Abstract
The PDS1000-He biolistic gun was used to bombard plasmid DNA harbouring gus and nptII genes into tender young leaves of in vitro grown shoots of Camellia sinensis (tea). Out of a total of 500 bombarded leaves, 217 (43.4 %) showed callusing after 5 weeks on selection medium containing 1.71 µM kanamycin. Only 15 of these regenerated into indirect shoot buds. Only 7 out of 15 putative transformants showed the expected 400 bp signal with gus gene specific primers during PCR analysis. On the other hand, all the 15 putative transformants tested positive with nptII gene specific primers. In Southern hybridization with nptII specific gene probe, all the six randomly selected PCR positive plants showed stable integration of nptII gene. Both the transgenic and not-bombarded control plants showed phenotypic similarity under polyhouse conditions. Although their growth parameters were significantly at par, significantly lesser shoot height was recorded in transgenic plants. The reproductive behavior of the transgenics was also depressed. Thus, floral bud and flower abscission, fruit drop as well as empty seed production was higher in the transgenics as compared to control. Viability and germination of transgenic seeds was also significantly lower than control. Survival of the transgenic seedlings was also negligible (about 3 %). Hence, the chances of germ-line transmission of the transgenes were remarkably reduced in case of gus-transgenics. Tea being a vegetatively propagated plant, the method described in the present paper is an important approach for developing transgenics of elite tea plants from leaf explants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tea (Camellia sinensis (L.) O. Kuntze) of family Theaceae is an important beverage crop of the world. It is cultivated in several countries of Asia, Africa and South America as an important employment generator and a foreign exchange earner (Sandal et al. 2007). The beverage produced from tea leaves is rich in medicinal, nutritional, anti-microbial, anti-cancer and anti-aging properties (Chen 1999; Hara 2003; Cooper et al. 2005). Thus, the demand for tea leaves of superior quality is ever increasing. Yet, the amounts of quality tea actually produced are failing to meet the increasing demands. Biotic (fungi, insect pests and mites) and abiotic (frost, hail, chilling, drought, nutritional deficiencies) stresses cause major yield losses in tea (Bhattacharya and Ahuja 2001). Therefore, stress tolerance in tea continues to be an important goal of conventional breeding programs. However, tea breeding is constrained by long life cycle (6–10 years), high inbreeding depression, self-incompatibility, low success rate of hand pollination and differences in the time of flowering and fruit bearing capacity of some clones (Mondal et al. 2004). Scarcity of land for tea improvement is another major constraint that cannot be ignored (Bhattacharya and Ahuja 2001). Alternatively, transgenic technology can serve as an attractive method for improving yield as well as quality or cup characteristics of tea (Bhattacharya et al. 2014; Song et al. 2014).
In this regard, transgenic tea plants were developed by the Agrobacterium as well as biolistic gun method of transformation in this laboratory (Mondal et al. 2001; Saini et al. 2012). The methods employed somatic embryos, derived from cotyledon explants of tea seeds. Seeds being the products of sexual reproduction are well known to be variable from each other. Thus, the use of seed explants requires labour intensive precautions such as maintenance of separate lines of somatic embryos derived from each seed (Saini et al. 2012). On the other hand, leaves and the selected elite mother plant from which they are plucked are true to type. Unlike seeds, all leaves of a plant retain the superior characteristics of its elite mother without any variation. Thus, when leaves are transformed with desirable genes, the superior traits of the mother plant are generally enhanced. This makes leaves more favoured as explants for genetic transformation as compared to cotyledons/seeds (Sandal et al. 2007). However, extensive optimization experiments are required before explants of a specific clone can become amenable to both regeneration and genetic transformation. In view of this, the aim of the present study was to optimize a biolistic gun method of transformation of tea leaf explants for the development of transgenic plants. The study also aimed at understanding the reproductive behavior of the transgenics with a view to generate primary information. The information is expected to serve as primary requisite for the control and maintenance of transgenic plants in the field and their commercial utilization.
