAGE) on 10 % resolving gels with Tris–glycine pH 8.3 (25 mM Tris, 192 mM glycine) running buffer, under constant voltage (120 V) at 4 °C (Laemmli 1970).
To visualize the peroxidases (POX; EC 1.11.1.7) activity, the gels were incubated with universal peroxidase substrate 4-chloro-1-naphthol (Dragišić-Maksimović et al. 2013). The staining solutions contained 50 mM K-phosphate buffer pH 6.5 with 0.56 mM 4-chloro-1-naphthol and 0.01 % H2O2 (v/v). Purple bands of produced insoluble precipitates appeared after about 20 min of staining.
The catalase (CAT; EC 1.11.1.6) was detected according to Woodbury et al. (1971). After separation of total proteins, the gels were preincubated in 0.01 % H2O2 (v/v) for 10 min, followed by a brief wash with distilled water and incubated for 10 min in staining solution containing 0.123 mM ferric chloride (FeCl3) and 0.061 mM potassium ferricyanide [K3Fe(CN6)].
For the separation of superoxide dismutases (SOD; 1.15.1.1) photochemical staining with riboflavin and nitroblue tetrazolium (NBT) as described by Beauchamp and Fridovitch (1971) was used. The gels were soaked in 100 mM K-phosphate pH 7.8 buffer containing 1 mM EDTA, 0.25 mM NBT, 0.08 mM riboflavin and 1.33 mM N,N,N′,N′-Tetramethylethylenediamine (TEMED) for 30 min, in darkness. The gels were then rinsed with distilled water and illuminated with white fluorescent lamp for 15 min to initiate the photochemical reaction. SOD isoforms were differentiated by incubating gels in selective inhibitors of SOD for 30 min before staining (Vitória et al. 2001). KCN at 1 mM was used to inhibit Cu/Zn-SOD activity, and 4 mM H2O2 was used to inhibit Cu/Zn-SOD and Fe-SOD activities, whereas Mn-SOD activity is resistant to both inhibitor treatments.
Spectrophotometric determination of POX, CAT and SOD activity
Total activities of respective enzymes were determined spectrophotometrically. The total POX activity was measured in a 1.5 ml reaction mixture consisting of 50 mM K-phosphate buffer pH 6.5, 10 mM pyrogallol and aliquots of 10 μl of crude protein extract. The reaction was started by addition of 0.07 % H2O2 (v/v), and an increase in absorbance at 430 nm was followed using Agilent 8453 spectrophotometer (Life Sciences, USA). Peroxidase activity was calculated using the extinction coefficient for formed purpurogallin (ε 430 = 12 mM−1 cm−1).
The activity of CAT was measured by reading absorbance decrease at 240 nm, as a consequence of H2O2 consumption (ε 240 = 0.0436 mM−1cm−1), according to the method of Aebi (1984). The reaction mixture (1.5 ml) contained 50 mM Na–K-phosphate buffer pH 7.0 and 10 μl of crude protein extract. The reaction was initiated by the addition of 0.07 % H2O2 (v/v).
Total SOD activity determination was based on capacity of the enzyme extract to inhibit the photochemical reduction of NBT to blue formazan (Beyer and Fridovich 1987). The reaction mixture contained 50 mM K-phosphate buffer pH 7.8, 0.1 mM EDTA, 12 mM l-methionine, 0.075 mM NBT, 0.002 mM riboflavin and 0-10 μl of crude protein extract. Reaction mixtures were exposed to illumination for 15 min at 25 °C. Absorbance was recorded at 540 nm using an ELISA microplate reader (LKB Vertriebs GmbH, Austria).
Specific activity of each enzyme was determined as the rates of respective product formation, or substrate disappearance per mg of total soluble proteins per min (U mg−1). Activity of respected enzymes was presented relative to Kin- or TDZ-untreated controls and values reported are the average of two biological replicates.
