Abstract
Doubled haploid technology is an important tool in plant breeding and research, but routine application requires the establishment of efficient protocols. Microspore culture is an attractive approach although its efficiency is strongly dependent on the genotype. In this study we evaluated the effects of the three ethylene inhibitors aminooxyacetic acid, 2,5-norbornadiene, and silver thiosulphate (STS) on embryogenesis, regeneration rate and green plant rate in triticale microspore culture. Our results show that STS at 20 µM in particular had a positive effect on the embryogenesis rate, suggesting that for many genotypes ethylene accumulates in the culture vessel above a critical threshold where it becomes inhibitory for embryogenesis. In addition, STS also appears to positively influence the green plant rate. Taken together, our results show that the addition of ethylene inhibitors at defined concentrations can counteract the negative effect of ethylene, increase the embryogenesis rate and potentially also the green plant rate in microspore culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doubled haploid (DH) technology is a valuable tool for plant breeding and research. It enables the rapid generation of completely homozygous lines, which is beneficial for genomic approaches and strongly reduces the time required to establish new cultivars (Forster et al. 2007; Wedzony et al. 2009; Ferrie and Caswell 2011). Consequently, DHs have been implemented in routine breeding programs for crops such as barley, for which an efficient DH protocol has been established. For other small grain cereals, high costs owing to a low efficiency of available protocols have hampered the routine application of DH technology. DHs can be generated by different methods, including wide crosses, gynogenesis by female gametophytes, and androgenesis through anther or microspore culture (Forster et al. 2007; Wedzony et al. 2009). For a number of crops including triticale (Triticosecale Wittmack L.), microspore culture is emerging as the DH method of choice due to the abundance of microspores per spike and the higher DH output per donor plant as compared to other approaches such as wide crosses (Castillo et al. 2009). The central process during microspore culture and a key factor limiting its application at a commercial level is the rate of embryogenesis, i.e. the frequency of embryo regeneration from isolated microspores. This requires a de-differentiation of microspores and subsequent development into embryos. This switch from the normal gametophytic developmental program to the formation of multicellular structures and subsequently to embryos can be achieved in vitro by subjecting the plants to different stress treatments. Despite this stress pre-treatment, the embryogenesis rate shows a strong genotype dependency and is often low, resulting in few generated DH plants and consequently a poor efficiency of the DH protocol. Besides the stress treatment, other factors such as colchicine, antibiotics, antioxidants, or inducer chemicals have also been reported to affect embryogenesis in different crops (Hansen and Andersen 1998; Zheng et al. 2001; Asif et al. 2013, 2014; Żur et al. 2014; Sinha and Eudes 2015), but with varying success.
Ethylene (C2H4) is the simplest olefin and is involved in the regulation of many physiological processes related to plant growth, development and senescence (Yang and Hoffman 1984). It is also known to affect plant tissue culture as it may accumulate in the culture vessel and affect growth and development of plant tissues (Williams et al. 1990; Biddington 1992; Tiainen 1996). The source of ethylene in the culture vessel is unclear but the pollen of many species contains high levels of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) (Hill et al. 1987), hence ethylene may be involved in the regulation of pollen germination and pollen tube growth (Cho and Kasha 1989). Reports on the effects of ethylene on tissue culture are scarce and provide contrasting results by either promoting or inhibiting microspore embryogenesis (Biddington et al. 1988; Cho and Kasha 1989). This is likely due to genotypic differences in endogenous ethylene production (Cho and Kasha 1989). Biddington and Robinson (1991) observed higher ethylene production during the early stages of anther culture of Brussels sprouts for poorly responsive as compared to more responsive cultivars. Furthermore, Dunwell (1979) found that in Nicotiana tabaccum anther culture, removal of ethylene from the culture vessel either enhanced or retarded embryo induction, depending on the size of the culture vessel and the age of the anthers in culture. Anthers with mature pollen produce much more ethylene compared to anthers with pollen at the first mitosis (Horner et al. 1977; Dunwell 1979). When ethylene was removed from cultures with more mature anthers, the embryo survival increased suggesting an inhibitory effect of high ethylene concentrations on embryogenesis and embryo growth (Dunwell 1979). Consequently, different substances known to inhibit ethylene action in plants have been found to affect in vitro embryo formation. In anther cultures of Brassica oleracea, for example, embryo production of three poorly responsive genotypes was increased by AgNO3, an inhibitor of ethylene (Biddington et al. 1988).