Materials and methods
Plant material
Twenty year old bushes of Chinary type tea (Camellia sinensis (L.) O. Kuntze) growing in the Institute’s Experimental Tea Farm at Banuri, Palampur, Himachal Pradesh, India (1292 m asl, 32.6°N and 78.19°E) were selected. Shoots were collected from these bushes during the seasons of fresh growth (March–May). Nodal segments (3.0 cm long) with a single axillary bud were excised from these shoots and surface sterilized by first washing in a solution of Tween-20 for 15 min followed by 0.04 % mercuric chloride for 5 min. After removing all traces of mercuric chloride by 4–5 rinses with sterile distilled water, the nodal segments (2.0 cm long after excision of exposed surfaces) were inoculated on 0.8 % agar (w/v) solidified half strength MS (Murashige and Skoog 1962) medium containing 3 % sucrose. After 2 weeks on this medium, the cultures were transferred to shoot multiplication medium (SMM) having full strength MS medium supplemented with 3 % sucrose, 8.88 µM 1-benzyl adenine (BA) and 0.98 µM indole 3-butyric acid (IBA) for further growth and multiplication. After three subcultures of 4 weeks each, completely folded first leaves (adjacent to the apical buds) were excised from the shoot cultures and used as explants for biolistic gun mediated genetic transformation. The pH of all the media were maintained at 5.8 prior to autoclaving and all the cultures were maintained at 25 ± 2 °C under a photoperiod of 16 h light (cool white lights with intensity of 52 µmol m−2 s−1) and 8 h dark.
Bacterial strain and plasmid
The plasmid pRT99gus (Topfer et al. 1988) contained in E. coli is popularly used for standardization of protocols for biolistic mediated genetic transformation. The plasmid is a 6.71 kb pUC18 derivative containing the selection marker gene coding for neomycin phospho-transferase (NPTII) and gusA gene coding for β-glucuronidase (GUS). Both the genes are driven by the CaMV 35S promoter and are flanked by the NOS terminator (Fig. 1).
Plasmid DNA isolation
The alkaline lysis method of Sambrook et al. (1989) was employed to isolate plasmid DNA from fresh bacterial cultures that were grown overnight in Luria broth (LB) supplemented with 0.86 µM kanamycin monosulphate (Sigma-Aldrich, Bangalore, India). The isolated plasmid was purified using the purification columns (Qiagen, USA), and the presence of the gus and nptII genes in the isolated DNA were confirmed by colony PCR with gene specific primers. These comprised of (i) nptII: 5′-CCA-TCG-GCT–GCT-CTG-ATG-CCG–CCG-T-3′ (25 mer) as forward and 5′- AAG-CGA-TAG-AAG-GCG-ATG-CGC-TGC-3′ (24 mer) as reverse primers and also (ii) gus: 5′-GGT-GGG-AAA- GCG-CGT-TAC-AAG-3′ (21 mer) as forward and 5′-TGG-ATC-CCG-GCA-TAG-TTA-AA-3′ (20 mer) as reverse primers. The plasmid DNA was then used for biolistic mediated genetic transformation.
Pre-bombardment preparations
The pre-bombardment preparations were done in four steps namely, (1) sterilization of gold particles, (2) precipitation of plasmid DNA onto the gold particles (3) coating of macrocarrier discs with plasmid DNA coated gold particles and finally (4) pretreatment and arrangement of the leaf explants on the medium for bombardment. Each step was performed as follows:
-
1.
Gold particles of 1.0 μm (BioRad Laboratories Inc., CA, USA) were sterilized thrice in 70 % ethanol and finally once in absolute ethanol. For this, the gold particles were vortexed for 5 min in 1 ml of 70 % or absolute ethanol followed by incubation for 15 min. The gold particles were pelleted by microfugation and suspended in 1 ml of sterile de-ionized water. Finally, these were dispensed as 50 µl aliquots in eppendorfs and stored at 4 °C.
-
2.
The 50 µl aliquots of gold particles were mixed with 1 µg µl−1 of plasmid DNA (10 µl volume), 10 µl of 2.5 M CaCl2 and 10 µl of 0.5 M spermidine free of phosphate salts by vortexing for 3 min followed by brief centrifugation at 9000 rpm. The obtained pellet was suspended in absolute ethanol and centrifuged again. The final pellet comprising of the gold particles coated with plasmid DNA were suspended in 60 μl absolute ethanol.
-
3.
60 μl suspension of plasmid DNA coated gold particles was vortexed and 10 μl suspension was coated uniformly on each macro-carrier disc with the help of a pipette. The macro-carrier discs were always washed in 70 % ethanol and dried under laminar hood prior to use.
-
4.
The leaf explants (average size of 1.2 × 0.8 cm) were immersed in PGR free liquid MS medium supplemented with 3 % sucrose (pH 5.8) for 4 h and then blotted on sterile filter paper. These were then inoculated on 0.8 % agar gelled MS medium supplemented with 3 % sucrose at same pH. Care was taken to inoculate the leaves closely at the centre of a 9.0 cm Petri plate with adaxial leaf surfaces touching the medium.