Data analysis
All cultures were placed in a completely randomized design. Percentage data were subjected to angular transformation and bud/root number data to square root transformation prior to analysis, followed by inverse transformation for presentation. All data were subjected to standard analysis of variance (ANOVA), and the means were separated using Fisher’s LSD test at p ≤ 0.05. The BFC index was calculated using appropriately transformed data and subsequently presented without inverse transformation. As only the SAM-containing cross sections responded by adventitious bud formation, only one number per plant was computed for statistical analysis. In the cases of two TCL sections response per plant, the number of buds data was computed as the sum of buds obtained from both sections per plant.
Results and discussion
Effect of cytokinin type on the regeneration capacity and plant growth
The basal SAM-containing section of chive plants, which usually belonged to the first section, enlarged within a week of the cultivation, without massive callus formation in all treatments. This was the only bud responding section since the remaining foliage leaves-containing sections elongated and seldom formed snowy calli, but buds were never regenerated, as it was observed in the previous study (Tubić et al. 2011). In SAM-sections cultivated on PGR-free medium spontaneous de novo bud formation has never been observed (Table 1). These explants only responded by forming a single whole plant developed from SAM (Fig. 1a). The regeneration of adventitious buds was observed only in the presence of cytokinin (Fig. 1b). First buds have been observed on 10 μM TDZ-treated explants as follows 5th day of culture with the aid of a stereomicroscope and 14th day by a naked eye. The quick action of TDZ was observed in other plant species as in geranium, where 2 days of cultivation of hypocotyl segments on media with TDZ were enough to induce somatic embryos, visible after 12 days (Visser et al. 1992).
Bud regeneration from the basal transverse section of chive plants. a Single plantlet developed from the shoot apical meristem of the basal section of chive plant cultivated on PGR-free medium. The apical shoot (AS) is indicated by the arrow. b Adventitious buds and the apical shoot (AS) developed from an explant grown on the medium with 5 μM BA following a month of cultivation. Scale bars a, b 1 mm
In SAM-sections of chive cultivated on 10 μM TDZ for 14 days scanning electron microscopy revealed swollen zone representing the basal plate tissue proliferation just at the base of leaf sheaths (Fig. 2a, b). Numerous bud-like structures could be recognized around the proliferated basal plate.
Shoot regeneration from the basal transverse section of chive plants grown on the medium supplemented with 10 μM TDZ. a, b Scanning electron micrographs of the explants following 14 days of culture. Note proliferated basal plate tissue at the bottom of leaf sheaths and adventitious bud primordia around the proliferated tissue (arrow). c–f Light microscopy micrographs of longitudinal sections through explants following 22 days of culture. c Adventitious bud primordium at the base of leaf sheath. Note the periclinal cell divisions (arrow) at the bottom of the abaxial side of leaf sheath. d Well developed adventitious buds at the base of leaf sheaths and adventitious bud primordium arising from leaf sheath (arrow). e Multiple adventitious buds (arrow) at different stages of development. Note bud primordium developing from the base of the leaf sheath (arrow), and root meristem in the inner stem region. f The chive shoot apex with young leaf primordia closing the apical meristem. Note the axillary bud that arises on axils of young leaf (arrow). AB abaxial side of leaf, AD adaxial side of leaf, BP basal plate, L leaf, PV provascular elements, RM root meristem
Histological analysis of 22-day-old cultures confirmed the presence of single or more often multiple, usually fasciated, adventitious buds (Fig. 2c–e). At the base of leaf sheaths and beneath the apical meristem of adventitious buds broad basal plate could be observed. The basal plate was composed mainly of meristematic, frequently dividing, cells and organized meristemoids. The procambium strands distributed throughout stem could also be distinguishable. Cell files produced by periclinally dividing cells positioned near the leaf base contributed the enlargement of the basal plate (Fig. 2c). Adventitious buds appeared to originate mainly from the bottom of abaxial or adaxial site of leaf sheaths, as well as from the meristemoids in superficial layers of the basal plate (Fig. 2c–e). The top region of the basal plate and the bases of the youngest leaf sheaths were also recognized as the most responsive tissues in onion and garlic (Kahane et al. 1992; Ayabe and Sumi 1998; Kenel et al. 2010). The axillary buds were rarely detected, and only on the axils of leaves near the shoot apical meristem (Fig. 2f). Adventitious or axillary buds were not observed in the explants at the starting point of the cultures (data not shown).