These results illustrate the potential of manipulating ethylene concentrations in tissue culture but this has yet to be exploited to improve DH production in microspore culture. The aim of our study was therefore to investigate the effect of ethylene inhibitors on the efficiency of DH production in hexaploid triticale. In particular, we evaluated three ethylene inhibitors in different concentrations and their effects on embryogenesis, plant regeneration and green plant rate, and validate the treatment on a large number of genotypes in an applied DH program.
Materials and methods
Plant material
Our study was based on winter triticale (Triticosecale Wittmack L.) F1 plants derived from three crosses between elite lines. The crosses are further referred to as Tcl 1 (TIW 671 × TIW 656), Tcl 2 (Mungis × SW Talentro), and Tcl 3 (Grenado × Cultivo). In addition, 30 F1 genotypes from the triticale DH program of the State Plant Breeding Institute of the University of Hohenheim were evaluated. Seeds of donor plants were sown in pots in September and kept outside the greenhouse for vernalization until February. Plants were then transferred to the greenhouse and grown at 24 °C with a photoperiod length of 16 h.
Microspore culture
Microspores were isolated following the general procedure described by Eudes and Amundsen (2005) and recently refined by Würschum et al. (2012, 2014). For Tcl 1, Tcl 2 and Tcl 3, 17-28 spikes from donor plants were used for isolation yielding between 4.5 and 10.5 million microspores. The microspore concentration was determined using a hemacytometer and the final concentration was set at 30,000 microspores per ml medium. Microspores were cultured in petri dishes with 3 ml culture medium. The microspores used to determine the effects of the different treatments on the three genotypes were always derived from a single isolation. The ethylene inhibitors investigated in this study were silver thiosulphate (STS), aminooxyacetic acid (AOA) and 2,5-norbornadiene (NBD). To assess the effect of these ethylene inhibitors, they were added to the culture medium. Two concentrations of each inhibitor were tested: 20 and 200 µM STS, 5 and 10 µM AOA, and 100 and 300 µM NBD. The concentrations were chosen based on existing literature to cover a broad range where the inhibitor is likely effective. The experiment included four replicates of the control without inhibitor and of each of the six inhibitor-concentration combinations. Embryo induction and plant regeneration was carried out as per Würschum et al. (2012). The protocol generates almost exclusively embryos and no calli and consequently the term ‘embryo’ refers to regenerated embryos and not regenerated calli. For the 30 F1 genotypes the number of replications with and without STS at 20 µM ranged from 8 to 24 (Fig. 2).
Statistical analyses
All statistical analyses were conducted using the statistical software R (R Development Core Team 2010). Mean comparisons were done by a Duncan test following ANOVA for the number of regenerated embryos and by calculating the confidence intervals with the R package binom (Pearson–Klopper method) for the binominal traits.
Results
In this study we evaluated the effects of three ethylene inhibitors on microspore culture in hexaploid triticale. The ethylene inhibitors AOA, NBD and STS were supplemented to the culture medium and tested in two concentrations each. The microspores of each genotype were obtained in a single isolation so that the effects of the different treatments are directly comparable. We observed differences between the three F1 genotypes with regard to the number of regenerated embryos in the control medium without ethylene inhibitor. Tcl 1 produced on average 19.3 embryos per 100,000 isolated microspores, Tcl 2 produced 47.5 while Tcl 3 was the most responsive genotype and produced 111.3 embryos (Table 1).
The addition of NBD to the culture medium resulted in an almost complete inhibition of embryogenesis for all three genotypes (Table 1; Fig. 1). By contrast, AOA had no effect on Tcl 1, but for Tcl 2 and Tcl 3 increased the number of regenerated embryos when supplied at 10 µM concentration. While STS had no or even a negative effect at 200 µM, it led to a substantial increase in the number of regenerated embryos when added to the medium at a concentration of 20 µM. This trend was most pronounced for Tcl 1, the least responsive genotype, but also for the highly responsive Tcl 3 genotype a slightly increased number of regenerated embryos could be observed.
The regeneration rate, i.e. the proportion of plantlets regenerated from the embryos, ranged in the control from 24.8 % for Tcl 2 to 41.5 % for Tcl 1. These differences were not significant and also the treatments with AOA and STS resulted in no significantly different regeneration rates, except for STS at 200 µM for Tcl 3. By contrast, the addition of AOA at 10 µM and even more so STS at 20 µM appeared to have a positive effect on the proportion of green plants among the regenerated plants, i.e. increased the proportion of green and reduced the proportion of albino plants.