Bombardment of leaf explants
A helium powered Particle Delivery system, PDS-1000/He (Bio-Rad) was used to bombard the leaves contained in each Petri plate twice. A macro-carrier disc coated with plasmid-DNA on gold particles was used for each bombardment. Based on previously optimized parameters of Sandal et al. (2006), the bombardment was performed under a chamber pressure of 94.8 kPa Hg at a 0.95 cm + 0.64 cm gap distance (the distance between the rupture disc and the macro-carrier), 16 mm macrocarrier flight distance (the distance between the macro-carrier and the stopping screen), 6 cm target distance (distance between stopping screen and target explant) and He burst pressure of 7584.23 kPa. Five Petri plates with 20 leaves in each were taken. For control, similar number of Petri plates each having 20 not-bombarded leaves were taken.
Regeneration and establishment of tissue culture plants from bombarded leaf explants
After 2 days in dark, the bombarded and not-bombarded control leaves were transferred to basal MS medium containing 0.8 % agar, 22.6 µM 2,4-dichlorophenoxyacetic acid (2,4-D), 3 % sucrose and 1.71 µM kanamycin (MS1K) for 5 weeks. Following which, the leaf calli were transferred to 0.8 % agar semi solid basal MS medium containing 8.88 µM BA, 3 % sucrose and 1.71 µM kanamycin (MS2K) for the regeneration of adventitious shoot buds as per the modified method of Sandal et al. (2003). When the shoot buds of the putative transformants grew to a height of 2.0 cm after 12 weeks, they were excised and multiplied in liquid basal MS medium containing 5.0 µM thidiazuron (TDZ), 2 % sucrose and 1716.5 µM kanamycin monosulphate (MS3K). The putatively transformed shoots from each transformation event and the not-bombarded shoots (3.0 cm long) serving as control were maintained as independent lines and sub-culturing was done at regular interval of 4 weeks each. The not-bombarded control lines were also maintained for 5 weeks on basal MS medium containing 0.8 % agar, 22.6 µM 2, 4-D and 3 % sucrose (MS1), and then similarly on MS medium containing 0.8 % agar, 8.88 µM BA and 3 % sucrose (MS2) for adventitious shoot regeneration as per the modified method of Sandal et al. (2003). After three sub-cultures of 4 weeks each, both the not-bombarded and bombarded shoots were rooted, transferred to 12 inches pots containing sand: garden soil (1:1) and hardened as per the method described by Sharma et al. (1999). The plants were finally planted in soil under contained polyhouse conditions and maintained as tea bushes. The distances between each plant were 3 ft and that between each row was 2 ft.
Histochemical GUS assay
Histochemical GUS assay was performed in ten randomly selected leaves after 2 days of bombardment as per the method described by Jefferson (1987). Leaves from 4 year old transgenic plants were also assayed. In both the cases, the not-bombarded leaves served as control. The selected leaves were infiltrated with 100 mM X-gluc buffer (pH 7.0) containing 1 mM potassium ferrocyanide, 1 mM potassium ferricyanide, 10 mM EDTA (pH 8.0), 0.1 % TritonX-100 and 1 mM of 5-bromo-3-chloro-2-indolyl-β, D-glucuronide sodium phosphate (Sigma Aldrich, Bangalore, India) under vacuum and incubated overnight at 37 °C in dark. The distinct indigo blue coloured scorable spots or sectors per explant were recorded and the percent GUS expression was calculated.
Molecular characterization of transgenic tea plants
DNA isolation
DNA was isolated from randomly chosen 15 independent transgenic shoots growing under in vitro conditions in MS3K supplemented with 1716.5 µM kanamycin. Plants growing under polyhouse conditions were also taken. Three not-bombarded lines growing in MS3K free of kanamycin as well as plants growing under polyhouse conditions served as control. The method of Doyle and Doyle (1990) was used to isolate genomic DNA from 1.0 g leaf tissues.
PCR analysis
The S1000 Thermal cycler (BioRad Laboratories (India) Pvt. Ltd., Gurgaon, India) was used to PCR amplify 50 ng of genomic DNA from 15 independent T0 transgenic and 3 lines of not-bombarded control. The plasmid DNA served as positive control. The amplification was done using gus and nptII specific forward and reverse primers by denaturation at 94 °C for 4 min followed by 35 cycles of 94 °C for 1 min, annealing at 57 and 58 °C for 30 s and extension at 72 °C for 7 min followed by further 2 min. All PCR products were separated on 1.2 % agarose gel and photographed under a UV trans-illuminator (Fotodyne MP-St) equipped with MP4 Polaroid Instant Camera System). Amplification products of 400 bp were expected for each of the internal sequences of gus and nptII genes, respectively. The forward and reverse primers comprised of: (1) 5′-GGTGGGAAAGCGCGTTACAAG-3′ and 5′-TGGATCCCGGCATAGTTAAA-3′ for gus gene and (2) 5′-AGGATCTCCTGCATCTCAC-3′ and 5′-CCAAGCTCTTCAGCAATATC-3′ for nptII gene.