Cytokinins as a sole PGR have only scarcely been used for the induction of regeneration in Allium species. Kahane et al. (1992) found cytokinins absolutely indispensable for bud regeneration from the basal section of onion plants. In the present study, the type of cytokinin significantly affected the frequency of bud regeneration (p ≤ 0.001), while cytokinin concentration, as well as their interaction, were insignificant. Actually, all cytokinins were effective in bud induction, with nearly all explants responded (Table 1). The only exceptions with slightly lower regeneration frequency of 85.1 and 85.7 % were Kin and ZEA at 1 μM, respectively (Table 1). The weak effect of Kin on regeneration response was also observed in Barleria greenii (Amoo et al. 2011).
Both cytokinin type and concentration, and their interaction as well, significantly affected the mean bud number per section (p ≤ 0.001, p ≤ 0.001, p ≤ 0.05, respectively). Generally, the mean bud number per section increased with cytokinin concentration, and it was the highest (20.0) with 10 μM TDZ (Fig. 3a), although it was not significantly different from 10 μM mT (15.4) (Table 1). The lowest mean bud number was obtained with Kin (1.2–2.8), followed by ZEA (1.6–7.7) while 5 or 10 μM BA, CPPU and mT, as well as 1 or 5 μM TDZ exhibited similar effect (8.3–15.4) (Table 1).
Accordingly, the index of bud-forming capacity (BFC) was significantly affected by cytokinin type (p ≤ 0.001) and concentration (p ≤ 0.01), and it was the highest (7.0) with 10 μM TDZ, followed by 10 μM mT (6.1), while 1 μM Kin or ZEA provoked the lowest BFC (1.6 both) (Table 1).
Altogether, the results showed 10 μM TDZ was the most efficient in promotion of bud induction among the cytokinins tested, as it was observed in numerous plant species (Murthy et al. 1998). Furthermore, the results indicated that cytokinins belonging to the same structural group might not necessarily have similar effect on the process of bud regeneration. Contrary to the phenylurea derivatives, the aromatic-side chain adenine derivatives displayed significantly different efficiencies. BA and mT were only slightly less efficient than TDZ and their effect was comparable to CPPU. However, Kin had quite weak effect, much more similar to ZEA, than to its counterparts BA and mT.
An increasing body of evidence found topolins being the most effective cytokinin in promotion of tissue culture response in a wide range of species, e.g. banana (Aremu et al. 2012a), Aloe polyphylla (Bairu et al. 2007) and Huernia hystrix (Amoo and Van Staden 2013). However, it was not an absolute case in chive, where mT was approximately efficient as BA and CPPU in promotion of bud regeneration. The exception was mT at 10 μM with close efficiency as the most potent TDZ (Table 1).
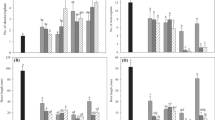
As culture period was extended to 8 weeks, regenerated shoots developed from explants and formed shoot bunches, typical for chive plants (Fig. 3a–c), with an exception of untreated control where only a single plant was developed (Fig. 1a). By the end of this period, growth parameters (the longest shoot and root length and the frequency of rooting and the mean root number per explant) were significantly influenced by cytokinin type and concentration (p ≤ 0.001 all), while their interaction was significant for all parameters (p ≤ 0.001 all), except for the root length.
Compared to untreated control, ZEA, BA and mT stimulated shoot growth only at lower concentrations, with the exception of Kin which was stimulant at all tested concentrations (Table 1). However, the longest shoot was obtained with 1 μM mT (113.7 mm) (Table 1; Fig. 3b), while the shortest shoots were obtained with 10 μM CPPU (39.6 mm) and 10 μM TDZ (42.0 mm).
Cytokinin type, concentration and their interaction significantly affected the frequency of rooting (p ≤ 0.001, p ≤ 0.001, p ≤ 0.05, respectively). The best results were obtained with all concentrations of Kin (Table 1). Other cytokinins were also effective in the induction of root formation, including untreated control. Generally, the frequencies decreased with increasing cytokinin concentration, although high frequencies of 80–100 % were obtained. The lowest frequency of rooting was achieved with 10 μM ZEA (74.2 %) or 10 μM mT (79.2 %).