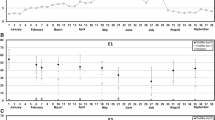
The results from this experiment with three genotypes suggested that STS at a concentration of 20 µM can have a strong positive effect on embryogenesis in triticale microspore culture and potentially also on the green plant rate without negatively affecting the plant regeneration rate. We therefore evaluated STS at 20 µM concentration on a larger number of F1 genotypes derived from our triticale breeding program. This analysis showed that with only few exceptions, the addition of STS led to a higher number of embryos as compared to the control without STS (Fig. 2). Across all 30 genotypes, the average number of embryos per 100,000 isolated microspores was 44.5 for the control and 68.2 for the STS variant (P < 0.05). While the regeneration rate was generally comparable between both variants, this analysis further supported the positive effect of STS on the green plant rate.
Effect of silver thiosulphate (STS at 20 µM) on embryogenesis, i.e. regenerated embryos per 105 microspores, plant regeneration rate, and green plant rate assessed on 30 different triticale genotypes. The bars show the mean and the whiskers the standard deviation; n Rep. refers to the number of replications. Significant differences (t test P < 0.05) between the STS variant and the control are indicated by an asterisk
Discussion
Doubled haploid production is a key technology in plant breeding and research. However, the lack of an efficient protocol as well as strong variation in the response among genotypes for in vitro approaches has so far limited its widespread use in many small grain cereals, including triticale. Ethylene has been shown to accumulate in the culture vessel during tissue culture but the results of experiments aimed at modifying ethylene concentrations in tissue culture are ambiguous and varied between species and even among cultivars of species (Cho and Kasha 1989; Biddington 1992; Tiainen 1996). While ethylene appears to be involved in microspore embryogenesis in anther culture, the effects of modifying ethylene levels in microspore culture of small grain cereals have not been evaluated yet. In this study, we therefore investigated the effects of ethylene inhibitors on microspore culture in hexaploid triticale.
The plant growth regulator ethylene is produced by most plant tissues including germinating seeds. The main component controlling ethylene biosynthesis is 1-aminocyclopropane (ACC) synthase which converts S-adenosylmethionine to ACC, the immediate precursor of ethylene (Yang and Hoffman 1984). Among the most commonly used inhibitors of ethylene biosynthesis are aminoethoxyvinylglycine (AVG) and AOA which inhibit ACC synthase. In addition to AOA as an inhibitor of ethylene biosynthesis, we evaluated two inhibitors of ethylene perception that differ in nature: silver ions and NBD. Silver ions are known as potent, specific, non-competitive inhibitors of ethylene binding (Beyer 1976) that have been widely used either as the nitrate (AgNO3) or as the more mobile thiosulphate (STS). By contrast, NBD is a volatile competitive inhibitor of the ethylene receptor (Sisler and Pian 1973; Locke et al. 2000).
We observed strong differences among the three triticale genotypes with regard to the number of regenerated embryos per 100,000 isolated microspores, which corroborates results on genotypic differences in responsiveness observed in a range of other crops (Wedzony et al. 2009). AOA at 10 µM concentration increased the number of regenerated embryos as compared to the control for two of the three genotypes while STS at 20 µM resulted in an increase for all three tested genotypes (Table 1). This indicates that during triticale microspore embryogenesis, ethylene accumulation in the culture vessel has a negative effect on embryogenesis that can be counteracted by the addition of ethylene inhibitors. The ethylene and polyamine biosynthetic pathways are linked through S-adenosylmethionine and Locke et al. (2000) reported an increased production of polyamines by blocking ethylene biosynthesis and a subsequent effect on germination and seedling growth of barley. Our finding that both the ethylene biosynthesis inhibitor AOA and the ethylene perception inhibitor STS resulted in a significant increase in the number of regenerated embryos suggests that this is due to reduced ethylene levels in the culture vessels and not to an indirect effect of increased polyamine levels which have been shown to increase embryo yield in potato anther culture (Tiainen 1992).