Southern analysis
Genomic DNA (30 µg) from the leaves of six independent lines of PCR positive T0 plants, two lines of T1 plants and one line each of not-bombarded controls of T0 and T1 plants were digested with HindIII (Invitrogen, Life Technologies Pvt. Ltd, Bengaluru, Karnataka, India). Digests were resolved on 0.8 % (w/v) agarose gels and blotted onto a Hybond-N+ nylon membrane (Amersham Biosciences Little Chalfont, UK) under alkaline conditions. This was then hybridized with PCR-amplified gus and nptII gene probes after radiolabeling with α-32P. For this, a mega-primer DNA labeling kit (Amersham, Biosciences, India) was used. The hybridized products were viewed on a phospho-imager and developed using X-Ray film (Kodak India Private Limited, India). Un-cut plasmid DNA served as control. Lighted up bands in each lane indicated transgene integration. In case of T1 plants however, the HindIII digested genomic DNA was hybridized using biotin labeled nptII gene probe. In this case, the chromogenic substrate, alkaline phosphate conjugate streptavidin and NBT/BCIP-T were used to detect dark purple signals.
Growth and reproductive performance of transgenic tea plants under contained poly-house conditions
Phenotypic traits like branching, length of stem and internodes, leaf morphology, percentage flowering and seed set were recorded in ten plants each of transgenic and control plants at 4 weeks interval for four consecutive years. Percent germination of seeds from the transgenic and control plants followed by their seedling establishment and survival were also recorded.
Results
Raising of transgenic tea plants
The bombarded and not-bombarded leaves showed variable responses to 1.71 µM kanamycin (Table 1) . Callusing was recorded in 65 % not-bombarded leaves after 5 weeks on MS1 free of kanamycin. When these were transferred to MS2, adventitious shoot buds developed (16.2 %) after 5 weeks. In case of MS1 K however, not-bombarded leaves (100 %) turned yellow and died, whereas, 217 out of a total of 500 bombarded leaves (43.4 %) showed callusing after 5 weeks on MS1 K (Fig. 2a, b) . Adventitious shoot buds developed only in 15 out of a total of 217 (6.9 %) kanamycin resistant putatively transformed leaf calli after 6–7 weeks of transfer to MS2 K (Fig. 2c). These grew within 1–2 weeks (Fig. 2d).
In vitro plant regeneration from tea leaf explants. a, b Callusing on leaf explant, c initiation of adventitious shoot buds from leaf calli, d elongation of putatively transformed adventitious shoots on selection medium containing 1.71 µM kanamycin, e multiplication of putatively transformed shoots in liquid MS medium containing 5.0 µM TDZ, 2 % sucrose and 1716.5 µM kanamycin monosulphate, f multiplication of not-bombarded control shoots in kanamycin free liquid MS medium containing 5.0 µM TDZ and 2 % sucrose, g hardened transgenic plants growing in pots, h 4 year old transgenic tea bush growing under polyhouse. Bars in a–d = 1.4 cm, e, f = 3 cm, g = 20 cm and h = 60 cm
Excised shoot buds derived from not-bombarded leaves died (100 %) after 15 weeks of transfer to liquid MS3K containing 1716.5 µM kanamycin, whereas, the putatively transformed shoots showed rapid multiplication and grew to a height of 3.0 cm (Fig. 2e). All the not-bombarded shoots (100 %) showed normal growth and multiplication in liquid SMM free of kanamycin (Fig. 2f). Root initiation was recorded in 83 and 89 % shoots derived from bombarded and not-bombarded leaves, respectively within 9–10 weeks of transfer to pots containing sand: garden soil. Even upon transfer to soil under polyhouse conditions, the hardened plants remained healthy (Fig. 2g) and continued to grow for 4 years into sturdy tea plants (Fig. 2h).
GUS expression
Transient GUS expression was evident from distinct indigo-blue coloured spots/sectors distributed over the surface of different leaf explants. These ranged from tiny to small spots and large patches. The spots/patches were recorded only in three out of ten putatively transformed leaf explants (Fig. 3a) and also in the leaves of seven out of 15 lines of 4 year old transgenics. Such expression was not observed in case of not-bombarded leaf explants (Fig. 3b).
Molecular characterization of transgenic tea plants
PCR analysis
Distinct bands corresponding to 400 bp of gus gene were detected in 7 (Fig. 4a) out of 15 lines of kanamycin resistant putative transformants in PCR. However, when nptII gene specific primers were used, the expected amplification product of 400 bp was observed in all the 15 kanamycin resistant lines (Fig. 4b). No amplification products were detected in the not-bombarded control lines (Fig. 4, lanes C, C1, C2 and C3), whereas, distinct bands corresponding to 400 bp were recorded in the positive control or plasmid DNA (Fig. 4, lane P).