Kin at all concentrations stimulated root initiation although the highest mean root number per plant bunch was obtained with 1 μM ZEA (7.5) (Table 1). With other cytokinins the root number decreased with concentration increasing. The root growth was also far more efficient with Kin than with any other cytokinin and untreated control (Table 1; Fig. 3c).
In general, chive plants with superior growth parameters were obtained with Kin, that promoted both shoot and root growth, contrary to the literature evidence of poor effect of Kin on the root systems’ growth (Aremu et al. 2012b). Although 1 μM mT induced the most vigorous shoot growth, as indicated in Table 1, it was ineffective for root growth, and the later effect was quite similar to BA, TDZ and CPPU. This is not in accordance with numerous reports that found mT to have beneficial effect on rooting compared to BA (Werbrouck et al. 1996; Magyar-Tábori et al. 2001).
Optimization of the regeneration procedure
Section thickness significantly affected the frequency of regeneration (p ≤ 0.01), the mean bud number per section (p ≤ 0.001) and the index of BFC (p ≤ 0.001) (Table 2). In the case of 2 mm- and 5 mm-thick sections, only one section per plant was responsive. It was also the case for the vast majority of 0.5 mm-thick sections (thin cell layer -TCL), but in a few cases two TCL sections responded per plant.
Some reports indicated TCL-explants as suitable solution to improve regeneration in many difficult-to-propagate species, like orchids (Teixeira da Silva 2013) or to speed up the regeneration process in responsive species (Ghnaya et al. 2008). However, in chive only 22.6 % TCL sections regenerated buds, with the mean of 2.07 buds per section while the thicker sections responded with much higher regeneration frequencies (up to 89 %) and the mean bud numbers (up to 23.4) (Table 2). The 5 mm-thick sections exhibited the highest regeneration capacity with BFC of up to 6.75 (Table 2).
Low regeneration capacity of TCLs was obtained probably because of the responsive tissue was injured during preparation. This suggests that only a small portion of the plant has regeneration capacity, as it could be destroyed by a section as thin as 0.5 mm. Histological analysis conducted in the present study confirmed this notion, and this is in agreement with Kenel et al. (2010), who found this particular site just above the basal plate to be the most responsive tissue for bud regeneration in garlic.
Shoot and root length, the frequency of rooting and the mean root number per explant were also significantly affected by the thickness of cross sections (p ≤ 0.001 all). All parameters were the highest with 5 mm-thick sections (Table 2).
As the highest regeneration capacity was obtained with 10 μM TDZ (Table 1; Fig. 3a), and the best plant growth was achieved with 1 μM mT (Fig. 3b) or 5 μM Kin (Fig. 3c), further protocol optimization included combination of these treatments; cultivation of 5 mm-thick sections on 10 μM TDZ for 4 weeks and then subcultivation on media supplemented with 5 μM Kin, 1 μM mT or PGR-free medium.
As compared to the control explants subcultivated on the medium supplemented with 10 μM TDZ, the replacement of TDZ did not significantly affect neither the frequency of regeneration nor the mean bud number per section and the index of BFC, as well as the shoot length (Table 3), while the frequency of rooting, the mean root number and root length were significantly affected (p ≤ 0.001 each). The highest root number was obtained with 5 μM Kin (6.7) or without PGR (6.2) and the same trend was observed with root length (Table 3). The replacement of TDZ with 1 μM mT gave unsatisfactory rooting response.
Since TDZ substantially improved regeneration capacity of chive plants compared to ones continuously grown on Kin-supplemented media (Table 1), but impaired plant quality which had shorter shoots and roots (Table 3), the best regeneration capacity and plant growth were achieved by a 4-week cultivation of explants on medium supplemented with 10 μM TDZ, and then a 4-week cultivation (or longer) on either PGR-free (Fig. 4) or 5 μM Kin-supplemented media.