The strongest and most consistent increase in regenerated embryos was obtained with STS at 20 µM concentration. Interestingly, we observed the strongest increase in the number of regenerated embryos relative to the control for Tcl 1, the least responsive genotype. By contrast, the most responsive genotype Tcl 3 showed the least relative increase in embryogenesis as compared to the control. Ethylene production in anther culture of Brussels sprouts was found to be ~20 times higher in a poorly responsive cultivar as compared to a highly responsive cultivar (Biddington and Robinson 1991). Our results therefore support previous findings and suggest that the lower embryogenesis rate of some cultivars might be caused by higher ethylene production and consequently a higher concentration of ethylene in the culture vessel. Consistent with this, Cho and Kasha (1989) reported different patterns of ethylene production and concentrations of ACC in barley anther cultures among different genotypes. Biddington et al. (1988) observed that the inhibitory effect of endogenous ethylene was counteracted over a wide range of AgNO3. By contrast, silver thiosulfate had a stimulatory effect on anther culture of potato at certain concentrations but was inhibitory at other concentrations (Tiainen 1992). We obtained a similar result in that STS at 200 µM had a slight positive effect for Tcl 1 but a strong negative effect for Tcl 2 and Tcl 3. This indicates that ethylene not only has a negative effect on microspore embryogenesis but also a stimulatory effect, depending on the concentration. Consistent with the assumption that the two more responsive genotypes, Tcl 2 and Tcl 3, have lower ethylene levels than Tcl 1, the higher concentration of STS is likely to result in a further reduction of ethylene below a critical threshold required for embryogenesis. This indicates that ethylene levels must be carefully balanced for optimum response in microspore embryogenesis. The source of ethylene in microspore culture is unclear but it could be derived from the endogenous precursor ACC, from hormones (e.g. IAA) or from the sensitivity of anthers to stress like for example dehydration or wounding (Cho and Kasha 1989). Notably, the concentration of ethylene in the culture vessel not only depends on the amount of ethylene produced by the genotype but also on its loss from the culture vessel (Biddington 1992). Taken together, our results illustrate that embryogenesis in microspore culture of triticale can be substantially enhanced by the addition of ethylene inhibitors to the culture medium. Importantly, the regeneration rate was not adversely affected by the addition of AOA or STS while the green plant rate also appeared to be positively affected by the two ethylene inhibitors, especially by STS.
While the genotypic differences observed for the control were still present when the ethylene inhibitors AOA or STS were added to the medium, the embryo yield was substantially increased, particularly for the least responsive genotype, thus increasing the overall efficiency of the DH production. To assess the effect of ethylene inhibition on microspore embryogenesis in a large number of triticale genotypes, we evaluated STS at 20 µM on 30 F1 genotypes from our routine DH program. This analysis substantiated the positive effect of STS on embryogenesis suggesting that for most triticale genotypes ethylene levels are above the threshold where it becomes inhibitory. Furthermore, this analysis supported another positive effect of STS on microspore culture as the green plant rate was generally higher compared to the control.
Taken together, our results suggest that ethylene accumulation above a critical threshold has an inhibitory effect on microspore embryogenesis in triticale. Differences in the capacity to produce ethylene and subsequently in ethylene concentrations in the culture vessel are likely to contribute to the observed genotypic differences in embryogenesis rate. Thus, ethylene inhibitors, especially STS, appear promising to adjust ethylene to an optimum level and thereby increase the efficiency of DH production based on microspore culture.