PCR amplification of transgenic plants growing under poly-house condition showing the expected 400 bp amplification product of, a gus gene and b nptII gene where lane M 100 bp marker, lane C, C1, C2, C3 untransformed control; lane P plasmid DNA as positive control; lanes 1–15 independent transgenic plants
Southern hybridization
Distinct but variable bands were observed in all the six randomly selected transgenic T0 plants (Fig. 5a). While a large 6.7 kb fragment was common to all the five transgenic lines, additional bands slightly larger than 6.7 kb were observed in each of lanes 1, 4, 5 and 6. Three bands, one higher than 6.7 kb, one of 6.7 kb and a band of about 3.1 kb were recorded in lane 1. A single band corresponding to 6.7 kb was recorded in lane 2 but a total of four bands of >6.7, 6.7, 2.5 and 1.8 kb were observed in lane 4. In lane 5 however, there were three bands of >6.7, 6.7 and <4.0 kb, while only two bands of >6.7 and 6.7 kb were recorded in lane 6. In case of lane 7, only a single band of 2.5 kb was recorded. However, no bands were detected in the not-bombarded control (Fig. 5b, lane C).
Southern hybridization of genomic DNA digested with HindIII, a T0 transgenic where lanes 1–7 independent transgenic lines, lane C not-bombarded control, P plasmid DNA as positive control, b T1 transgenic where lanes 1–2 independent transgenic lines, lane C not-bombarded control, lane P plasmid DNA as positive control. A 1–8 kb marker was used
In case of independent T1 transgenic plants, lane 1 had two bands of size larger than 6.7 kb, whereas, lane 2 had a band higher and one corresponding to 6.7 kb (Fig. 5c). No bands were detected in lane C representing not-bombarded control.
Growth and reproductive performance of transgenic tea plants under poly-house conditions
The transgenic lines were phenotypically similar to the not-bombarded control plants both visually as well as statistically. Thus, both the transgenic and control plants had low spreading branches, thin and hardy stems and dull but dark green colored, oblong to ovate-oblong leaves with wavy margins. However, the shoot height of transgenics was significantly shorter than control (Table 2).
Under poly-house, both the transgenic and control plants produced a large number of flower buds throughout the year. Maximum number of flowering occurred when the mean diurnal temperature was above 20 °C i.e., during April to June (Fig. 6a, b). Continuous bud-abscission was also recorded in both transgenic and control plants but the rate of bud-abscission was invariably higher in the transgenic plants (Table 3). Many fully-opened transgenic flowers underwent drying and abscission (Fig. 6c), while only a small number of floral buds showed normal development (Fig. 6d1–5). The actual number of transgenic flower buds that opened during October to December (the peak season for fully opened flowers) was remarkably lower than control. While a small number of flowers also opened during January to March, and July to September, only a negligible number opened during April to June.
Reproductive behaviour in 4 year old not-bombarded control and transgenic plants growing under polyhouse condition. a Profuse flowering and flower abscission in transgenic, b a fully opened normal flower, c desiccation of flowers and necrosis of fertilized ovaries in transgenic, d stages of fruit/seed formation in control plants where 1–3 bud growth; 4 half opened flower; 5 fully opened flower; 6 fertilization; 7–8 fertilized ovary; 9–10 fruit development; 11 dehisced fruit with mature seeds, e abscission and necrosis at each stage of fruit/seed development in transgenic where 1 flower; 2–3 fertilized ovary; 4 dehisced fruit with under-developed dried seeds and fruits, f high rate of necrosis of fertilized ovaries in transgenic, g aborted transgenic seeds, h dehisced not-bombarded control fruit with mature and viable seeds, i transgenic seedling. Bars in a = 30 cm, b, c = 2 cm, d, e = 2 cm, f = 0.5 cm, g, h = 1.5 cm and i = 5 cm
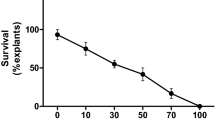
Fertilization (as indicated by flowers with withered petals but persistent style and stigma) was observed (Fig. 6d6–8) in all the seasons with exception to spring i.e., April to June. Maximum number of fruits were recorded during January to March, and the number in case of control plants was always higher than that of transgenics. Thus, in case of transgenic and control plants, 25 and 86 % of the fertilized ovaries produced fruits which abscised in high numbers i.e., 75 and 14 %, respectively (Fig. 6c, e, f). Fruit abscission in the transgenic plants was also accompanied by empty seeds or fruits with shrunken seeds (Fig. 6g). Finally, 7 and 47 % germination was recorded in the normally developed seeds of transgenic and control, respectively (Fig. 6h, i). Although the number of transgenic seedlings that finally survived was significantly lower than not-bombarded control (Table 3), both the transgenic and control plantlets grew normally and were phenotypically similar.