Some cytokinins, especially strong ones, like TDZ, display carry-over effect, which is actually prolonged effect after its exclusion, as it is less susceptible to enzymatic degradation and, therefore, more persistent than adenine-based cytokinins. Sometimes this effect is responsible for low rooting capacity and poor acclimatization to greenhouse conditions, so the duration of treatment should be kept as short as possible (Makara et al. 2010).
Plantlets obtained in the present study are suitable donor material for new cycles of regeneration, as was proposed for onion (Kahane et al. 1992).
Antioxidant enzymes activities
The activity of POX, CAT and SOD was investigated during the first 14 days of induction treatment, in order to understand the potential role of antioxidant system in Kin- or TDZ-induced morphogenesis from the basal sections of chive plants (Figs. 5, 6, 7).
Effect of kinetin (Kin) and thidiazuron (TDZ) on activity of peroxidases (POX) during the course of cytokinin-induced morphogenesis (1, 3, 5, 7, 10 and 14 days) and in untreated control (UC) of chive explants. a Native PAGE revealed the activity of three isoforms (I, II and III). b Spectrophotometrically measured total POX activity. Results are expressed relative to UC and presented values are the average of two independent biological repetitions. Treatments denoted by the same letter are not significantly different (p ≤ 0.05) according to the LSD test
Effect of kinetin (Kin) and thidiazuron (TDZ) on the activity of catalase (CAT) during the course of cytokinin-induced morphogenesis (1, 3, 5, 7, 10 and 14 days) and in untreated control (UC) of chive explants. a Native PAGE revealed the activity of a single CAT isoform. b Spectrophotometrically measured total CAT activity. Results are expressed relative to UC and presented values are the average of two independent biological repetitions. Treatments denoted by the same letter are not significantly different (p ≤ 0.05) according to the LSD test
Effect of kinetin (Kin) and thidiazuron (TDZ) on activity of superoxide dismutases (SOD) during the course of cytokinin-induced morphogenesis (1, 3, 5, 7, 10 and 14 days) and in untreated control (UC) of chive explants. a Native PAGE revealed the activity of five SOD isoforms. b Spectrophotometrically measured total SOD activity. Results are expressed relative to UC and presented values are the average of two independent biological repetitions. Treatments denoted by the same letter are not significantly different (p ≤ 0.05) according to the LSD test
Native-PAGE analysis revealed three bands with specific POX activity (Fig. 5a). The most prominent band, with the highest mobility (III), and the band corresponding to the biggest protein isoform (I) were present in all samples including untreated controls, while the middle faint band (II) was observed only in TDZ-treated explants after 3rd day of treatment. Additionally, spectrophotometric measurements revealed statistically significant increase in total POX activity from the level recorded in controls only in TDZ-treated explants in which the additional band appeared (Fig. 5b).
CAT activity was visualized on gels as a single band in all analyzed samples (Fig. 6a), with observed different patterns of induction for Kin and TDZ, and these differences reflected in spectrophotometrically measured activity (Fig. 6b). During the regeneration, Kin induced weak response with highest CAT activity observed following 10 days of cultivation, while TDZ caused a sharp rise in CAT activity with faster response reaching the significant increase after just 3 days.
The activity staining of enzymes separated by native gels demonstrated five bands with SOD activity in all samples (Fig. 7a). Preincubation with specific inhibitors determinate these isoforms as follows: bands I and II constitute MnSODs and the other three bands III, IV and V constitute the Cu/ZnSOD isoforms (Fig. 8). The most prominent bands in all samples were III and IV Cu/ZnSOD together with one MnSOD assigned as II. The cumulative SOD activity measure showed that Kin induced slower response with gradual elevation in SOD activity that reached maximum after 10 days of treatment (Fig. 7b). By contrast, in TDZ-treated explants SOD activity sharply increased to maximal value detected just after 1 day, and remained high until the 7th day, when it sharply declined to the levels revealed in controls.