References
Asif M, Eudes F, Goyal A, Amundsen E, Randhawa H, Spaner D (2013) Organelle antioxidants improve microspore embryogenesis in wheat and triticale. In Vitro Cell Dev Biol Plant 49:489–497. doi:10.1007/s11627-013-9514-z
Asif M, Eudes F, Randhawa H, Amundsen E, Spaner D (2014) Phytosulfokine alpha enhances microspore embryogenesis in both triticale and wheat. Plant Cell, Tissue Organ Cult 116:125–130. doi:10.1007/s11240-013-0379-y
Beyer EM (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58:268–271
Biddington NL (1992) The influence of ethylene in plant tissue culture. Plant Growth Regul 11:173–187
Biddington NL, Robinson TH (1991) Ethylene production during anther culture of Brussel sprouts (Brassica oleracea var. gemmifera) and its relationship with factors that affect embryo production. Plant Cell Tissue Cult 25:169–177
Biddington NL, Sutherland RA, Robinson HT (1988) Silver nitrate increases embryo production in anther culture of Brussels sprouts. Ann Bot 62:181–185
Castillo AM, Cistué L, Vallés MP, Soriano M (2009) Chromosome doubling in monocots. In: Touraev A, Forster BP, Mohan Jain S (eds) Advances in haploid production in higher plants. Springer, Berlin, pp 329–338
Cho UH, Kasha KJ (1989) Ethylene production and embryogenesis from anther cultures of barley (Hordeum vulgare). Plant Cell Rep 8:415–417
Dunwell JM (1979) Anther culture in Nicotiana tabacum: the role of culture vessel atmosphere in pollen embryo induction and growth. J Exp Bot 30:419–428
Eudes F, Amundsen E (2005) Isolated microspore culture of Canadian 6× triticale cultivars. Plant Cell, Tissue Organ Cult 82:233–241. doi:10.1007/s11240-005-0867-9
Ferrie AMR, Caswell KL (2011) Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell, Tissue Organ Cult 104:301–309
Forster BP, Heberle-Bors E, Kasha KJ, Touraev A (2007) The resurgence of haploids in higher plants. Trends Plant Sci 12:368–375. doi:10.1016/j.tplants.2007.06.007
Hansen NJP, Andersen SB (1998) In vitro chromosome doubling with colchicine during microspore culture in wheat (Triticum aestivum L.). Euphytica 102:101–108
Hill SE, Stead AD, Nichols R (1987) Pollination-induced ethylene and production of 1-aminocyclopropane-1-carboxylic acid by pollen of Nicotiana tabacum cv. White Burley. J Plant Growth Regul 6:1–13
Horner M, McComb JA, McComb AJ, Street HE (1977) Ethylene production and plantlet formation by Nicotiana anthers cultured in the presence and abscence of charcoal. J Exp Bot 28:1365–1372
Locke JM, Bryce JH, Morris PC (2000) Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J Exp Bot 51:1843–1849
R Development Core Team (2010) R: a language and environment for statistical computing, R foundation for statistical computing. http://www.R-project.org
Sinha RK, Eudes F (2015) Dimethyl tyrosine conjugated peptide prevents oxidative damage and death of triticale and wheat microspores. Plant Cell, Tissue Organ Cult 122:227–237. doi:10.1007/s11240-015-0763-x
Sisler EC, Pian A (1973) Effect of ethylene and cyclic olefins on tobacco leaves. Tob Sci 17:68–72
Tiainen T (1992) The role of ethylene and reducing agents on anther culture response of tetraploid potato (Solanum tuberosum L.). Plant Cell Rep 10:604–607
Tiainen T (1996) Influence of ethylene in microspore embryogenesis. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 1, pp 177–187
Wedzony M, Forster BP, Zur I, Golemice E, Szechynska-Hebda M et al (2009) Progress in doubled haploid technology in higher plants. In: Touraev A, Forster BP, Mohan Jain S (eds) Advances in haploid production in higher plants. Springer, Berlin, pp 1–33
Williams J, Pink DAC, Biddington NL (1990) Effect of silver nitrate on long-term culture and regeneration of callus from Brassica oleracea var. gemmifera. Plant Cell, Tissue Organ Cult 21:61–66
Würschum T, Tucker MR, Reif JC, Maurer HP (2012) Improved efficiency of doubled haploid generation in hexaploid triticale by in vitro chromosome doubling. BMC Plant Biol 12:109. doi:10.1186/1471-2229-12-109
Würschum T, Tucker MR, Maurer HP (2014) Stress treatments influence efficiency of microspore embryogenesis and green plant regeneration in hexaploid triticale (×Triticosecale Wittmack L.). In Vitro Cell Dev Biol Plant 50:143–148. doi:10.1007/s11627-013-9539-3
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol 35:155–189
Zheng MY, Liu W, Weng Y, Polle E, Konzak CF (2001) Culture of freshly isolated wheat (Triticum aestivum L.) microspores treated with inducer chemicals. Plant Cell Rep 20:685–690. doi:10.1007/s00299-001-0393-0
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K et al (2014) Antioxidant activity and ROS tolerance in triticale (×Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell, Tissue Organ Cult 119:79–94. doi:10.1007/s11240-014-0515-3
Acknowledgments
We greatly acknowledge the lab work of Alexandra Appel and Sylvia Kaiser.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
The authors declare that the experiments comply with the current laws of Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Würschum, T., Tucker, M.R., Maurer, H.P. et al. Ethylene inhibitors improve efficiency of microspore embryogenesis in hexaploid triticale. Plant Cell Tiss Organ Cult 122, 751–757 (2015). https://doi.org/10.1007/s11240-015-0808-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0808-1