Discussion
Somatic embryos derived from cotyledon explants of seeds have been generally used for the genetic transformation of tea (Mondal et al. 2001; Saini et al. 2012). However, the seeds are the products of sexual reproduction and are genetically variable from their mother plant. Therefore, they pose a threat of additional variability in the genome targeted for genetic modification. In contrast, leaf explants are more desirable (Sandal et al. 2007) because the leaves and the mother plant from which they are plucked are true to type. Leaves retain the superior characteristics of an elite mother without any variation. As a result, the elite characteristics of the mother plant are generally not lost when additional desirable transgenes are added into leaves through genetic transformation methods. Moreover, as compared to other explants, the higher surface area of the leaves further increases the ease of biolistic gun mediated transformation (Sandal et al. 2006). Despite these advantages, leaves were not used as explants for genetic transformation of tea in previous studies because of the lack of a reproducible regeneration system. In the present study however, the methods of Sandal et al. (2003, 2005, 2006) were modified and used to produce transgenic tea plants from leaf explants. As opposed to our earlier report, the step for rhizogenesis on medium containing BAP and IBA was omitted in the present study. This reduced the time frame for regenerating shoot buds and finally transgenic plants by almost 3–8 weeks. Although this time frame was higher than or at par with previous reports where somatic embryos were used (Mondal et al. 2001; Lopez et al. 2004; Saini et al. 2012), it was lower than that of previous report wherein leaves were used as explants (Sandal et al. 2006). Moreover in the present study, a target distance of 6 cm and a gap distance of 3/8 + ¼ inches were used to deliver plasmid DNA containing gus and nptII genes into a total of 500 tender in vitro leaves. In our earlier study, Saini et al. (2012) had optimized a target distance of 9 cm and gap distance of ¼ inches for the genetic transformation of somatic embryos. Often, optimization of parameters is necessary to avoid injury as well as undesirable transgene integration in delicate tissues (Birch and Franks 1991; Jagga-Chugh et al. 2012; Kim et al. 2012). Optimization of bombardment parameters has often resulted in the production of several commercially important tropical crops (De Guglielmo-Cróquer et al. 2010; Saini et al. 2012; Bhattacharyya et al. 2015).
Out of the 500 leaf explants that were bombarded, only 43.4 % (217) were resistant to 1.71 µM kanamycin and developed further to produce 15 independently transformed putative adventitious shoots (Fig. 2c–e). When these were subjected to PCR analysis, all the lines tested positive for nptII, whereas, only 7 out of 15 transformants amplified the expected band for gus gene (Table 1; Fig. 4a). The fact that this was not an outcome of failed PCR reaction was evident from the distinct band obtained in case of positive control or plasmid DNA (Fig. 4a, lane P). This indicated that the gus transgene was perhaps either truncated, deleted or rearranged in 8 out of the 15 lines during transformation and/or plant regeneration. Transgene rearrangement and breakage are commonly associated with biolistic mediated transformation (Wilson et al. 2006; Chen et al. 2007; Kim et al. 2012; Tassy et al. 2014). Thus, direct methods of transgene delivery and integration often lead to chimerism in the target genome (Christou 1990). Chimerism is reported to persist in tissues of several herbaceous and woody plants even after rigorous selection of transformants (Bhat and Srinivasan 2002; Dalla Costa et al. 2014). It is implicated to result from transient expression of transgene(s) during the early stages of in vitro plant regeneration (Faize et al. 2010). In the present study, GUS expression was evident in the form of indigo blue spots to large sectors in almost all the in vitro leaves assayed after 2 days of bombardment, whereas, no GUS expression was recorded in the leaves of 8 out of 15 lines of 4 year old transgenics. This, thereby indicated the transient expression of gus gene in these lines. The results of histochemical GUS assay of the leaves of 4 year old plants also corresponded with PCR results when gus gene specific primers were used (Fig. 4a).
Despite the above observations, stable integration of nptII gene in tea genome was evident from the fact that all the randomly selected lines tested positive in both PCR and Southern hybridization with nptII gene specific primers and probe, respectively. Finally, a total of 15 true transgenic lines for nptII were generated (Fig. 4b) and multiplied as individual lines. In previous reports of Mondal et al. (2001) and Sandal et al. (2006, 2007), the actual number of Southern positive transgenic lines and final stable transformation efficiency were not mentioned. In the present study however, all the six lines tested positive in Southern hybridization. A similar observation was reported by Lopez et al. (2004) and Saini et al. (2012).