All antioxidative enzymes activities analyzed in the present study changed much faster with TDZ than with Kin. Moreover, the unique POX isoform was observed only in TDZ-treated explants after 3rd day of treatment. It is well known that TDZ pronounces a stress response of tissues, as it induces the expression of stress-related genes (Zhang et al. 2006). Elevated cytokinin content in tobacco plants overexpressing ipt gene correlated well with increased POX activities (Schnablová et al. 2006), while BA reduced levels of ROS by enhancing CAT and POX activities (Zavaleta-Mancera et al. 2007). Similarly, TDZ, BA and 2iP stimulated POX activity during multiplication of saffron meristems (Díaz-Vivancos et al. 2011). In light of this, POX seems to have a significant role in plant growth and development. Increased POX activity was found in dividing protoplasts, in contrast to non regenerating protoplasts (Papadakis et al. 2001; Xu et al. 2013). Furthermore, the reprogrammed expression of genes encoding peroxidases during the process of plant cell dedifferentiation and subsequent redifferentiation implies their significant roles in morphogenesis (Che et al. 2006).
Recently, there is an increasing body of evidence that indicates the involvement of antioxidant enzymatic system in differentiation processes (Tian et al. 2003; Filipović et al. 2015). It has been proposed that the severity of oxidative stress and the antioxidant machinery scavenging capabilities determines the cell’s fate. While the state of oxidative stress causes irreversible damage and the loss of regenerative potential (Papadakis et al. 2001; Xu et al. 2013), ROS at low levels could account for increased morphogenetic response. However, despite numerous publications on this issue, knowledge about relation of oxidative stress and tissue culture response is still fragmentary. There are no general rules with regard to antioxidative enzymatic activity and tissue culture response. In that sense, parallel systems allowing direction of tissue response to somatic embryogenesis or caulogenesis from the same explant are very valuable (Dutta Gupta and Datta 2003/2004; Filipović et al. 2015).
In conclusion, an efficient and reliable method of in vitro regeneration was established for chive. Both high regeneration efficiency and high performances of regenerated plants were achieved by treatments combination including cultivation of 5 mm-thick basal plate sections on 10 μM TDZ for 4 weeks following subcultivation on either 5 μM Kin-containing or PGR-free medium. Since the presented protocol requires intact meristematic tissue for regeneration and considering the high competence of this tissue for transformation and regeneration (Kenel et al. 2010), it is very useful for genetic transformation and virus-free plant production as well. Finally, this work has contributed to the expansion of knowledge about the role of antioxidative enzymes in plant organogenesis.
Abbreviations
- ANOVA:
-
Analysis of variance
- BA:
-
N6-Benzyladenine
- BFC:
-
Bud-forming capacity
- CAT:
-
Catalase
- CPPU:
-
N-(2-chloro-4-pyridyl)-N′-phenylurea
- MS:
-
Murashige and Skoog (1962)
- mT:
-
meta-Topolin [6-(3-hydroxybenzylamino) purine]
- Kin:
-
Kinetin (N6-furfuryl aminopurine)
- PGR:
-
Plant growth regulator
- PPFD:
-
Photosynthetic photon flux density
- POX:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- TCL:
-
Thin cell layer
- TDZ:
-
Thidiazuron (N-phenyl-N′-1,2,3-thiadiazol-5-ylurea)
- ZEA:
-
trans-Zeatin [6-(4-hydroxy-3-methylbut-trans-2-enylamino) purine]
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Amoo SO, Van Staden J (2013) Influence of plant growth regulators on shoot proliferation and secondary metabolite production in micropropagated Huernia hystrix. Plant Cell Tiss Organ Cult 112:249–256
Amoo S, Finnie JF, Van Staden J (2011) The role of meta–topolins in alleviating micropropagation problems. Plant Growth Regul 63:197–206
Aremu AO, Bairu MW, Szüčová L, Doležal K, Finnie JF, Van Staden J (2012a) Shoot and root proliferation in ‘Williams’ banana: Are the topolins better cytokinins? Plant Cell Tiss Organ Cult 111:209–218
Aremu AO, Bairu MW, Doležal K, Finnie JF, Van Staden J (2012b) Topolins: A panacea to plant tissue culture challenges? Plant Cell Tiss Organ Cult 108:1–16
Ayabe M, Sumi S (1998) Establishment of a novel tissue culture method, stem-disc culture, and its practical application to micropropagation of garlic (Allium sativum L.). Plant Cell Rep 17:773–779
Bairu MW, Stirk WA, Doležal K, Van Staden J (2007) Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tiss Organ Cult 90:15–23
Beauchamp C, Fridovitch I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Che P, Lall S, Nettleton D, Howell SH (2006) Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol 141:620–637
Cui K, Xing G, Liu X, Xing G, Wang Y (1999) Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci 146:9–16
Díaz-Vivancos P, Majourhat K, Fernández JA, Hernández JA, Piqueras A (2011) Study of the antioxidant enzymatic system during shoot development from cultured intercalar meristems of saffron. Plant Growth Regul 65:119–126
Dragišić-Maksimović JJ, Milivojević JM, Poledica MM, Nikolić MD, Maksimović VM (2013) Profiling antioxidant activity of two primocane fruiting red raspberry cultivars (Autumn bliss and Polka). J Food Compos Anal 31:173–179
Dutta Gupta S, Datta S (2003/2004) Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regenerations. Biol Plant 47:179–183
Eady CC, Kamoi T, Kato M, Porter NG, Davis S, Shaw M, Kamoi A, Imai S (2008) Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol 147:2096–2106
Filipović BK, Simonović AD, Trifunović MM, Dmitrović SS, Savić JM, Jevremović SB, Subotić AR (2015) Plant regeneration in leaf culture of Centaurium erythraea Rafn. Part 1: the role of antioxidant enzymes. Plant Cell Tiss Organ Cult 121:703–719
Ghnaya AB, Charles G, Branchard M (2008) Rapid shoot regeneration from thin cell layer explants excised from petioles and hypocotyls in four cultivars of Brassica napus L. Plant Cell Tiss Organ Cult 92:25–30
Kahane R, Rancillac M, Teyssendier de la Serve B (1992) Long-term multiplication of onion (Allium cepa L.) by cyclic shoot regeneration in vitro. Plant Cell Tiss Organ Cult 28:281–288
Kenel F, Eady C, Brinch S (2010) Efficient Agrobacterium tumefaciens-mediated transformation and regeneration of garlic (Allium sativum) immature leaf tissue. Plant Cell Rep 29:223–230
Keusgen M (2002) Health and Alliums. In: Rabinowitch HD, Currah L (eds) Allium crop science: recent advances, 1st edn. CAB International, Wallingford, pp 357–378
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Magyar-Tábori K, Dobránszki J, Jámbor-Benczúr E, Bubán T, Lazányi J, Szalai J, Ferenczy A (2001) Post-effects of cytokinins and auxin levels of proliferation media on rooting ability of in vitro apple shoots (Malus domestica Borkh.) ‘Red Fuji’. Int J Hort Sci 7:26–29
Makara AM, Rubaihayo PR, Magambo MJS (2010) Carry-over effect of thidiazuron on banana in vitro proliferation at different culture cycles and light incubation conditions. Afr J Biotechnol 9:3079–3085
Mukhopadhyay MJ, Sengupta P, Mukhopadhyay S, Sen S (2005) In vitro stable regeneration of onion and garlic from suspension culture and chromosomal instability in solid callus culture. Sci Hortic 104:1–9
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 4:267–275
Papadakis AK, Siminis CI, Roubelakis-Angelakis KA (2001) Reduced activity of antioxidant machinery is correlated with suppression of totipotency in plant protoplasts. Plant Physiol 126:434–444
Rattanachaikunsopon P, Phumkhachorn P (2008) Diallyl sulfide content and antimicrobial activity against food-born pathogenic bacteria of chives (Allium schoenoprasum). Biosci Biotechnol Biochem 72:2987–2991
Rauber M, Grunewaldt J (1989) In vitro regeneration in Allium species. Plant Cell Rep 7:426–429
Schnablová R, Synková H, Vičánková A, Burketová L, Ederc J, Cvikrová M (2006) Transgenic ipt tobacco overproducing cytokinins overaccumulates phenolic compounds during in vitro growth. Plant Physiol Biochem 44:526–534