Transgene re-arrangements are known to alter the phenotype, the growth behavior or the trait for which a particular crop is useful or beneficial (Wilson et al. 2006). Although rearrangement of the integrated transgene was evident in the transgenic tea plants developed in the present study, yet their phenotypic characteristics remained largely unaltered with exception to lesser height of transgenics as compared to control (Table 2). While there was no mention of the phenotypic traits of the transgenics generated through Agrobacterium mediated transformation of leaf explants (Sandal et al. 2007), all previous reports described the transgenic lines generated from somatic embryos to be normal (Mondal et al. 2001; Lopez et al. 2004; Saini et al. 2012). While none of the previous studies reported about the reproductive behavior of the transgenic plants generated by them, distinct differences in the reproductive behavior of the transgenics and control plants were observed in the present study (Table 3). Higher rates of floral bud and fruit abscission in the transgenics were accompanied by the production of lower number of normal and viable seeds as compared to control. Finally, normal germination was recorded only in 7 % transgenic seeds as opposed to 47 % in not-bombarded control seeds. In case of transgenics, only 2.6 % seedlings finally survived to grow into normal plants. Thus, a marked suppression in the reproductive capacity was evident in the transgenics. Besides a strong possibility of sterile gamete(s) formation, such suppression could be the result of one or more of the different factors such as position effect of integrated transgene, homology dependent silencing of endogenous genes, genetic and/or epigenetic changes induced during the process of in vitro culturing and genetic transformation (Curtis et al. 2000; Aragão et al. 1996). However, the observed reproductive behavior could also be specific to the ten transgenic tea plants that were analysed in the present study.
Suppression of reproductive behavior in tea is advantageous because it can reduce the chances of transgene out-flow. Adding to the benefit is the fact that germ-line transmission in tea is automatically prevented as it is propagated vegetatively. In this regard, the present work will prove useful for stacking genes of important agricultural traits and quality parameters into selected elite plants of tea.
References
Aragão FJL, Barros LMG, Brasileiro ACM, Ribeiro SG, Smith FD, Sanford JC, Faria JC, Rech EL (1996) Inheritance of foreign genes in transgenic bean (Phaseolus vulgaris L.) co-transformed via particle bombardment. Theor Appl Genet 93:142–150
Bhat SR, Srinivasan S (2002) Molecular and genetic analyses of transgenic plants: considerations and approaches. Plant Sci 163:673–681
Bhattacharya A, Ahuja PS (2001) Transgenic tea and its scope in tea crop improvement. In: Singh RP, Jaiwal PK (eds) Plant genetic engineering. Sci-Tech Publishing Company, Houston, USA, pp 1–4
Bhattacharya A, Saini U, Joshi R, Kaur D, Pal AK, Kumar N, Gulati A, Mohanpuria P, Yadav SK, Kumar S, Ahuja PS (2014) Osmotin expressing transgenic tea plants have improved stress tolerance and quality parameters. Transgenic Res 23:211–223
Bhattacharyya J, Chakraborty A, Roy S, Pradhan S, Mitra J, Chakraborty M, Manna A, Sikdar N, Chakraborty S, Sen SK (2015) Genetic transformation of cultivated jute (Corchorus capsularis L.) by particle bombardment using apical meristem tissue and development of stable transgenic plant. Plant Cell Tiss Organ Cult 121:311–324
Birch RG, Franks T (1991) Development and optimization of micro-projectile systems for plant genetic transformation. Aust J Plant Physiol 18:453–469
Chen Z (1999) Pharmacological functions of tea. In: Jain NK (ed) Global advances in tea science. Aravali Books International (P) Ltd, New Delhi, pp 333–358
Chen JM, Yao QH, Xiong AS, Ma HX, Hong YH (2007) Structures of transgenic integration loci in transformed plants. Yi Chuan 29:157–162
Christou P (1990) Morphological description of transgenic soybean chimeras created by the delivery, integration and expression of foreign DNA using electric discharge particle acceleration. Ann Bot 66:379–386
Cooper R, Moore DJ, Morre DM (2005) Medicinal benefits of green tea: Part I. Review of anti-cancer properties. J Altern Complement Med 11:639–652
Curtis IS, Power JB, Hedden P, Phillips A, Lowe KC, Ward DA, Davey MR (2000) Transformation and characterization of transgenic plants of Solanum dulcamara—Incidence of transgene silencing. Ann Bot 86:63–71