Tang W, Newton RJ (2005) Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobus L.) zygotic embryos. Plant Physiol Biochem 43:760–769
Teixeira da Silva JA (2013) The role of thin cell layers in regeneration and transformation in orchids. Plant Cell Tiss Organ Cult 113:149–161
Tian M, Gu Q, Zhu M (2003) The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci 165:701–707
Timité G, Mitaine-Offer AC, Miyamoto T, Tanaka C, Mirjolet JF, Duchamp O, Lacaille-Dubois MA (2013) Structure and cytotoxicity of steroidal glycosides from Allium schoenoprasum. Phytochemistry 88:61–66
Tubić Lj, Zdravković-Korać S, Mitić N, Milojević J, Ćalić-Dragosavac D, Vinterhalter B (2011) Plant regeneration from transverse stalk sections of chive plants. Rom Biotechnol Lett 16:55–59
Umehara M, Sueyoshi T, Shimomura K, Iwai M, Shigyo M, Hirashima K, Nakahara T (2006) Interspecific hybrids between Allium fistulosum and Allium schoenoprasum reveal carotene-rich phenotype. Euphytica 148:295–301
Valero-Aracama C, Kane M, Wilson S, Philman N (2010) Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul 60:43–49
Van Staden J, Zazimalova E, George EF (2008) Plant growth regulators II: cytokinins, their analogues and antagonists. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, vol. 1: the background, 3rd edn. Springer, Dordrecht, pp 205–226
Visser C, Qureshi JA, Gill R, Saxena PK (1992) Morphoregulatory role of TDZ. Substitution of auxin and cytokinin requirement for the induction of somatic embryogenesis in geranium hypocotyl cultures. Plant Physiol 99:1704–1707
Vitória AP, Lea PJ, Azevedo RA (2001) Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry 57:701–710
Werbrouck SPO, Strnad M, Van Onckelen HA, Debergh PC (1996) Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol Plant 98:291–297
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Xu X, Xie G, He L, Zhang J, Xu X, Qian R, Liang G, Liu JH (2013) Differences in oxidative stress, antioxidant systems, and microscopic analysis between regenerating callus-derived protoplasts and recalcitrant leaf mesophyll-derived protoplasts of Citrus reticulata Blanco. Plant Cell Tiss Organ Cult 114:161–169
Zavaleta-Mancera HA, López-Delgado H, Loza-Tavera H, Mora-Herrera M, Trevilla-García C, Vargas-Suárez M, Ougham H (2007) Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J Plant Physiol 164:1572–1582
Zdravković-Korać S, Milojević J, Lj Tubić, Ćalić-Dragosavac D, Mitić N, Vinterhalter B (2010) Somatic embryogenesis and plant regeneration from root sections of Allium schoenoprasum L. Plant Cell Tiss Organ Cult 101:237–244
Zhang CR, Huang XL, Wu JY, FengBH Chen YF (2006) Identification of thidiazuron-induced ESTs expressed differentially during callus differentiation of alfalfa (Medicago sativa). Physiol Plant 128:732–739
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E, Bączek-Kwinta R, Płażek A (2014) Antioxidant activity and ROS tolerance in triticale (x Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tiss Organ Cult 119:79–94
Acknowledgments
The authors would like to express their gratitude to the Ministry of Education, Science and Technological Development of the Republic of Serbia for financial support through contract No. 173015.
Author contribution
S.Z.K. and N.M. designed research; Lj.T., J.M. and N.M. performed tissue culture experiments; Lj.T. and J.S. performed antioxidative enzymes analysis, D.J. and S.B. performed histological analysis; Lj.T. did statistical analyses and prepared tables; S.Z.K. prepared Figs. 1, 3 and 4, D.J. prepared Fig. 2, and J.S. prepared Figs. 5–8; S.Z.K. wrote the manuscript and J.S., N.M., D.J. and S.B. contributed in writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tubić, L., Savić, J., Mitić, N. et al. Cytokinins differentially affect regeneration, plant growth and antioxidative enzymes activity in chive (Allium schoenoprasum L.). Plant Cell Tiss Organ Cult 124, 1–14 (2016). https://doi.org/10.1007/s11240-015-0869-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0869-1