Dalla Costa L, Pinto-Sintra AL, Campa M, Poletti V, Martinelli L, Malnoy M (2014) Development of analytical tools for evaluating the effect of T-DNA chimeric integration on transgene expression in vegetatively propagated plants. Plant Cell Tiss Organ Cult 118:471–484
De Guglielmo-Cróquer Z, Altosaar I, Zaidi M, Menéndez-Yuffá A (2010) Transformation of coffee (Coffea arabica L. cv. Catimor) with the cry1ac gene by biolistic, without the use of markers. Braz J Biol 70(2):387–393
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Faize M, Faize L, Burgos L (2010) Using quantitative real-time PCR to detect chimeras in transgenic tobacco and apricot and to monitor their dissociation. BMC Biotechnol 10:1–8
Hara Y (2003) Health benefits and industrial applications of tea catechins. Intern J Tea Sci 3:225
Jagga-Chugh S, Kachhawaha S, Sharma M, Kothari-Chajer A, Kothari SL (2012) Optimization of factors influencing microprojectile bombardment-mediated genetic transformation of seed-derived callus and regeneration of transgenic plants in Eleusine coracana (L.) Gaertn. Plant Cell Tiss Organ Cult 109:401–410
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:389–405
Kim JY, Gallo M, Alpeter F (2012) Analysis of transgene integration and expression following biolistic transfer of different quantities of minimal expression cassette into sugarcane (Saccharum spp. hybrids). Plant Cell Tiss Organ Cult 108:297–302
Lopez SJ, Kumar RR, Pius PK, Muraleedharan N (2004) Agrobacterium tumefaciens mediated genetic transformation in tea (Camellia sinensis [L.] O. Kuntze). Plant Mol Biol Rep 22:201a–201j
Mondal TK, Bhattacharya A, Ahuja PS, Chand PK (2001) Transgenic tea (Camellia sinensis (L.) O. Kuntze cv. Kangra Jat) plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep 20:712–720
Mondal TK, Bhattacharya A, Laxmikumaran M, Ahuja PS (2004) Recent advances of tea (Camellia sinensis) biotechnology. Plant Cell Tiss Organ Cult 76:195–254
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Saini U, Kaur D, Bhattacharya A, Kumar S, Singh RD, Ahuja PS (2012) Optimising parameters for biolistic gun-mediated genetic transformation of tea [Camellia sinensis (L.) O. Kuntze]. J Hortic Sci Biotechnol 87:605–612
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning—A laboratory manual, edition 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sandal I, Bhattacharya A, Sharma M, Ahuja PS (2003) Methods for micropropagation of tea plants from leaf explants. US Patent 6599743
Sandal I, Kumar A, Bhattacharya A, Sharma M, Shanker A, Ahuja PS (2005) Gradual depletion of 2,4-D in the culture medium for indirect shoot regeneration from leaf explants of Camellia sinensis (L.) O. Kuntze. Plant Growth Regul 47:121–127
Sandal I, Bhattacharya A, Ahuja PS (2006) Transgenic tea through biolistic using leaf explants. US Patent 7129394
Sandal I, Saini U, Bhattacharya A, Lacroix B, Kumar S, Ahuja PS, Citovsky V (2007) Agrobacterium-mediated genetic transformation of tea leaf explants: effects of counteracting bactericidity of leaf polyphenols without loss of bacterial virulence. Plant Cell Rep 26:169–176
Sharma M, Sood A, Nagar PK, Prakash O, Ahuja PS (1999) Direct rooting and hardening of tea microshoots in the field. Plant Cell Tiss Org Cult 58:111–118
Song DP, Feng L, Rana MM, Gao MJ, Wei Shu (2014) Effects of catechins on Agrobacterium-mediated genetic transformation of Camellia sinensis. Plant Cell Tiss Organ Cult 119:27–37
Tassy C, Partier A, Beckert M, Feuillet C, Barret P (2014) Biolistic transformation of wheat: increased production of plants with simple insertions and heritable transgene expression. Plant Cell Tiss Organ Cult 119:171–181
Topfer R, Schell J, Steinbiss HH (1988) Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acids Res 16:8725
Wilson AK, Latham JR, Steinbrecher RA (2006) Transformation-induced mutations in transgenic plants: analysis and biosafety implications. Biotechnol Genet Eng 23:209–234
Acknowledgments
The authors thank Mr. Pabitra Gain for his photographic inputs and also Senior Technical Officers, Mr. R. S. Shekhawat and Mr. Om Prakash for establishing and maintaining the plants under contained polyhouse conditions. This work was supported by the financial grant from Council of Scientific and Industrial Research (CSIR), India. Indra Sandal and Uksha Saini thank CSIR for their Senior Research Fellowships, while Rajash Koul acknowledges Research Fellowship from DBT, New Delhi, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandal, I., Koul, R., Saini, U. et al. Development of transgenic tea plants from leaf explants by the biolistic gun method and their evaluation. Plant Cell Tiss Organ Cult 123, 245–255 (2015). https://doi.org/10.1007/s11240-015-0828-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0828-x